Severe Complications Due to THFR SNP in ALL T Type Recent Diagnosed Teenager, after Induction Protocol with Methotrexate: Case Report
Melendi GA1, Hernández A1, Picón A1, Chiesa I2, Maria S Perez2, Hernández Y1, Pablo Moreno1 and Gustavo Cardigni1
1Sanatorio Trinidad Palermo, Pediatric Intensive Care Unit, South America
2Medicine genomic, Laboratorio Manlab, USA
Submission: March 01, 2017; Published: June 01, 2017
*Corresponding author: Guillermina AM, Sanatorio Trinidad Palermo, Pediatric Intensive Care Unit, South America.
How to cite this article: Melendi GA, Hernández A, Picón A, Chiesa I, Maria S P. Severe Complications Due to THFR SNP in ALL T Type Recent Diagnosed Teenager, after Induction Protocol with Methotrexate: Case Report. Acad J Ped Neonatol. 2017; 4(4): 555701. DOI: 10.19080/AJPN.2017.04.555701
Abstract
Severe drugs toxicities in pediatric ALL patients could cause life-threatening situations and consequent PICU admition. Genomics and pharmacogenomics bring new insights on management and treatment. We describe a ALL pediatric patient with severe bone marrow aplasia with high blood levels of methotrexate after a standard IV dose for ALL induction block treatment.
Keywords: Leucemia children; Methotrexate adverse event; MTHFR
Abbrevations: ALL: Acute Lymphoblastic Leukemia; GATLA: Grupo Argentino de Tratamiento de la Leucemia Aguda; LTC: Long Term Catheter; MTHFR: Methylene Tetra Hydrofolate Reductase; PICU: Pediatric Intensive Care Unit; qPCR: quantitive Protein C Reactive; SNPs: Single Nucleotide Polimorphisms; TPN: Total Parenteral Nutricion; WBC: White Blood Cell Count
Introduction
There are 370 registered acute lymphoblastic leukemia (ALL) cases per year in Argentina, 30% of which will be admitted in PICU at some point of their disease history. Methotrexate is one of the drugs that ALL patients will be early exposed. Its metabolism by the MTFHR (methylene tetra hydrofolate reductase) is key to its clearance [1-4]. Over the past decades, the medical advance of genomics and pharmacogenetics enhanced the knowledge of drugs toxicity.
Case Report
A 13-year-old teenager with recently ALL diagnose, is admitted to PICU with shock clinical signs after the first exposure to IV methotrexate (2gr) the patient initially required vasoactive drugs, antibiotic therapy and supplementary oxygen. Methotrexate toxicity was suspected therefore blood levels were measure, over 4 times expected levels were found. Folinic Acid rescue treatment started [5].
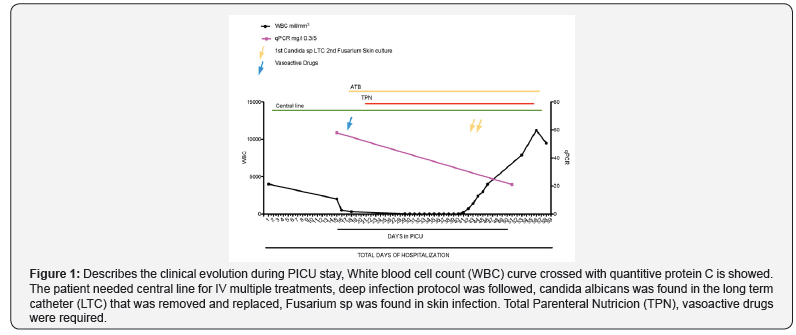
Due to severe mucositis total parenteral nutrition was needed. White blood cell count descended since addition, after fifteen days at PICU the patient started with pancytopenia that persisted for 26 days; fever was associated therefore deep infection protocol was followed. Skin and mucosa infection by Fusarium sp. And Candida albicans in blood cultures were found. Total PICU days of stay were 61, days with TPN 35, antibiotics and antifungal were 45 Figure 1.
MTHFR’s Single nucleotide polimorphisms (SNPs) were analyzed, a mutation A1298C a (glutamic acid/ alanine change) was found. The patient continued with an alternative ALL treatment protocol, achieving remission. No PICU admission was needed again.
Discussion
Two MTHFR SNPs are associated with severe methotrexate toxicity, C677T and A1298C. Both, widely described in the literature as methotrexate metabolism inhibitor. Associated clinical manifestations are leucopenia and mucocitis from moderated to severe. [1-8]. The patient described in this Case Report was found hetorocygote for the A1298C SNP. Felice et al. described the association of mutations above with high risk of severe leucopenia in a pediatric population, encompassing different countries in a multicentre international study [9]. Latest recommendations on SNPs screening are not in GATLA protocols. SNPs for THFR are found mostly in jew population, Argentina has 1:238 6th highest ratio of Jews population per habitant in the world. The cost of the assay in Argentina, in a private clinical laboratory is around 40 US dollars (Man Lab, Argentina). Here and now, critical ill patients can benefit on their disease prognosis with the current knowledge on epigenetics, pharmacogenetics, custom biologics treatment, therefore these approaches are not the future any more, they are the present, therefore we should think about them also as prevention [10-12].
References
- Tanaka Y, Manabe A, Nakadate H, Kondoh K, Nakamura K, et al. (2013) Methylenetetrahydrofolate reductase gene haplotypes affect toxicity during maintenance therapy for childhood acute lymphoblastic leukemia in Japanese patients. Blood 121(26): 5145-53.
- Bellampalli R, Phani NM, Bhat KG, Prasad K, Bhaskaranand N, et al. (2015) Significance of 5,10-methylenetetrahydrofolate reductase gene variants in acute lymphoblastic leukemia in Indian population: an experimental, computational and meta-analysis. Leuk Lymphoma 56(5): 1450 -1459.
- Radtke S, Zolk O, Renner B, Paulides M, Zimmermann M, et al. (2014) Germline genetic variations in methotrexate candidate genes are associated with pharmacokinetics, toxicity, and outcome in childhood acute lymphoblastic leukemia. harmacogenomics J 14(2): 115-119.
- Wang SM, Sun LL, Zeng WX, Wu WS, Zhang GL (2014) Influence of genetic polymorphisms of FPGS, GGH, and MTHFR on serum methotrexate levels in Chinese children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 74(2):283-289.
- Murphy NM, Diviney M, Szer J, Bardy P, Grigg A, et al. (2012) The effect of folinic acid on methylenetetrahydrofolate reductase polymorphisms in methotrexate-treated allogeneic hematopoietic stem cell transplants. Biol Blood Marrow Transplant 18(5): 722-730.
- Salazar J, Altés A, del Río E, Estella J, Rives S, et al. (2012) Methotrexate consolidation treatment according to pharmacogenetics of MTHFR ameliorates event-free survival in childhood acute lymphoblastic leukaemia. Med Oncol 29(3): 2053-2062
- Aráoz HV, D’Aloi K, Foncuberta ME, Sanchez La Rosa CG, Alonso CN, et al. (2014) Pharmacogenetic studies in children with acute lymphoblastic leukemia in Argentina. Cancer Chemother Pharmacol 74(2): 283-289.
- El-Khodary NM, El-Haggar SM, Eid MA, Ebeid EN (2011) Study of the pharmacokinetic and pharmacogenetic contribution to the toxicity of high-dose methotrexate in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 68(5): 1339-1346.
- D’Angelo V, Ramaglia M, Iannotta A, Crisci S, Indolfi P, et al. (2011) Methotrexate toxicity and efficacy during the consolidation phase in paediatric acute lymphoblastic leukaemia and MTHFR polymorphisms as pharmacogenetic determinants. Cancer Chemother Pharmacol 68(5): 1339-1346.
- Hagleitner MM, Coenen MJ, Aplenc R, Patiño-Garcia A, Chiusolo P, et al. (2013) The role of the MTHFR 677C>T polymorphism in methotrexateinduced liver toxicity: a meta-analysis in patients with cancer. Leuk Lymphoma 54(12): 2639-2644.
- D’Angelo V, Ramaglia M, Iannotta A, Francese M, Pota E, et al. (2013) Influence of methylenetetrahydrofolate reductase gene polymorphisms on the outcome of pediatric patients with non-Hodgkin lymphoma treated with high-dose methotrexate. Leuk Lymphoma 54(12): 2639- 2644.
- Lopez-Lopez E, Martin- Guerrero I, Ballesteros J, Garcia -Orad A (2012) A systematic review and meta-analysis of MTHFR polymorphisms in methotrexate toxicity prediction in pediatric acute lymphoblastic leukemia. Biol Blood Marrow Transplant 18(5): 722-730.






























