Abstract
Background: The study aimed to compare the effect of menstruation on salivary flow rate (SFR) and pH in a healthy female population. The investigation focuses on understanding the changes in salivary parameters during menstruation and its impact on oral health.
Materials and Methods: A convenience sample of 32 healthy females with regular menstrual cycles of 28-30 days, aged 18 to 30 years, was considered. The sample included females with regular menstrual cycles. Salivary parameters were analyzed using flow rate (ml/min) and pH (color indicators on pH strips).
Results: The mean salivary pH during menstruation was higher (7.925) compared to the absence of menstruation (7.016). The mean Salivary Flow Rate (SFR) during menstruation was higher (0.616 ml/min) compared to the absence of menstruation (0.363 ml/min). There was a significant difference in SFR and pH between the two groups, with P > 0.05.
Conclusions: The results suggest that salivary secretion plays a critical role in oral homeostasis by modifying the oral cavity’s ecology. The study highlights the need to develop new technologies for easier monitoring of oral health status, disease onset, and progression.
Keywords:Menstruating females; Salivary flow rate; Salivary pH; Oral Health
Introduction
Maintaining oral health is crucial for overall well-being and plays a pivotal role in sustaining a high quality of life [1]. Various physical, hormonal, and psychological changes in females can influence their overall health, impacting the oral environment, particularly in terms of salivary flow rate, salivary pH, and buffering capacity. Saliva, an enigmatic fluid, contains a variety of host mucosal defence factors from numerous salivary organs and crevicular fluids. Although saliva has been studied in relation to various physiological and pathological conditions, the association between different properties of saliva and different stages of the female menstrual cycle remains to be elucidated [2]. Saliva, the most readily available and non-invasive biological fluid in the human body, continuously “bathes” the oral cavity and adapts to the constantly changing environment. Saliva contains a large amount of organic and inorganic compounds, which act as a “mirror reflecting the body’s health”. Thus, it provides a diagnostic window into the body from both a health and disease perspective. The ability to monitor patient health and disease using saliva represents a highly desirable goal for health promotion and healthcare research [3].
Saliva plays a critical role in oral homeostasis, as it modifies the ecology within the oral cavity. Its functions include alimentary bolus lubrication, protection against viruses, bacteria, and fungi, buffering capacity, protection and repair of the oral mucosa, and dental remineralization. Against this background, quantitative and/or qualitative changes in salivary secretion may cause local caries, oral mucositis, candidiasis, oral infections, chewing disorders or extraoral dysphagia, halitosis, weight loss side effects.
These reasons are convincing enough to use saliva as a diagnostic fluid to probe health and disease [4]. In general, the release of ovarian hormones has a substantial influence on women’s lives. Throughout the menstrual cycle, there are alterations in the levels of estrogen and progesterone hormone. In several women, the appearance of monthly menstruation causes associated changes in the oral cavity. Oral inconveniences have been reported, such as mild burning sensation, bleeding along with mild irritation, red gums, recurrent oral ulcers, canker sores, herpes labialis, Candida albicans infection, increased tooth mobility, and emotional disturbances. Discrete stages of the menstrual cycle and varying hormone levels are associated with gingival disease. Hormonal imbalance is thought to cause gingival abnormalities during menstruation, and this can sometimes occur in conjunction with a propensity for ovarian dysfunction. Fluctuations in steroidal sex hormone levels during menstruation may be due to variations in the immune system [2].
Aim
The aim of this pilot study is to compare the effect of menstruation on salivary flow rate and pH in a healthy female population.
Materials And Methods
This study was conducted in the department of Oral Medicine and Radiology, MGVs KBH Dental College, Nashik. A convenience sample of 32 healthy females was taken from the outpatient department consisting of individuals aged 18 to 30 with normal 28-30-day menstrual cycles. Salivary samples were collected on the 2nd day of their menstrual cycle from subjects, who came to the clinics for routine dental check-ups. Basic information such as age, sex, systemic disease, daily medication and various oral symptoms was recorded for each individual. Dietary and oral hygiene practices, as well as any accompanying xerostomia symptoms, were also documented. Before initiating the study procedures, all individuals provided informed consent. Ethical clearance was obtained from the Ethical Research Committee of MGVs KBH Dental College in Nashik, Maharashtra, India, prior to the commencement of the study.
Salivary flowrate and salivary pH were measured. Unstimulated whole saliva samples were collected by expectorating saliva in a container and pH was measured using commercially available pH strips. After 15 days, following the cessation of the menstrual cycle, the same patients were recalled and their unstimulated whole saliva was collected, and pH was measured for comparison. The inclusion criteria comprised healthy menstruating women on the second day of their cycle maintaining adequate dental hygiene. Women without systemic or local illnesses influencing salivary secretions, normal masticatory abilities, no evidence of dry mouth or salivary gland problems, and no acute or chronic diseases of the oral mucosa or salivary glands were included. Women who do not complain of dry mouth or a burning feeling in their mouth and do not have acute infectious infections, systemic illness, or cardiac, renal, respiratory, or hepatic failure were also eligible. Volunteers were excluded from the study if they did not meet inclusion criteria or did not sign an informed consent form.
Exclusion criteria were patients having any oral diseases such as oral submucous fibrosis and candidiasis, systemic diseases including Diabetes, nutritional deficiency, and endocrinal disorders, diseases affecting the salivary properties like salivary stones, hypertension, neurological disorders, patients with the habit of smoking and alcohol consumption, patients with the habit of smoking and alcohol consumption, patients on medication affecting salivary properties like antidiabetic, antihypertensive, anticholinergic, antidepressants, pregnant women, patients with a history of radiotherapy in the head and neck region, and those unwilling to participate in the study or not signing an informed consent. Every patient was examined by the same dentist to rule out any acute or chronic disorders of the oral mucosa or saliva glands. Demographic features (age, height, and weight) were collected, along with information on smoking status and alcohol consumption. The subject was classified as a smoker if she smoked, regardless of how many cigarettes smoked, and an alcohol consumer if she consumed more than 40 g of alcohol per day.
Collection of Samples
Under normal temperature and humidity circumstances, the research of salivary secretion was carried out in the morning (9 to 11 a.m.) in the absence of any stimulation. Prior to saliva collection, all individuals were instructed to abstain from eating, drinking or smoking and any type of tobacco use for a minimum of two hours. All measurements were recorded in a comfortable room that was quiet and distraction-free. Salivary samples of the menstruating group were collected on the second day of menstruation.
Estimation of flow rate of saliva
Brief case history of the subject was recorded for name, age, day of cycle, any medications. Subjects were comfortably seated and were asked not to eat/ drink for 30 minutes prior to study, after a few minutes of relaxation, they were instructed to avoid swallowing saliva and to lean forward in order to spit all of the saliva they generated into a graded, sterile plastic container for five minutes. The whole volume collected for 5 minutes was then measured.
Estimation of salivary pH
To determine salivary pH, commercially available pH strips were used as per manufacturer’s instructions. We examined the salivary flow rate (ml/min) and pH, which were expressed as colour indicators on pH strips, as markers of salivary secretion. After obtaining and recording all the data of salivary flow rate and pH on a chart, the data was statistically analysed.
Statistical Methods
Descriptive statistical analysis has been carried out in the present study and the paired t test was applied.
Descriptive Statistics
Results
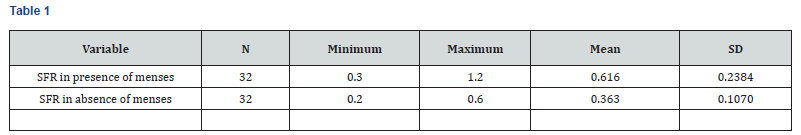
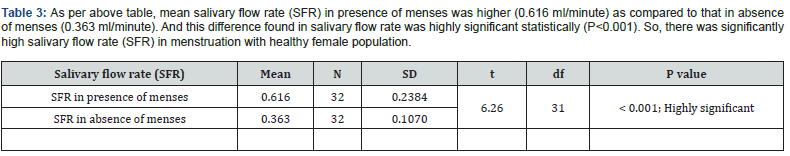
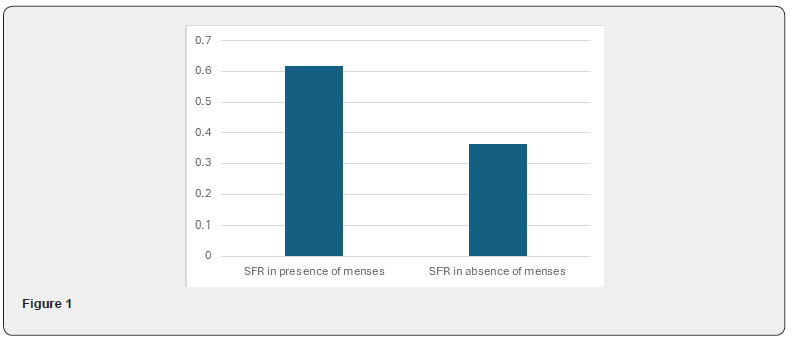
Table 3 & Table 4 As per above table 3, mean salivary flow rate (SFR) in presence of menses was higher (0.616 ml/minute) as compared to that in absence of menses (0.363 ml/minute). And this difference found in salivary flow rate was highly significant statistically (P<0.001). So, there was significantly high salivary flow rate (SFR) in menstruation with healthy female population (Figure 1).
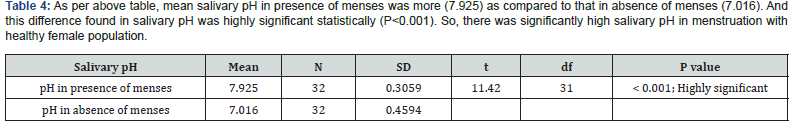
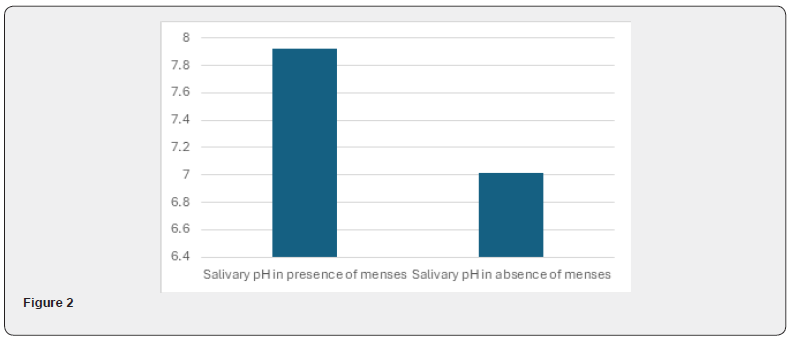
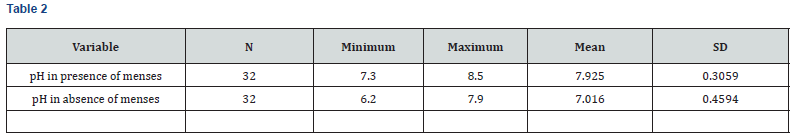
As per the above table 4, mean salivary pH in presence of menses was more (7.925) as compared to that in absence of menses (7.016). And this difference found in salivary pH was highly significant statistically (P<0.001). So, there was significantly high salivary pH in menstruation with healthy female population (Figure 2).
Discussion
Women may be more susceptible to alterations in salivary flow due to the distinct hormonal fluctuations they undergo [5]. In humans, the process of oral digestion is only of marginal importance; yet saliva plays a crucial role in preparing food for mastication and swallowing. Without saliva, mealtimes are challenging, awkward, and humiliating. Alterations in salivary function may lead to impairment of oral tissues and have a significant effect on the patient’s quality of life. Different techniques can be used to measure salivary secretion: a) resting or unstimulated whole saliva secretion; b) stimulated whole saliva secretion; and c) glandular saliva collection (mainly from parotid glands) with or without stimulation. Unstimulated whole saliva, which is the secretion that protects oral tissues, is found in our oral cavity for around 14 hours per day and represents the basal salivary flow rate. Stimulated saliva represents secretion during food intake (physiological stimulation) and remains in the mouth for up to 2 hours. So, while stimulated salivary secretion is helpful for studying the functional reserve, unstimulated salivary production provides an accurate way to analyse the state of the salivary glands [7]. Numerous studies have demonstrated that the effects of sex hormones, such as oestrogen and progesterone, can affect the oral mucosa. As their levels fluctuate, they also have an impact on the oral cavity. Similarly, some women experience oral changes during the menstrual cycle due to hormonal changes particularly the increase in progesterone such as bright red swollen gums, swollen salivary glands, the formation of canker sores, or bleeding gums. Menstruation gingivitis often appears a day or two before the period commences and goes away quickly after [3]. We have opted to test unstimulated saliva in our study since it is a simple, comfortable, and non-invasive process that favours its use in population studies..
The composition and flow rate affects the salivary functions. It is essential to maintain a healthy flow rate for maintenance of the whole-body health along with the oral well-being. The salivary pH is also modulated by the salivary flow rate. At low flow rates, pH decreases due to reduction in bicarbonate release. The pH of women’s saliva also appears to vary significantly. A study by Beedubali SR. et al. [5] found that among females with regular menstrual cycle changes in salivary pH was found to have no statistically significant difference in contrast to this study which presented the highly statistically significant difference. Numerous cardiometabolic risk factors also have an impact on salivary flow rate. In individuals with diabetes and dyslipidaemia, degenerative changes in the acinar cells are commonly seen. These alterations cause the salivary pH to drop and the salivary flow rate to decrease. Additionally, obesity, age, and hypertension have all been linked to hyposalivation [6]. Another study by Agrawal AT et al. [2] regarding the salivary stream rate showed that menstruating women showed no statistically significant change which states that there is no change in the salivary flowrate during menstruation. Another study by Kullander and Sonesson [7] demonstrated that histaminestimulated saliva production was marginally higher during the luteal phase of menstruation. The current study evaluated changes in pH and salivary flowrate among females with regular menstrual cycle. In order to determine the impact of the menstrual cycle on salivary flow rate and pH, the current study used a reasonably simple method of flow rate and pH determination. The prevention and treatment of dental diseases will benefit from a thorough understanding of the role hormones play in salivary flow rate, pH, and oral health. Consequently, a direct correlation between dental health of a female and their fluctuating hormonal condition may exist. The limitations of the study are the smaller size of the sample group and shorter duration of the study. This research also gives further scope to contribute valuable insights into the potential associations between hormonal fluctuations during menstruation and oral health, providing a foundation for future studies in this field [8-15].
Conclusion
In conclusion, the assessment of salivary pH and flow rate offers a practical avenue for evaluating potential oral health risks associated with the menstrual cycle. It is crucial to ensure that menstrual fluctuations do not detrimentally affect oral health outcomes. Preserving oral health holds significant importance, as existing literature underscores its linkage to broader health concerns. This study suggests that during menstruation, hormonal changes may lead to increased salivary flow rates, potentially causing discomfort for some individuals despite being within normal ranges. However, this heightened flow rate might aid in the natural cleansing of the oral cavity, thereby potentially enhancing oral health indices. Moreover, a slight elevation in saliva pH during the menstrual bleeding phase, compared to nonmenstruating periods, may contribute to improved oral hygiene indicators. Nevertheless, it has been noted that saliva with excessively high alkaline levels can lead to enamel erosion and the development of dental caries. Utilizing salivary pH and flow rate as oral health markers proves to be a cost-effective and easily accessible approach, requiring minimal professional expertise and resources. This underscores the utility of these parameters as a screening tool applicable across diverse clinical settings, catering to the needs of both developed and developing nations.
References
- Jahan SS, Hoque Apu E, Sultana ZZ, Islam MI, Siddika N (2022) Oral healthcare during pregnancy: its importance and challenges in lower-middle-income countries (LMICs). International Journal of Environmental Research and Public Health 19(17): 10681.
- Agrawal AT, Hande A, Reche A, Paul P, Agrawal AT (2022) Appraisal of Saliva and Its Sensory Perception in Reproductive Transitions of Women: A Review. Cureus 14(11).
- Saluja P, Shetty V, Dave A, Arora M, Hans V, et al. (2014) Comparative evaluation of the effect of menstruation, pregnancy and menopause on salivary flow rate, pH and gustatory function. Journal of clinical and diagnostic research: JCDR 8(10): ZC81.
- FenolI-Palomares C, Muñoz-Montagud JV, Sanchiz V, Herreros B, Hernández V, et al. (2004) Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Revista espanola de enfermedades digestivas 96(11): 773-783.
- Beedubail SP, Kashyap RR (2022) Assessment of Salivary pH Changes During Various Phases of Menstrual Cycle. International Journal of Dentistry Research 7(2): 31-33.
- Tremblay M, Loucif Y, Methot J, Brisson D, Gaudet D (2012) Salivary pH as a marker of plasma adiponectin concentrations in women. Diabetology & metabolic syndrome 4(1): 1-7.
- Kullander S, Sonesson B (1965) Studies on saliva in menstruating, pregnant and post-menopausal women. European Journal of Endocrinology 48(2): 329-336.
- Sreebny LM (2000) Saliva in health and disease: an appraisal and update. International dental journal 50(3): 140-161.
- Mahesh DR, Komali G, Jayanthi K, Dinesh D, Saikavitha TV, et al. (2014) Evaluation of salivary flow rate, pH and buffer in pre, post & post-menopausal women on HRT. Journal of clinical and diagnostic research: JCDR 8(2): 233.
- Minicucci EM, Pires RB, Vieira RA, Miot HA, Sposto MR (2013) Assessing the impact of menopause on salivary flow and xerostomia. Australian dental journal 58(2): 230-234.
- Bonnans SR, Noble AC (1995) Interaction of salivary flow with temporal perception of sweetness, sourness, and fruitiness. Physiology & behaviour 57(3): 569-574.
- Delilbasi C, Cehiz T, Akal UK, Yilmaz T (2003) Evaluation of gustatory function in postmenopausal women. British dental journal 194(8): 447-449.
- Ochsenbein‐Kölble N, Von Mering R, Zimmermann R, Hummel T (2005) Changes in gustatory function during the course of pregnancy and postpartum. BJOG: An International Journal of Obstetrics & Gynaecology 112(12): 1636-1640.
- Dangore‐Khasbage SB, Degwekar SS, Bhowate RR, Motwani MB, Indurkar AD, et al. (2010) Comparative evaluation of gustatory function between postmenopausal women and age‐matched men. Oral Diseases 16(5): 469-475.
- Fasunla AJ, Nwankwo U, Onakoya PA, Oladokun A, Nwaorgu OG (2019) Gustatory function of and non-women in a Tertiary Health Institution. Ear, Nose & Throat Journal 98(3): 143-148.






























