Decreased Phosphorylated CREB and AKT in Individuals with Autism Normalized after Zinc Therapy
Russo AJ1*, Albert Mensah2 and Judith Bowman2
1Department of Biology, Drew University, USA
2 Mensah Medical, Warrenville, IL, USA
Submission: June 20, 2017; Published: August 09, 2017
*Corresponding author: Russo AJ, Department of Biology, Drew University, Mensah Medical Center, Warrenville, Il 60555, USA.
How to cite this article: Russo A, Albert M, Judith B. Decreased Phosphorylated CREB and AKT in Individuals with Autism Normalized after Zinc Therapy. Acad J Ped Neonatol. 2017; 5(3): 555721. DOI: 10.19080/AJPN.2017.05.555721
Abstract
Introduction: CREB (cAMP response element-binding protein) is a cellular transcription factor, which plays a role in neuronal plasticity and long-term memory formation in the brain. Protein kinase B (PKB), also known as Akt, is a serine/threonine-specific protein kinase, which plays a key role in multiple cellular processes, such as cell survival and apoptosis, cell proliferation, and transcription. Akt and CREB are involved in the same cellular pathways. We report here that both CREB and Akt are decreased in individuals with autism, these decreased levels are associated with high symptom severity and zinc therapy normalizes both Akt and CREB levels in this patient group.
Abbrevations: CREB: Camp Response Element-Binding Protein, PKB: Protein Kinase B, PDD-NOS: Pervasive Developmental Disorder-Not Otherwise Specified (PDD-NOS), HRI: Health Research Institute, AGRE: Autism Genetic Resource Exchange, ADI-R: Autism Diagnostic Interview-Revised, HRI: Health Research Center, BDNF: Brain-Derived Neurotrophic Factor
Introduction
CREB (cAMP response element-binding protein) is a cellular transcription factor which plays a role in neuronal plasticity and long-term memory formation in the brain [1,2].
There is some evidence to suggest that low-functioning CREB is associated with major depressive disorder [3]. For instance, depressed rats, with an over expression of CREB in the dentate gyrus, behaved similarly to rats treated with antidepressants. Also, cortices of patients with untreated major depressive disorder contain reduced concentrations of CREB compared to both healthy controls and patients treated with antidepressants [4]. The function of CREB can be modulated by a signaling pathway associated with the binding of serotonin and noradrenaline to post-synaptic G-protein coupled receptors. Dysfunction of these neurotransmitters is also implicated in major depressive disorder [5]..
Protein kinase B (PKB), also known as Akt, is a serine/threonine-specific protein kinase, which was originally identified as the oncogene in the transforming retrovirus, AKT8 [6]. The kinase plays a key role in multiple cellular processes, such as cell survival and apoptosis [7], cell proliferation [8,9] and transcription [10].
Our lab previously reported that Akt levels are significantly decreased in autism [5]. Data presented here demonstrates that CREB levels are also significantly lower in individuals with autism. In addition, these results show that Akt levels correlate significantly with CREB levels in autism.
There is evidence that suggests a role for zinc and copper in the stimulation of the Akt pathway [11] and in an animal model, zinc supplementation may increase protein synthesis by affecting the Akt/mTOR pathway [12].
The results presented here also demonstrate that zinc and anti-oxidant therapy may significantly raise (normalize) both Akt and CREB levels, and that the increase in Akt and CREB parallels improved symptom severity.
This data suggests that zinc supplementation may improve symptom severity, in part, because it raises Akt and CREB levels.
Materials and Methods
Subjects
Cellular phosphorylated Akt and CREB concentration was measured in 37 autistic children and 12 age and gender similar neurotypical, controls the diagnostic criteria used in this study were defined by DSM-IV criteria. In 2012, the separate diagnostic labels of Autistic Disorder, Asperger’s Disorder, and Pervasive Developmental Disorder-not otherwise specified (PDD-NOS) were replaced by one umbrella termed “Autism Spectrum Disorder”.
Plasma and white blood cells from consecutive individuals with diagnosed autism (n=35; 27 male; mean age 10.2 years) and controls (n=18; 12 male; mean age 9.1 years) were obtained from patients presenting at the Health Research Institute (HRI)* over a two year period. All autistic individuals who presented to HRI were asked to participate, and patients who participated in this study were randomly chosen from all patients who volunteered. Neurotypical control plasma was obtained from HRI and the Autism Genetic Resource Exchange (AGRE)** and randomly chosen from a selection of about 200 samples. The autistic individuals in this study met the DSM-IV criteria and many were diagnosed using The Autism Diagnostic Interview-Revised - ADI-R before presenting to the HRI.
Patient consent was obtained from all patients involved in this study and this study was approved by the IRB of the HRI.
ELISA’s to measure cellular Akt and CREB (e biocsiences, San Diego, CA)
a. 50μL/well of 1X Cell Lysis Mix (negative control) and 50μL/well Positive
b. Control Cell Lysate (positive control) to separate assay wells for controls.
c. 40μl of lysis buffer (contains a combination of detergents, phosphatase inhibitors, salts and buffers) was added to each of the control and experimental wells.
d. 10μl of buffy coat cells (experimental and controls) were added to appropriate wells and mixed gently
e. 50μL/well of Antibody Cocktail mix (detection antibody and HRP conjugated antibody) was added to all the assay test wells. The plate was incubated for 1hr at room temperature on a microplate shaker (~300rpm).
f. Wells were washed with 300μL/well 1X Wash Buffer 4 times.
g. 100μL of Detection Reagent (TMB) was added to each well and the wells were incubated for 10-30 minutes
h. After color development, 100μL of Stop Solution was added to each well.
i. Absorbance was measured using a colorimetric (spectrophotometric) plate reader (BioRad) set at 450nm.
To ensure reproducibility of results, samples were run in duplicate and reported concentrations were the result of the average of at least two separate assays.
Zinc and anti-oxidant therapy
Individuals in this study who presented to the HRI with autism were tested for zinc, copper and anti-oxidant levels. Based on deficiencies, they were then prescribed the appropriate dose of anti-oxidants. Pre-therapy patients represent those who were tested when they first presented and were not previously taking any zinc or anti-oxidants. Post-Therapy patients received antioxidant therapy (Vitamin C, E, B-6 as well as Magnesium, and Manganese if warranted), and zinc supplementation (as zinc picolinate), daily, for a minimum of 8 weeks.
Buffy coat white blood cells
All experimental and control cells were separated from whole blood using centrifugation and were treated in an identical fashionrefrigerated (4C) immediately after collection and cell/serum separation, then used within 4 hours for inductively-coupled plasma-mass spectrometry for zinc concentration determination. Frozen buffy coat was placed at -70C and used for ELISAs within 6 months of retrieval.
Serum/plasma
All experimental and control plasmas were treated in an identical fashion-refrigerated (4C) immediately after collection and cell/serum separation, then used to measure zinc levels (LabCorp, Warrenville Il) within 4 hours using inductively-coupled plasma-mass spectrometry.
Statistics
Inferential statistics were derived from t-test with 95% confidence intervals.
Severity of disease
The HRI questionnaire, severity criteria, and statistical methodology has been previously reported [13].
An autism symptom severity questionnaire was used to evaluate symptoms. The questionnaire (HRI Questionnaire) asked parents or caregivers to assess the severity of the following symptoms: Awareness, Expressive Language, Receptive Language, (Conversational) Pragmatic Language, Focus, Attention, Hyperactivity, Impulsivity, Perseveration, Fine Motor Skills, Gross Motor Skills, Hypotonia (low muscle tone), Tip Toeing, Rocking/ Pacing, Stimming, Obsessions/Fixations, Eye Contact, Sound Sensitivity, Light Sensitivity, and Tactile Sensitivity. The symptoms were rated by parents/guardians on a scale of 0-5 (5 being the highest severity) for each of these behaviors.
*The Health Research Center (HRI) is a comprehensive treatment and research center, specializing in the care of with neurological disorders, including Depression
Results
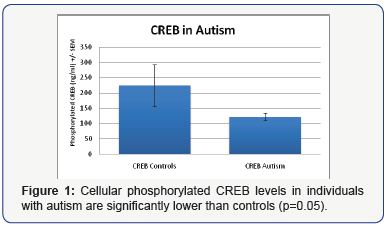
We used an ELISA to measure cellular phosphorylated CREB and Akt in individuals with autism (N=35) and age and gender similar neurotypical controls (N=18). We found CREB levels to be significantly decreased in these individuals compared (p=0.05) (Figure 1).
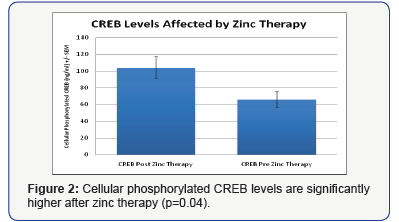
We also found that after zinc therapy, CREB levels in these individuals increased significantly (p=0.04) (Figure 2).
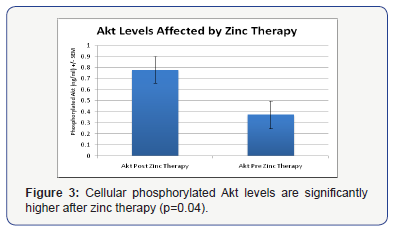
We previously reported that phosphorylated Akt levels were significantly lower in individuals with autism [5]. Here, we found that the low levels of Akt are significantly increased after zinc therapy (p=0.04) (Figure 3).
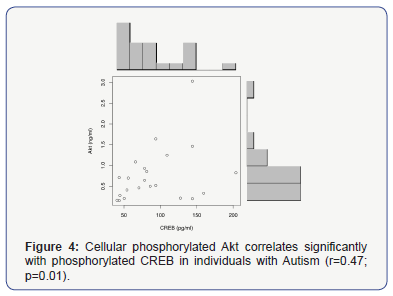
We also found that there was a significant correlation between Akt and CREB levels in this autism group (r=0.47; p=0.01) (Figure 4).
In this autistic group, Akt and CREB levels are associated with certain symptom severity. Low Akt levels are related to high severity of impulsivity (p=0.03463), rocking and pacing (p = 0.00406), stimming (p = 0.03934) and obsessions and fixations (p=0.02565). Low CREB Levels are related to high severity of impulsivity (p=0.002), stimming (p=0.02), and obsessions/ fixations (p = 0.03).
Discussion
CREB down regulation is implicated in the pathology of Alzheimer’s disease and increasing the expression of CREB is being considered as a possible therapeutic target for Alzheimers disease [13]. CREB is a transcription factor for BDNF (Brainderived neurotrophic factor) and BDNF levels are also abnormal in individuals with autism [14].
We have found Akt levels decreased in individuals with autism [5]. Others have also found a down-regulation of the Akt/mTOR pathway in autism [15].
CREB is a regulatory target for the protein kinase Akt [16]. Akt has been shown to block cellular apoptosis and to promote cell survival in response to growth factor induction [17]. Following activation Akt translocates to the nucleus where it is thought to regulate specific genetic programs by catalyzing the phosphorylation of specific nuclear factors [18].
A number of growth factors and hormones have been shown to stimulate the expression of cellular genes by inducing the phosphorylation of the nuclear factor CREB [19]. CREB is important for cell survival. CREB-deficient mice, for example, exhibit a spermatogenesis defect secondary to enhanced apoptosis of germ cells [20]. Overexpression of a CREB transgene induces apoptosis in T cells, following growth factor stimulation [21], and CREB may contribute importantly to cell survival in response to growth factor stimulation [16].
Our data supports a strong relationship between Akt and CREB in this autistic group. We found that levels of Akt and CREB correlate significantly in patients, and low levels of these markers are associated with high symptom severity in the behaviors - impulsivity, stimming and obsessions and fixations.
Other results support the notion that Akt/PKB promotes cell survival, at least in part, by stimulating the expression of cellular genes via the CREB/CBP nuclear transduction pathway [22]. Our findings that individuals with autism have low Akt and CREB could indicate that low Akt leads to low CREB, or that both proteins are produced abnormally.
We show here that the low levels of CREB and Akt, which are associated with high symptom severity, are normalized by zinc and anti-oxidant therapy, suggesting that zinc therapy may help improve symptoms in these individuals by helping to increase marker concentration.
References
- Bourtchuladze, Frenguelli B, Blendy J, Cioffi D, Schutz G, et al. (1994) Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell 79(1): 59-68.
- Purves D, Augustine GJ, Fitzpatrick D, Hall WC, LaMantia AS, et al. (2008) Neuroscience. (4th edn), Sinauer Associates, USA, pp. 170-176.
- Belmaker RH, Agam G (2008) Major depressive disorder. New England Journal of Medicine 358: 55-68
- Blendy JA (2006) The role of CREB in depression and antidepressant treatment. Biol Psychiatry 59(12): 1144-1150.
- Russo AJ (2015) Decreased Phosphorylated Protein Kinase B (Akt) in Individuals with Autism Associated with High Epidermal Growth Factor Receptor (EGFR) and Low Gamma-Aminobutyric Acid (GABA). Biomark Insights 10: 89-94.
- Staal SP, Hartley JW, Rowe WP (1977) Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci USA 74(7): 3065-3067.
- Song G, Ouyang G, Bao S (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9(1): 59-71.
- Ramaswamy S, Nakamura N, Vazquez F, Batt DB, Perera S, et al. (1999) Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway. Proc Natl Acad Sci USA 96(5): 2110-2115.
- Kandel ES, Skeen J, Majewski N, Di Cristofano A, Pandolfi PP, et al. (2002) Activation of Akt/protein kinase B overcomes a G(2)/m cell cycle checkpoint induced by DNA damage. Mol Cell Biol 22(22): 7831- 7841.
- Faissner A, Heck N, Dobbertin A, Garwood J (2006) DSD-1- Proteoglycan/Phosphacan and receptor protein tyrosine phosphatasebeta isoforms during development and regeneration of neural tissues. Adv Exp Med Biol 557: 25-53.
- Ohashi K, Nagata Y, Wada E, Zammit PS, Shiozuka M, et al. (2015) Zinc promotes proliferation and activation of myogenic cells via the PI3K/ Akt and ERK signaling cascade. Exp Cell Res 333(2): 228-237.
- McClung JP, Tarr TN, Barnes BR, Scrimgeour AG, Young AJ (2007) Effect of supplemental dietary zinc on the mammalian target of rapamycin (mTOR) signaling pathway in skeletal muscle and liver from postabsorptive mice. Biol Trace Elem Res 118(1): 65-76.
- Saura C, Valera J (2011) The role of CREB signaling in Alzheimer’ s disease and other cognitive disorders. Rev Neurosci 22(2): 153-169.
- Kasarpalkar NJ, Kothari ST, Dave UP (2014) Brain-Derived Neurotrophic Factor in children with Autism Spectrum Disorder Ann Neurosci 21(4): 129-133.
- Nicolini C, Ahn Y, Michalski B, Rho JM, Fahnestock M, et al. (2015) Decreased mTOR signaling pathway in human idiopathic autism and in rats exposed to valproic acid Acta Neuropathol Commun 3: 3.
- Du K, Montminy M (1998) CREB is a regulatory target for the protein kinase Akt. The Journal of Biological Chemistry 273(49): 32377-32379.
- Downward J (1998) Mechanisms and consequences of activation of protein kinase B/Akt. Curr Opin Cell Biol 10(2): 262-267.
- Meier R, Alessi D, Cron P, Andjelkovic M, Hemmings B (1997) Mitogenic Activation, Phosphorylation, and Nuclear Translocation of Protein Kinase Bβ. J Biol Chem 272: 30491-30497
- Montminy M (1997) Transcriptional Regulation by Cyclic AMP. Annu Rev Biochem 66: 807-822.
- Nantel F, Monaco L, Foulkes NS, Masquilier D, LeMeur M, et al. (1996) Spermiogenesis deficiency and germ-cell apoptosis in CREM-mutant mice. Nature 380(6570): 159-162.
- Barton K, Muthusamy N, Chanyangam M, Fischer C, Clendenin C, et al. (1996) Defective thymocyte proliferation and IL-2 production in transgenic mice expressing a dominant-negative form of CREB Nature 379(6560): 81-85.
- Du K, Montminy M (1998) CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273(49): 32377-32379.






























