Nanofibrillated Cellulose from Natural Fibers for Food Applications
Imed Ben Marzoug*, Sana Ridene, Lassed Ghali, and Slah Msahli
Textile Engineering Laboratory, University of Monastir, Tunisia
Submission: August 06, 2024; Published: September 27, 2024
*Corresponding author: Imed Ben Marzoug, Textile Engineering Laboratory, University of Monastir, Tunisia, Email: imedbenmarzoug@gmail.com
How to cite this article: Imed Ben Marzoug*, Sana Ridene, Lassed Ghali, and Slah Msahli. Nanofibrillated Cellulose from Natural Fibers for Food Applications. Academ J Polym Sci. 2024; 6(4): 555688. DOI: 10.19080/AJOP.2024.06.555688
Abstract
Interest in nanofibrillated cellulose has been growing rapidly due to its ease of preparation, high yield, specific surface area, strength, stiffness, lightweight nature, and biodegradability. Alfa fibers, with a cellulose content of over 45%, have been recognized as a sustainable source for producing cellulose nanofibers because of their renewability and availability. By subjecting raw Alfa fibers to alkali, bleaching, and chlorhydric acid treatments, nanofibers with high yields have been successfully produced. Microscopy tests have confirmed that Alfa nanofibers, with an average diameter of 12 μm, were obtained after bleaching treatments. Transmission electron microscopy has shown that these nanofibers have a needle-like shape, with an average diameter and length of 5 ± 3 nm and 400 ± 30 nm, respectively. These nanofibers will be further explored for synthesizing nanomaterials, as additives for paper and paperboard, in biomedical applications, and for food packaging purposes.
Keywords: Alfa ultimate fiber; Cellulose; Nanofibrillated; Food packaging
Introduction
Cellulose, the most abundant component of plant biomass, is primarily found in plant cell walls in nature. However, it is also produced by some animals, algae, and a few bacteria [1,2]. Cellulose is a semi-crystalline polysaccharide that appears in nature in the form of fibers with widths ranging from 5 to 20 μm and lengths ranging from 0.5 up to several millimeters.
In recent decades, natural cellulose materials have been utilized for various purposes such as energy sources, building materials, paper, textiles, and clothing [3]. Despite their versatility, cellulosic materials have limitations, such as incompatibility with hydrophobic polymers, which has hindered their use as reinforcement in polymers [4]. Nanocellulosics, which are classified into three main subcategories based on their dimensions, functions, and preparation methods, offer a potential solution to these limitations. The classification of nanocellulosics is dependent on the source of cellulose and the processing conditions. This classification is detailed in (Table 1).
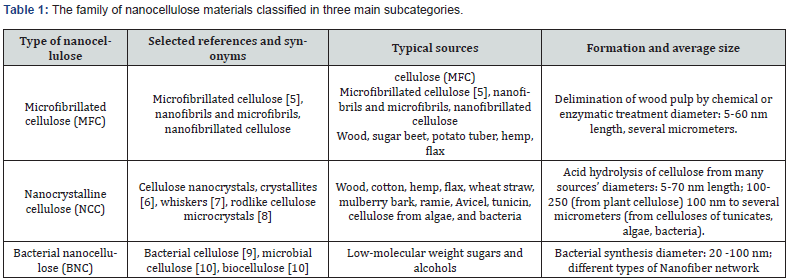
The purity and properties of nanocellulose are influenced by the plant source, including the fiber dimension structure of the cell wall, as well as the relative percentage of cellulose, hemicelluloses, and lignin. Additionally, the extraction method used plays a crucial role in determining the final quality of the nanocellulose.
Experimental
Cellulose Nanofiber Preparation
Cellulose nanofibers hold great promise for a wide range of applications, especially as a reinforcement in the creation of nanocomposites. Numerous studies have been conducted on the isolation and characterization of cellulose nanofibers from different sources. These nanofibers can be extracted from cell walls using either simple mechanical methods or a combination of chemical and mechanical processes. In our study, the extraction of Alfa cellulose nanofibers involved two distinct steps: pretreatment/bleaching and the hydrolysis process. This method allows for the efficient isolation of high-quality cellulose nanofibers for use in various industries.
Pretreatment and Bleaching Process of Alfa
In order to achieve a high level of cellulose fiber purity, it is essential to undergo a pretreatment and bleaching process to eliminate hemicelluloses, lignin, and other impurities such as waxes and ashes. In this particular study, a combined approach involving the use of sodium hydroxide and hydrogen peroxide was employed. Interestingly, when esparto fibers were bleached solely with hydrogen peroxide, the lignin content remained unaffected. However, the introduction of sodium hydroxide into the bleaching solution proved to be effective in ensuring the complete destruction of lignin [11]. During the experimentation phase, 5 grams of dried esparto grass fibers were submerged in a solution containing 30g/L of sodium hydroxide and 35g/L of hydrogen peroxide. The fibers were treated for a duration of 90 minutes at a temperature of 120°C [12].
Hydrolysis of the cellulose Alfa fiber
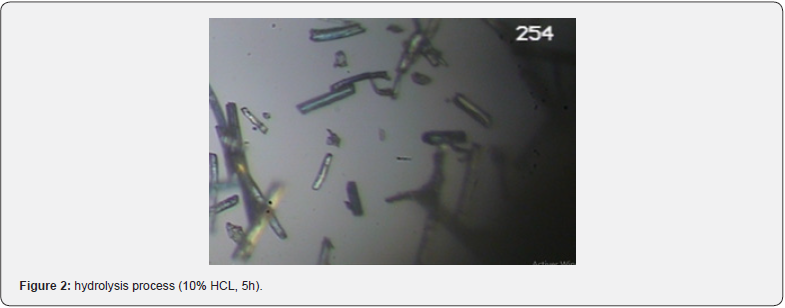
The bleached pulp underwent treatment with a 10% HCl (1 N) solution and was mixed using an ultrasonicator at a temperature of approximately 60 ± 1°C for durations of 2 and 5 hours. Subsequently, the fibers were removed and thoroughly washed with distilled water to achieve a neutral pH before being dried. The fibers were then suspended in water and subjected to continuous stirring with a high-shear homogenizer for 15 minutes. This high-shearing action effectively breaks down fiber agglomerates, resulting in the formation of nano-fibrils (Figure 1).

Results and discussion
Cellulose Alfa Nanofiber Preparation
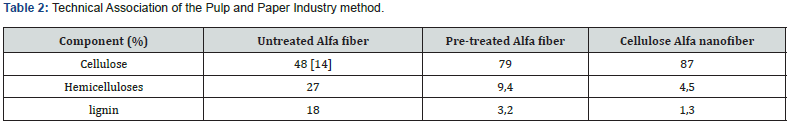
The pretreatment and bleaching processes result in the production of purer and whiter Alfa fiber strands. During the pretreatment process, the use of HCL and temperature causes the fibers to explode, effectively removing hemicelluloses and lignin [13]. This process, known as steam explosion techniques, was originally developed by W. H. Mason in 1928. Different source materials require varying steam explosion temperatures and retention times. Some materials may need longer retention times and higher temperatures than others.
Previous research has shown that steam explosion effectively separates hemicelluloses and lignin, improving the subsequent processes following pretreatment [14]. Composition analysis using the Technical Association of the Pulp and Paper Industry method, as shown in (Table 2), confirms that a significant amount of hemicelluloses and lignin are removed during pretreatment. Acid hydrolysis of cellulose Nanofiber further removes hemicelluloses and lignin, resulting in a higher percentage of cellulose content in the final product.
(Figures 2 & 3) displays the transmission electron microscopy image illustrating the morphology of the fiber after undergoing an acid hydrolysis process using hydrochloric acid. The fiber was broken down into nano-sized diameters ranging between 2-8 nm as a result of the acid treatment. The strong acid effectively cleaved the stack of cellulose microfibrils, resulting in the production of fibers with nano-sized diameters.

Conclusion
The pretreatment process was conducted to eliminate hemicelluloses, lignin, and other extractives in order to obtain a purer cellulose fiber. The cellulose nanofiber from Alfa was obtained through the hydrolysis process using hydrochloric acid. The optimal conditions for producing effective nanofibers were found to be using 10% hydrochloric acid for 5 hours. The purpose of this study was to assess the potential of Alfa nanofibers in creating new biopolymer-based nanocomposite films.
Conflict of Interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Henriksson M, Berglund LA (2007) Structure and properties of cellulose nanocomposite films containing melamine formaldehyde. J Appl Polym Sci 106(4): 2817-2824.
- Iwamoto S, Nakagaito AN, Yano H (2007) Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl Phys A Mater 89: 461-466.
- Brinchi L, Cotana F, Fortunati E, Kenny JM (2013) Production of nanocrystalline cellulose from lignocellulosic biomass: technology and applications. Carbohydr Polym 94(1): 154-169.
- Kalia S, Thakur K, Celli A, Kiechel MA, Schauer CL (2013) Surface modification of plant fibers using environment friendly methods for their application in polymer composites, textile industry and antimicrobial activities: a review. J Environ Chem Eng 1(3): 97-112.
- Mathew AP, Thielemans W (2008) Dufresne nanocrystals and related polymer nanocomposites. J Appl Polym Sci 109: 4065Cellulose.
- Jiang F, Hsieh Y (2013) Chemically and mechanically isolated nanocellulose and their self-assembled structures. Carbohydr Polym 95(1): 32-40.
- Saito T, Hirota M, Tamura N, Kimura S, Fukuzumi H, et al. (2009) Individualization of nano-sized plant cellulose fibrils by direct surface carboxylation using TEMPO catalyst under neutral conditions. Biomacromolecules 10(7): 1992-1996.
- Samir MASA, Alloin F, Gorecki WJ, Sanchez Y (2004) Dufresne: Cellulose nanocrystal-filled poly(acrylic acid) nanocomposite fibrous membranes. J Phys Chem B 108: 10845.
- Helbert W, Cavaille JY, Dufresne A (1996) Thermoplastic nanocomposites filled with wheat straw cellulose whiskers. Part I: processing and mechanical behavior. Polym Compos 17(4): 604-611.
- Alemdar A, Sain M (2008) Isolation and characterization of nanofibers from agricultural residues-wheat straw and soy hulls. Bioresour Technol 99: 1664-1671.
- Ben Marzoug I, Sakli F, Roudesli S (2010) Separation of ultimate and technical esparto grass Fibres: comparison between extraction methods. J The Textile Institute 101(12): 1050-1056.
- Ghali L, Msahli S, Zidi M, Sakli F (2009) Effect of pre-treatment of luffa fibers on the structural properties. Materials Letters 63(1): 61-63.
- Venkatesh Chaturvedi, Pradeep Verma (2013) An overview of key pretreatment processes employed for bioconversion of lignocellulosic biomass into biofuels and value added products, Biotech 3(5): 415-431.
- El Achabya M, Kassabab Z, Barakatc A (2018) Alfa fibers as viable sustainable source for cellulose nanocrystals extraction: Application for improving the tensile properties of biopolymer nanocomposite films, Industrial Crops and Products 112: 499-510.






























