Decoding the Role of Serum IL-12 as a Prognostic Biomarker in Pegylated IFN-Α-2a /Ribavirin- Treated Chronic Hepatitis C Patients Using Luminex Xmap Technology
Muhammad Hamed1, Peter Natesan Pushparaj2, Salem Bazarah3, Saleh Karim1, Khalid Alghamdy1 and Ishtiaq Qadri1*
1Department of Biological Sciences, King Abdulaziz University, Saudi Arabia
2Center of Excellence in Genomic Medicine Research, King Abdulaziz University, Saudi Arabia
3College of medicine, King Abdulaziz University hospital, Saudi Arabia
Submission: January 01, 2017; Published: January 12, 2017
*Corresponding author: Ishtiaq Qadri, Department of Biological Sciences, King Abdulaziz University, Jeddah, Saudi Arabia, Tel: 00966535168434; Email; ishtiaq80262@yahoo.com
How to cite this article: Muhammad H, Peter N P, Salem B, Saleh K, Khalid A. Decoding the Role of Serum IL-12 as a Prognostic Biomarker in Pegylated IFN-Α-2a /Ribavirin-Treated Chronic Hepatitis C Patients Using Luminex Xmap Technology. Adv Res Gastroentero Hepatol 2017; 2(3): 555589. DOI: 10.19080/ARGH.2017.02.555589
Abstract
HCV infects 3% of the world population. Its ultimate consequences lead to Liver morbidity and transplantation. Various liver function tests are used as a biomarker for the disease progression but they are not robust. Immune system of the host triggers when HCV invades the body. so pro-inflammatory cytokines like IL-12 might be a novel biomarker for the therapy outcome, also for the progression of hepatocellular carcinoma.
Keywords: IL-12, ALT, AST, HCV, Cytokines.
Introduction
Hepatitis C virus (HCV) infection almost 3% of the world’s population [1]. Almost 70% infected patients get chronic hepatitis while around 30% express complete recovery [2]. The pathogenicity of viral infections are highly predisposed by the host immune response. Immune systems of the body eradicate several viruses at their acute stage, Though HBV (hepatitis B virus) and HCV (hepatitis C virus) develop persistent infection the host immune systems and develop persistent infection [3]. Interleukin 12 (IL-12) is a 70-kilo Dalton hetero-dimer protein, comprised of p40 and p35 subunits.These subunits are encoded by two separate genes present on two different chromosomes [4]. IL-12 performs its role as a linkage among the innate and the adaptive immune reactions and also a perilous part in immune pathogenesis of numerous ailments [5]. IL-12 expresses antiviral role as an activator of NK cells, inducer of interferon , and cytotoxic lymphocytes. These all factors are vital part of anti-HCV immune responses [6]. IFN- stimulation results the production of antigen which causes the creation of pro-inflammatory cytokine IL-12 [7]. Therefore IL-12 is considered as a distinct Th1 and Th2 differentiation determining factor [8]. Therefore this study was intended to explore the expression of IL-12 with hepatitis C infestation in Interferon responders and non-responders and to assess their possible role as new biomarker for the treatment response.
Materials and Methods
Subjects
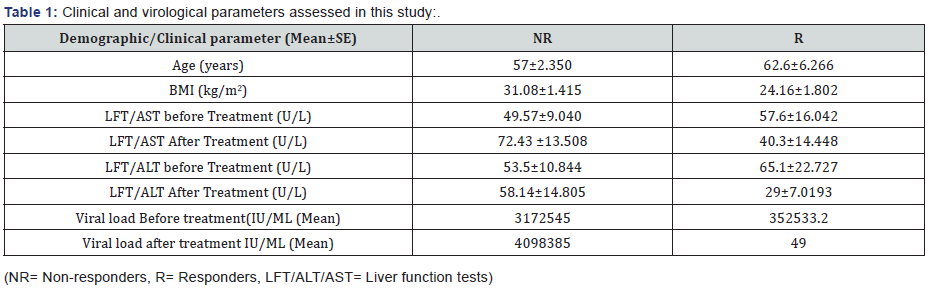
Ethical approval was observed by ethical committee of King Abdul Aziz University and patients were recruited with their informed consent. The medical history of the patients was obtained (viral load) and different biochemical changes (AST, ALT AFT) were observed in their blood before and after the interferon treatment. All data is shown in results Table 1.
A total of 20 patients with HCV infection and 10 healthy controls were enrolled in this study. All patients were hospitalized or present for followed-up examinations in the King Abdul Aziz University Hospital Jeddah KSA. Diagnoses were made in accordance with the standard of the hospital for Prevention and Treatment of Viral Hepatitis. Ten (10) healthy individuals who matched for sex ratio and mean age were also enrolled for normal controls (NC). No subjects were co-infected with HIV or other hepatitis viruses. Eighteen patients with HCV
infection received peg-interferon and ribavirin treatment for 48 weeks, out of nine patients showed Resistance to the treatment. Blood sampling were made on at start and end of the treatment. Based on the therapeutic response to antiviral treatment, those 18 patients could divide into two groups: Treated (Responder, R) 9 patients, and Resistant (Non-responder, NR) 9 patients.
Criteria of responder and non-responder was as following
- Responders (R) when HCV RNA in patient’s sera was undetectable at 48 weeks at end of the treatment. (Also known as SVR i.e. sustained virological response)
- Non-responders (NR) when HCV RNA in patient’s sera was detectable at the end of therapy.
Whole blood samples
Blood was collected from recruited patients. Serum samples were collected by centrifugation of blood at 3,000 g for 10 min, and stored immediately to -80°C until use.
Viral load
Plasma HCV RNA levels were measured by using of the COBAS TaqMan HCV test, version 2.0 (Roche), with a lower limit of detection of 50 IU/ml (Table 1).
(NR= Non-responders, R= Responders, LFT/ALT/AST= Liver function tests)
Chemokines assay (pg/mL) and bead preparation
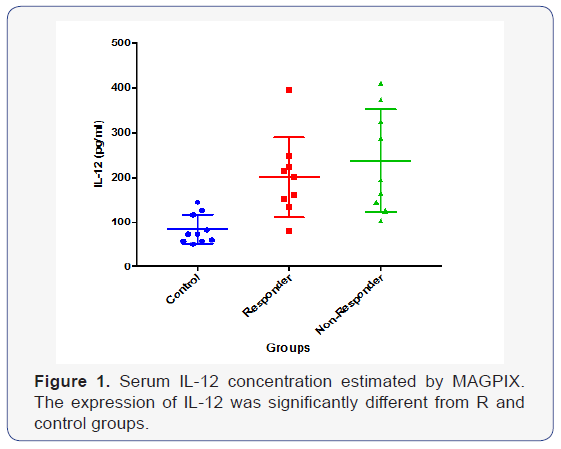
Preparation of beads was done according the manufactures guidelines by using Novex (Life Technologies), Human Cytokine Magnetic 30-plex panel, the sera from interferon responders vs. non responders were compared. This system is very robust and quantitative differences can measured easily. The results indicate a significant increase of IL-12 in non-responder patients (Graph 1).
Statistical analyses
Data were analyzed using MINITAB version 17 for Windows. Bonferroni simulations test was used for the comparison among groups. P values of <0.05 were deliberated to indicate a significant variance.
Results
Biochemical responses in responder and non-responders:
It is clearly stated that biochemical parameters like LFT, AST and ALT are correlated with therapy outcome. The values of these parameters are decreased in responders while increased in non-responders indicating hepatitis C virus among these patients played a role to make changes in non-responders. Also the values of viral load show the same trend between NR and R, details are in Table 1. (R) is less significant but in healthy control group its expression is very less.
Discussion
Cytokines play a vital role in viral clearance, control of infection, swelling, restoration, and fibrosis and also are concerned in the irrational procedures arising in the liver throughout viral contagion [9].Therefore, our study was aimed to explore serum level of IL-12 in prolonged liver disease and their association with HCV infection (non-responders to the therapy) and to appraise as new biomarkers in chronic inflammation progression leading to HCC. IL-12 is one of the most important pro-inflammatory cytokines presented with the initiation of immune response, determining Th1 and Th2 differentiation [1]. Serum level of different chemokines, growth factors and cytokines were analyzed by capone et al. [10], in this study the findings showed the higher level of mean concentrations in hepatocellular carcinoma patients compared to the normal. Our results are in consistence with these findings where serum levels of IL-12 was more elevated in interferon non-responders ( < 0.0001) patients than in those with responders ( < 0.001). These findings
explore that IL-12, pro-inflammatory molecules, tend to rise in chronic HCV [9]. Another study also supports
our findings in which IL-12 is significantly elevated in HV infected patients compared to healthy control group
[11,12]. Our study was unique as no comparison was found from the literature where responder and non
respondersare compared. We concluded that these strong pro-inflammatory responses might play a vital
role to develop hepatic injury. Also this could be used as immunological biomarker to the pathogenicity of liver manifestation [13].
Conclusion
The significantly elevated levels of ALT, AST and IL-12 in nonresponders to the therapy indicate that these
Factors may influence the disease progression resulting hepatocellular carcinoma and IL-12 might be considered as a novel biomarker for hepatic carcinoma.
Acknowledgment
Financial support was provided by king Abdulaziz City for Science and technology (KACST) large grant 162-34, to Ishtiaq Qadri.
References
- SS Youssef, AM Abd El-Aal, A Saad, MH Omran, T El Zanaty, at al. (2013) Impact of IL12B gene rs 3212227 polymorphism on fibrosis, liver inflammation, and response to treatment in genotype 4 Egyptian hepatitis c patients. Disease Markers 35(5): 431-437.
- MH Omran, NE Ibrahim, SS Youssef, et al. (2013) Relation of interleukin-1 gene to treatment response in chronic patients infected with HCV genotype 4. Journal of Infection in Developing Countries 7(11): 851-858.
- E Billerbeck, TB ottler, R Thimme (2007) Regulatory T cells in viral hepatitis. World Journal of Gastroenterology 13(36): 4858-4864.
- Miguel, A Xiaojing, M Alexandrina, SGiorgio T (1998) Molecular mechanisms of the induction of il-12 and its inhi- bition by il-10. J Immunol 160(12): 5936-5944.
- https://www.ncbi.nlm.nih.gov/pubmed/10419892
- Poynard T, Ngo Y, Munteanu M, Thabut D, Massard J, Moussalli J, et al. (2010) Biomarkers of liver injury for hepatitis clinical trials: a metaanalysis of longitudinal studies. Antiviral Ther 15(4): 617-631.
- Del Vecchio M, Bajetta E, Canova S, Lotze MT, Wesa A, et al. (2007) Interleukin-12: biological properties and clinical application. Clin Cancer Res 13(16): 4677-4685.
- Watford WT, Moriguchi M, Morinobu A, O’Shea JJ (2003) The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev 14(5): 361-368.
- Costantini S, Capone F, Guerriero E, Maio P, Colonna G (2010) Serum cytokine levels as putative prognostic markers in the progression of chronic HCV hepatitis to cirrhosis. Eur Cytokine Netw 21(4): 251-256.
- Capone F, Costantini S, Guerriero E, Calemma R, Napolitano M, et al. (2010) Serum cytokine levels in patients with hepatocellular carcinoma. Eur Cytokine Netw 21(2): 99-104.
- Kakumu S, Okumura A, Ishikawa T, Iwata K, Yano M, et al. (1997) Production of interleukins 10 and 12 by peripheral blood mononuclear cells (PBMC) in chronic hepatitis C virus (HCV) infection. Clin Exp Immunol 108(1): 138-143.
- Sarih M, Bouchrit N, Benslimane A (2000) Different cytokine profiles of peripheral blood mononuclear cells from patients with persistent and self-limited hepatitis C virus infection. Immunol Lett 74( 2): 117-120.
- Gigi E, Raptopoulou-Gigi M, Kalogeridis A, Masiou S, Orphanou E et al. (2008) Cytokine mRNA expression in hepatitis C virus infection: TH1 predominance in patients with chronic hepatitis C and TH1-TH2 cytokine profile in subjects with self-limited disease. J Viral Hepat 15(2): 145-154.






























