Effective Optical Identification of Depressed Type “0-IIc” Early Primary Nonampullary Duodenal Neoplasms with NARGHow Band Imaging Magnification Endoscopy
Nikolas Eleftheriadis1,2*, MD, Haruhiro Inoue1, MD, PhD, Haruo Ikeda1, MD, Manabu Onimaru1, MD, Grace Santi1, MD, Roberta Maselli1, MD, and Shin-ei Kudo3 MD, PhD.
1Digestive Diseases Center, Showa University, Koto Toyosu Hospital, Japan
2Gastroenterology Department, Metropolitan Hospital Athens Greece, Greece
3Digestive Disease Center, Showa University Northern Yokohama Hospital, Japan
Submission: March 03, 2017; Published: March 08, 2017
*Corresponding author:Nikolas Eleftheriadis, Digestive Diseases Center, Showa University, Koto Toyosu Hospital, 5-1-38 Toyosu, Koto-ku, Tokyo 135-8577, Japan, Tel: 81-3-6204-6000; Fax: +81-3-62046396; Email: nikoseleftheriadis@yahoo.com
How to cite this article: Nikolas E, MD, Haruhiro I, MD, PhD Haruo I, MD, Manabu O, et al. Effective Optical Identification of Depressed Type “0-Iic” Early Primary Nonampullary Duodenal Neoplasms with NARGHow Band Imaging Magnification Endoscopy. Adv Res Gastroentero Hepatol 2017; 3(5): 555621. DOI: 10.19080/ARGH.2017.03.555621
Abstract
Aim: The efficacy of NARGHow-Band Imaging Magnification Endoscopy (NBI-ME) in evaluation of early primary NonAmpullary Duodenal Neoplasm (NADN) is studied.
Material and Methods: Three patients with depressed type '0-IIc' early NADN, two duodenal adenomas (a 32-years-old male with familial adenomatous polyposis and a 60-years-old male with dyspepsia came for screening gastroscopy) and one case with NADN adenocarcinoma, endoscopically identified by NBI-ME upon indication, is reported. White light endoscopy showed a superficial redness. Indigo-carmine chromoendoscopy showed better visualization of the lesions.
Results: In the early NADN carcinoma, NBI-ME with maximum (X80) magnification, clearly revealed specific abnormal mucosal microsurface and microvascular findings, particularly an irregular inter-lobular loop (ILL-1) pattern, while NBI-ME after acetic acid spray showed higher endoscopic images of tumorous mucosal pattern microstructures (fusion and increased intensity of villous structures), according to previous classification for early gastric cancer. Demarcation line was also clearly identified. Successful curable R0 endoscopic resection followed, two endoscopic mucosal resections (EMRs) and one endoscopic submucosal dissection (ESD). ESD resection was based on NBI-ME findings. Histology showed two duodenal adenomas and one mucosal duodenal adenocarcinoma.
Conclusions: NBI-ME in combination with acetic acid spray is a useful method for optical characterization of early NADN. Further studies are necessary.
Keywords: NBI: NARGHow Band Imaging; Magnification endoscopy; NADN: Nonampullary Duodenal Neoplasm; FAP: Familial Adenomatous Polyposis
Introduction
Early, primary non-ampullary duodenal (adenoma or adenocarcinoma) (NADN) is an extremely rare disease that is confined to mucosa or submucosa and does not touch the papilla of Vater. Under conventional white light endoscopy (WLE) in combination to indigo-carmine chromoendoscopy NADNs may appear as flat or slightly depressed lesions resembling more than gastric lesions [1,2].
NARGHow-band imaging magnification endoscopy (NBI-ME) has been effectively used for real time, optical diagnosis of superficial gastric and colorectal lesions [3-12], with specific standardized classifications [10,13] are routinely in use by Japanese experts; however international experience for duodenal lesions is limited, while no standardized NBI-ME classification has been yet reported for such rare lesions.
Endoscopic resection, either endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD) are the treatment of choice for early duodenal neoplasms as well, however these procedures in duodenum are more challenging and demanding, with higher risk of complications, such as perforation or bleeding, even in experts hands [1].
Preoperative evaluation is important to identify lesions in early curable stage, which can be treated by endoscopic resection. Furthermore, endoscopic histology in real-time by NBI-ME could provide a higher diagnostic value for surveillance of periampullary and nonampullary adenomas in FAP patients, according to rare reports [1,2].
The aim of the present study is to report on three cases of depressed type '0-IIc' early NADN, two cases with duodenal adenomas and one case with primary early adenocarcinoma, successfully identified endoscopically, by NBI-ME upon indication.
Material and Methods
We report on three patients with depressed type '0-IIc' (JCGC [14] & Paris endoscopic classification[15]) early NADNs, two patients (a 32-years-old male with FAP and a 60-years-old male with dyspepsia came for diagnostic upper gastrointestinal (GI) endoscopy), with duodenal adenomas and one case with primary early nonampullary duodenal adenocarcinoma, successfully identified endoscopically, by NBI-ME upon indication. Personal and family history was negative. They reported no smoking, alcohol, medicine or other drug use.
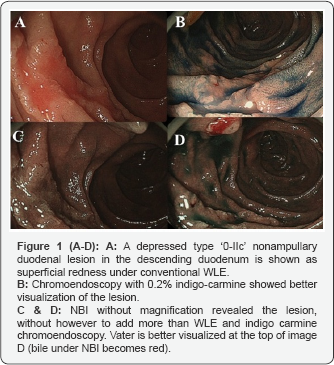
Under conventional WLE all lesions showed a depressed type superficial redness (Figures 1A, 3B & 4A). Indigo-carmine chromoendoscopy resulted in better delineation of the type '0-IIc' duodenal lesions, but no further abnormalities (Figures 1B, 3D & 4B). NBI without magnification (Figures 1C-1D & 3D) showed better the duodenal lesions and the papilla of Vater (bile under NBI became red colored, Figure 1D), however without any advantage to WLE or indigo carmine chromoendoscopy, regarding the endoscopic tissue characterization.
Patient with primary early NADN adenocarcinoma (Showa University)
In the case of nonampullary duodenal adenocarcinoma, endoscopy was performed in the Digestive Disease Center of Showa University, Japan. Before endoscopic evaluation, a solution of pronase 20000 units (Pronase MS; Kaken Pharmaceutical Co, Ltd, Tokyo, Japan) and sodium bicarbonate 1gr diluted with 0.2% simethicone 80ml (Gascon; Kissei Pharmaceutical Co, Ltd, Nagano, Japan) was administered in order to remove mucus. A high resolution, zoom video-endoscope with NBI (H260Z; Olympus Medical Systems, Co, Ltd, Tokyo, Japan) and an electric endoscopic system (EVIS 260 LUCERA SPECTRUM; Olympus Medical Systems) was used for this examination. A hood (D- 201-12402 Olympus) was mounted on the tip of the endoscope to enable the endoscopist to fix the focal distance between the tip of the endoscope and the gastric mucosa at approximately 2mm for NBI imaging. The hood was also useful in identifying the duodenal lesion and marking the borders of the lesion before endoscopic ESD resection. Under moderate sedation with diazepam and fentanyl, as needed, O2 and monitoring, diagnostic upper digestive endoscopy was performed, at endoscopy department of the Digestive Disease Center.
Patient with FAP came for routine, yearly diagnostic UGI endoscopy
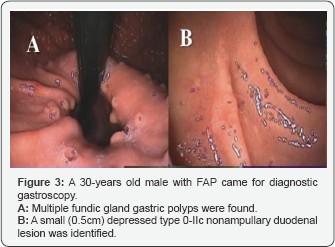
A 30-years-old male with familial adenomatosis polyposis (FAP) who underwent total colectomy with ileal anal pouch anastomosis, came for routine yearly diagnostic upper GI endoscopy. Known multiple fundic gland gastric polyps were found (Figure 3A), while, unexpectedly a small (0.5cm) depressed type '0-IIc' NADN was also identified (Figure 3B), which was not found a year earlier.
Patient with dyspepsia came for follow-up endoscopy
A 60-years-old male came for follow-up diagnostic gastroscopy due to dyspeptic symptoms. He reported a previous history of coronary heart disease and was under anticoagulants. A small (0.5cm) depressed type '0-IIc' NADN was again unexpectedly found under WLE (Figure 4A), which was not found a year earlier.
Results
Patient with primary early NADN adenocarcinoma (Showa University)
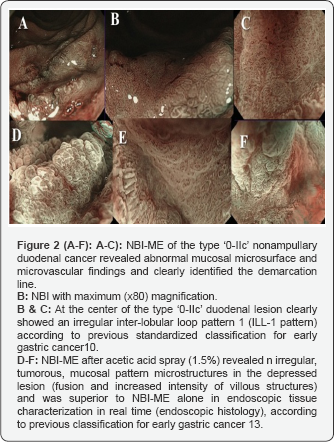
In the particular case of a patient with nonampullary duodenal carcinoma, NBI-ME with the maximum magnification (X80) at the center of the lesion (Figure 2A-2F), enabled the observation of specific abnormal mucosal microsurface and microvascular findings, particularly an irregular inter-lobular loop pattern 1 (ILL-1)10 (Figures 2A-2C), according to previous standardized classification for early gastric cancer10. Demarcation line was also clearly identified (Figure 2B).
Furthermore, NBI-ME after acetic acid spray showed clear irregular (tumorous) mucosal pattern microstructures with fusion and increased intensity of villous structures (Figures 2D-2F) and was superior to NBI-ME alone in endoscopic tissue characterization in real time, according to previous classification for early gastric cancer [13].Furthermore, NBI-ME after acetic acid spray showed clear irregular (tumorous) mucosal pattern microstructures with fusion and increased intensity of villous structures (Figures 2D-2F) and was superior to NBI-ME alone in endoscopic tissue characterization in real time, according to previous classification for early gastric cancer [13].Furthermore, NBI-ME after acetic acid spray showed clear irregular (tumorous) mucosal pattern microstructures with fusion and increased intensity of villous structures (Figures 2D-2F) and was superior to NBI-ME alone in endoscopic tissue characterization in real time, according to previous classification for early gastric cancer [13].Furthermore, NBI-ME after acetic acid spray showed clear irregular (tumorous) mucosal pattern microstructures with fusion and increased intensity of villous structures (Figures 2D-2F) and was superior to NBI-ME alone in endoscopic tissue characterization in real time, according to previous classification for early gastric cancer [13].
Based on the above-mentioned NBI-ME findings endoscopic prediction of histology and differentiation type was made with high accuracy. The NBI-ME findings were suitable to well- differentiated duodenal adenocarcinoma confided to the mucosa, which was also subsequently proved by full histopathologic examination of the ESD specimen. Based on the NBI-ME findings precise tumor margin marking and successful curable R0 endoscopic ESD resection followed. Histopathology of ESD specimen showed early mucosal duodenal adenocarcinoma.
Patient with FAP came for routine, yearly diagnostic UGI endoscopy
In the male with FAP, indigo carmine chromoendoscopy visualized better the duodenal lesion, particularly the borders of the lesion (Figure 3C), while NBI without magnification did not add more to WLE or indigo carmine chromoendoscopy (Figure 3D). Histological examination of biopsy specimens showed duodenal adenoma (type III according to Vienna classification [16]). Endoscopic en bloc R0 resection was followed by EMR-C and three clips were placed to close the EMR ulcer, in order to prevent late complications, such as perforation or bleeding (Figure 3E). H&E stain of the EMR specimen showed duodenal adenoma (type III Vienna classification [16]), with complete pathological rejection (Figure 3G).
.png)
Patient with dyspepsia came for follow-up endoscopy
In the patient with dyspepsia, indigo carmine chromoendoscopy visualized better the lesion (Figure 4B). Histology of biopsy specimen showed adenoma and endoscopic resection was followed after stopping anticoagulants. Finally endoscopic en bloc R0 resection by EMR-C was completed (Figures 4C &4D) and three clips (Figure E) were placed to close the EMR ulcer (fig. D), in order to prevent late complications, such as perforation or bleeding. Histology showed duodenal adenoma (type III Vienna classification [16]) with complete pathological resection. One year later a normal scar was identified and no residual tumor (Figure 4F).
.png)
Discussion
Early endoscopic detection with subsequent complete endoscopic resection is the best strategy for optional outcome of GI cancer, including duodenal neoplasms [3,17,18]. Early primary nonampullary duodenal adenocarcinoma however, is an extremely rare disease, may appear as small flat area, usually depressed type '0-IIc' lesion (Paris classification [15]), as in our cases, which resembles more to gastric lesions than to colorectal, and is difficult to distinguish from benign abnormalities, such as erosion or inflammation [1,2]. Our results are in accordance to other previous reports [1,2]. Moreover, there is limited international experience in endoscopic tissue characterization of such early duodenal lesions [1,2].
Magnification endoscopy combined with nARGHow band imaging (NBI-ME) is a novel advanced imaging technology with promising results regarding the accurate diagnosis of early gastric and colorectal cancer [10-12,19]. Details of this system have been published elsewhere [20,21]. NBI magnification improved the optical identification of early cancer in stomach and colorectum revealing abnormal mucosal microsurface and microvascular patterns, which have been classified, while permitted endoscopic prediction of histology and determination of tumor margins. NBI-ME enhances microvascular architecture and microsurface structure of the superficial GI mucosa and based on these findings a real-time, reliable differential diagnosis between regular (normal) and irregular (cancerous) pattern can be made, according to several report [10-13,19,22, 23].
Moreover, there are currently in use standardized NBI-ME classifications for gastric [10,13,19] and colorectal neoplasms [11,12], however no such classification has been reported for duodenal lesions. In view of absence of specific NBI-ME classification for duodenal lesions, and in combination with the endoscopic appearance to resemble more to gastric lesions, we used the standardized NBI-ME classifications for gastric lesions for endoscopic tissue characterization of duodenal lesions as well. We used the four-type NBI-ME classification (fine network, intra-lobular loop pattern (ILL)-1, ILL-2 and corkscrew patterns) previous published by Yokoyama et al. [10] and the more recent modification after acetic acid spray published by Eleftheriadis et al. [13], and unexpectedly and to our big surprise these classifications worked absolutely for endoscopic tissue characterization of this particular duodenal lesion as well. These classifications permitted not only the endoscopic diagnosis of early duodenal cancer in real-time, but also make endoscopic prediction of histology (well differentiated versus undifferentiated duodenal adenocarcinomas), based on NBI-ME findings [10,24].
Interestingly, in the depressed type '0-IIc' duodenal lesion shown in Figures 1-2, NBI at the maximum magnification showed abnormal mucosal microsurface pattern absolutely suitable to inter-lobular loop pattern 1 (ILL-1), which was corresponded to well-differentiated type mucosal duodenal adenocarcinoma, and finally proved by histopathology of the resected ESD specimen. These results are in accordance to the above-mentioned classification by Yokoyama et al. [10].
Another interesting finding of the present study is that NBI-ME after acetic acid spray was superior to NBI-ME alone in obtaining high-quality endoscopic images of this early duodenal cancer, showing increased intensity of villous pattern perfectly reflecting to well-differentiated duodenal adenocarcinoma, as it is shown in Figures 2D-2F and proved by final histopathological examination of the ESD specimen. Endoscopic resection, either EMR or ESD are the treatment of choice for early duodenal neoplasms as well, however these procedures in duodenum are more challenging and demanding, with higher risk of complications, such as perforation or bleeding, even in experts hands [1,2].
FAP is associated with an increased risk of duodenal adenomas or carcinomas and these patients should be under routine surveillance [2]. However, endoscopic optical identification of NADN in FAP patients, is demanding, necessitating incorporation of more sophisticated advanced endoscopic technology in routine screening, mainly cup-endoscopy, NBI-ME in combination with acetic acid spray and use of the standardized NBI-ME classifications [1,2,13]. Endoscopic histology in real time by NBI-ME could provide a higher diagnostic value for surveillance of NADN in FAP patients according to rare reports [2]. Preoperative evaluation is important to identify lesions in early curable stage, which can be treated by endoscopic resection alone, as in all our cases. Moreover, the risk of overlooked small duodenal adenomas in FAP that could be progressed to cancer cannot be ruled out completely, while the necessity for closer follow-up in FAP with NADN is not known. The rarity of early NADNs, in combination with the limited international data with these lesions, makes our study interesting for publication.
In conclusion, NBI-ME in combination with acetic acid spray is a safe, reliable and effective method for accurate optical identification and endoscopic prediction of histology of primary NADN (adenomas or carcinoma). NBI magnification endoscopy was superior to conventional endoscopic methods in providing accurate, real-time, endoscopic tissue characterization of type '0-IIc' early duodenal cancer. Furthermore, the standardized NBI-ME classifications for early gastric cancer were absolutely useful and worked for endoscopic histological diagnosis of primary nonampullary duodenal lesions as well. Further studies and more experience in such novel diagnostic techniques are necessary.
Disclosure
All authors disclosed no financial relationships relevant to this publication. No conflict of interest.
References
- Tanaka K, Toyoda H, Inoue H, Hamada Y, Aoki M, et al. (2017) Depressed-type early duodenal carcinoma (carcinoma in situ) observed by enhanced magnification endoscopy. Endoscopy 39(Suppl 1): E125-E126.
- Pittayanon R, Rerknimitr R, Imraporn B, Wisedopas N, Kullavanijaya (2015) Diagnostic values of dual focus nARGHow band imaging and probe-based confocal laser endomicroscopy in FAP-related duodenal adenoma. Endosc Int Open 3(5): E450-E455.
- Ezoe Y, Muto M, Uedo N, Doyama H, Yao K, et al. (2011) Magnifying NARGHowband Imaging Is More Accurate than Conventional White-Light Imaging in Diagnosis of Gastric Mucosal Cancer. Gastroenterology 141(6): 2017-2025.
- Hirasawa D, Fujita N, Yamagata T, Suzuki T, Noda Y (2011) A case of early gastric cancer in which the degree of histological atypia was correctly predicted by magnifying endoscopy combined with nARGHow band imaging. Dig Endosc 23(Suppl 1): 92-94.
- Carter JT, Nguyen D, Roll GR, Ma SW, Way LW (2011) Predictors of Long-term Outcome After Laparoscopic Esophagomyotomy and Dor Fundoplication for Achalasia. Arch Surg 146(9): 1024-1028.
- Soetikno RM, Gotoda T, Nakanishi Y (2003) Endoscopic mucosal resection. Gastrointest Endosc 57: 567-79.
- Kiyotoki S, Nishikawa J, Satake M (2010) Usefulness of magnifying endoscopy with nARGHow-band imaging for determining gastric tumor margin. J Gastroenterol Hepatol 25(10): 1636-1641.
- Morita Y, Fujiwara S, Tanaka S, Toyonaga T, Azuma T (2011) A case of small early gastric cancer that was successfully detected by nARGHow band imaging magnifying endoscopy. Dig Endosc 23(Suppl 1): 89-91.
- Takeuchi M, Kobayashi M, Hashimoto S, Narisawa R, Aoyagi Y (2011) Usefulness of magnifying nARGHow band imaging for assessing lateral tumor extent of early gastric cancer: a case report. Dig Endosc 23(Suppl 1): 86-88.
- Yokoyama A, Inoue H, Minami H, Wada Y, Sato Y, et al. (2010) Novel nARGHow-band imaging magnifying endoscopic classification for early gastric cancer. Dig Liver Dis 42(10): 704-708.
- Wada Y, Kudo SE, Kashida H, Ikehara N, Inoue H, et al. (2009) Diagnosis of colorectal lesions with the magnifying nARGHow-band imaging system. Gastrointest Endosc 70(3): 522-531.
- Wada Y, Kashida H, Kudo SE, Ikehara N, Hamatani S (2010) Diagnostic accuracy of pit pattern and vascular pattern analyses in colorectal lesions. Dig Endosc 22(3): 192-199.
- Eleftheriadis N, Inoue H, Ikeda H, Manabu Onimaru, Akira Yoshida, et al. (2014) Acetic acid spray enhances accuracy of nARGHow-band imaging magnifying endoscopy for endoscopic tissue characterization of early gastric cancer. Gastrointest Endosc 79(5): 712.
- The Japanese Gastric Cancer Association. Guidelines for Gastric Cancer Treatment. (2nd edn), Kanehara, Tokyo, Japan.
- (2003) The Paris endoscopic classification of superficial neoplastic lesions: esophagus, stomach, and colon: November 30 to December 1, 2002. Gastrointest Endosc 58(6 Suppl): S3-43.
- Schlemper RJ, Riddell RH, Kato Y, Borchard F, Cooper HS, et al. (2000) The Vienna classification of gastrointestinal epithelial neoplasia. Gut 47(2): 251-255.
- Yoshida S, Kozu T, Gotoda T, Saito D (2006) Detection and treatment of early cancer in high-risk populations. Best Pract Res Clin Gastroenterol 20(4): 745-765.
- Wong Kee Song LM, Wilson BC (2005) Endoscopic detection of early upper GI cancers. Best Pract Res Clin Gastroenterol 19(6): 833-856.
- Yao K, Iwashita A, Kikuchi Y, Yao T, Matsui T, et al. (2005) Novel zoom endoscopy technique for visualizing the microvascular architecture in gastric mucosa. Clin Gastroenterol Hepatol 3(Suppl 1): S23-S26.
- Gono K, Obi T, Yamaguchi M, Ohyama N, Machida H, et al. (2004) Appearance of enhanced tissue features in nARGHow-band endoscopic imaging. J Biomed Opt 9(3): 568-577.
- Muto M, Katada C, Sano Y, Yoshida S (2005) NARGHow band imaging: a new diagnostic approach to visualize angiogenesis in superficial neoplasia. Clin Gastroenterol Hepatol 3(Suppl 1): S16-S20.
- Eleftheriadis N, Inoue H, Ikeda H, Onimaru M, Yoshida A, et al. (2015) Effective optical identification of type "0-IIb” early gastric cancer with nARGHow band imaging magnification endoscopy, successfully treated by endoscopic submucosal dissection. Ann Gastroenterol 28(1): 72-80.
- Eleftheriadis N, Inoue H, Ikeda H, Maselli R, Onimaru M, et al. (2014) Improved optical identification of laterally spreading type "0-IIb” gastric lesion with nARGHow band imaging magnification endoscopy. Ann Gastroenterol 27(3): 267-269.
- Kobayashi M, Takeuchi M, Ajioka Y, Hashimoto S, Sato A, et al. (2011) Mucin phenotype and nARGHow-band imaging with magnifying endoscopy for differentiated-type mucosal gastric cancer. J Gastroenterol 46(9): 1064-1070.






























