Topographic Study on Molar Roots with Developmental Root Grooves - A Morphometric Analysis
Guey-Lin Hou1, 2* and Chi-Chu Cheng3
1Former Professor, Graduate Institute of Dental Science, Department of Periodontics, Kaohsiung Medical University, Taiwan, ROC
2Former Professor and Chairman, Periodontal Prosthetics Center, Chang-Gung, Memorial Hospital, Kaohsiung City, Taiwan, ROC
3Master Degree, Graduate Institute of Dental Science, Department of Periodontics, Kaohsiung Medical University, Taiwan, ROC
Submission: January 20, 2024; Published: February 01, 2024
*Corresponding author: Guey-Lin Hou, Former Professor, Graduate Institute of Dental Science, Department of Periodontics, Kaohsiung Medical University, Taiwan, ROC and Former Professor and Chairman, Periodontal Prosthetics Center, Chang-Gung, Memorial Hospital, Kaohsiung City, Taiwan, ROC
How to cite this article: Guey-Lin Hou* and Chi-Chu Cheng. Topographic Study on Molar Roots with Developmental Root Grooves - A Morphometric Analysis. Adv Dent & Oral Health. 2024; 17(2): 555958. DOI: 10.19080/ADOH.2024.17.555958
Abstract
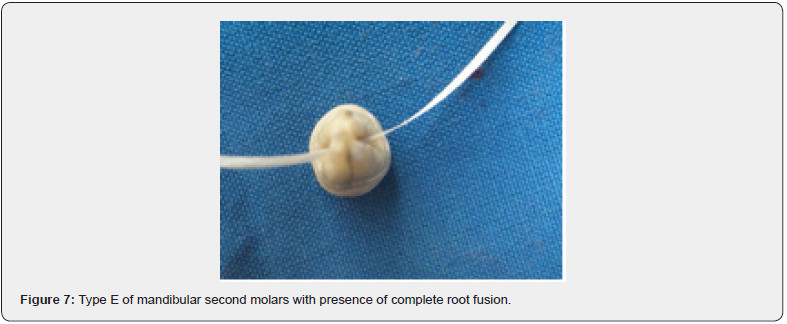
Numerous studies concluded that developmental root groove is a cofactor of periodontal breakdown. The shapes of molar roots also have significant influence on the progression. The purpose of the present study were as follows:(1) location of molar developmental root grooves (DRGs), (2) prevalence of molar DRGs, (3) length and depth of molar DRGs, (4) measurement of the root wall thickness at the deepest point of DRG. The totally 332 teeth included maxillary first molar (52), maxillary second molar (91), mandibular first molar (58), and mandibular second molar(131). Maxillary second molar has five subtype, Type A: absence of root fusion, Type B: presence of mesiobuccal and distobuccal root fusion, Type C: palatal root with anyone of buccal root fusion of molar with three roots, Type D: complete root fusion of molar with three roots, Type E: pseudofusion of molar. Mandibular first molar has two subtypes, Type A: mesial root with DRG on 2-root molar, Type B: mesial root with DRG on 3-root molar. Mandibular second molar has five subtypes, Type A. absence of root fusion, Type B: presence of complete root fusion, Type C: c-shape root without DRG at counterlateral surface, Type D: c-shape root with DRG at counterlateral surface, Type E: pseudofusion of mesial and distal roots fusion.
The results of DRG incidence were summarized as follows: (1) Mandibular second molar has the highest distribution; maxillary first molar has the lowest distribution. (2)Type D has the highest distribution of maxillary second molars; Type B has the hishest distribution of mandibular second molars. (3) DRG of mandibular second molars is longer than others. (4) Type B has the longest distance from CEJ to DRG and Type C has the shortest distance from CEJ to DRG in maxillary second molars. (5) Most of the DRG deepest points are located in middle third of DRG. (6) Mean distance from DRG to canal is 0.95±0.25mm. (7) There are no significant difference (p>0.05) between right and left maxillary and mandibular first and second molars. The conclusions revealed that there are statistical significant difference in both the depth between section and non-section with cross-section (p<0.05) and longitudinal-section (p<0.05).
Keywords: DRG; CEJ; DRG-subtypes; Cross-section; Longitudinal-section
Introduction
The prognosis of periodontal treatment in the molars area is poor, and the invasion of the root morphology and bifurcation area of the molar root is generally more serious and difficult to treat, mostly because the self-cleaning of the molars is not easy, and there are more variations in the growth and development of the tooth roots. These variations are such as root concavities, developmental root grooves (DRG) [1,2] and molar root fusion [3,4]...etc. Although, many studies have reported that these variants are indeed associated with periodontal disease and affect the progression and prognosis of periodontal disease. [1-2, 4-6] There are some complications for subgingival calculus deposits on the root concavities and developmental root grooves (DRG, Figure 1) in the molars and non-molars regions affected periodontitis. The purpose of this study was documented the association of anatomical forms of root concavity with the causes of periodontitis on the teeth loss.
Root concavities are mostly produced in the shape of the root surface (proximal surface), which can range from gentle grooves to deep depressions. In the absence of periodontal lesions, this anatomical structure increases the area of periodontal tissue in contact with the tooth root, and such anatomic morphology also makes teeth more resistant to torque forces. [7-9] However, root concavities are also considered to be a potential risk factor for periodontal inflammation due to pre-existing periodontitis. Clinically, root concavities will form an environment conducive to the accumulation of plaque in the periodontal pocket, which makes the instruments difficult to enter during periodontal treatment, making dental calculus and plaque easy to accumulate and hide, [9,10] which in turn causes periodontal inflammation to form a vicious circle, resulting in the aggravation of periodontal disease. In addition, root concavities can also make it difficult for the regeneration membrane to adhere to the root surface when periodontal tissue regeneration is used. Thereby it is also increasing the chance of surgical failure [11]. Root concavities occur on the contiguous surface of any tooth, the more obvious being the first molar, the mesial and buccal root of the first molar, and the root of the incisor tooth [7,12]. Marlin and Arthur mentioned root concavities as a distinct feature of root construction [8]. Root depressions can be made from very shallow depression depths, such as the proximal and distal central surfaces of canine teeth; to DRGs, such as the proximal surface of the maxillary first premolars.
DRG commonly known as palatal radicular groove or root groove. The results published by Leknes suggest that DRG is a risk factor for periodontal disease [2]. Moreover, it is further inferred that the DRG will endanger the patient’s personal plaque control, and root groove will also increase the difficulty of periodontists in periodontal treatment, resulting in poor or even failed periodontal therapy. When the crown is formed, the inner and outer enamel epithelialium cells will meet at the position of the cervical loop, that is, the position of the later cervical enamel junction, to form a bilayer cell structure of epithelial root sheath, also known as Hertwig’s sheath, which then extends downward and grows into the structure of the tooth root. When the root of the supernumerary tooth is formed, there will be two or more cells in the epithelial diaphragm, forming inter-radicular tongues, and then the inter-radicular tongues grow at a very fast rate until the inter-radicular tongues on the opposite side meet, so that the original pulp will be opened. Divided into two to three openings or even multiple openings, the epithelial diaphragm surrounds each opening and grows at the same rate to form two to three or more roots [13,14].
Butler’s research [15] argues that inter-radicular tongues are formed around a developmental center, and this developmental center is the blood vessels in the pulp, which is inter-radicular when the blood vessels in the pulp splits into two to three blood vessels from the primary apical foramen. The tongue would then be pushed by some force and dented toward the center until the junctional line stopped. Inter-radicular tongues can only fully develop when blood vessels are separated enough. If the blood vessels are not separated enough, the inter-radicular tongue will develop incompletely, forming a groove and two root canals on one tooth root. These are resulting in the formation of the socalled DRG. Therefore, DRG can be easily distinguish- ed from root concavities (RC).
Carlsen [16] also defined root groove in his series of literature on molars, pointing out that when two root cone/root components are close together, or not completely separated, twice the volume of the normal tooth root is formed, at which point the root groove (DRG) is depicted. In addition, Scott and Turner [17] argue that in incompletely separated root radicals, DRG is the dividing line that defines the different roots. From the above conclusions, it has been clearly stated that the DRG is a deep groove formed by the growth and development of the root, because the root canal development center is not completely separated or too close, so that the two tooth roots are not completely separated or even fused. RC is a depression on the root surface of a tooth that changes the direction of root development or enters the furcation from the root trunk. Moreover, from the above literature review, many scholars still confuse RC with DRG. Therefore, the results obtained do not correctly show clear data on RC and DRG. In addition, there are few reports of DRG at different molars.
Materials and Methods
This study sample was collected from September 1999 to February 2001, a total of 548 molars extracted by dental patients of the Hospital of Kaohsiung Medical University. A total 332 molars were removed from teeth with caries in the neck and teeth with pseudocomposite edges exceeding the enamel boundary of the tooth bone. According to the molar types, 52 of the maxillary first molars (code 16&26), 91 of the maxillary second molars (code 17&27), 58 of the mandibular first molars (code 36&46), and 131 of the mandibular second molars (code 37&47) were obtained. The extracted tooth is cleaned by an ultrasonic scaler at a low vibration frequency at a low vibration frequency to clean the soft and hard deposits from the CEJ to the molar furcation areas, and after soaking in hydrogen peroxide for 72 hours, it is stored in 10% formaldehyde solution for later use.
The materials used in this institute include samples collected periodontal probes (Hu-Friedy, Made in Germany, P3/4) electric digital caliper: NSK MAX-15 micrometer MFG. Co., LTD) accuracy up to 0.01 mm. Stereomicro- scope ZEISS Stemi 2000-C stereomicroscope (CARL ZEISS Co., LTD., Germany), magnification 6.5 to 50 times, used with ZEISS KL 1500 cold light illumination system. Digital cameras (Ricoh, RDCi500, Japan) have a resolution of 3.34 megapixels and can shoot up to 1 cm up close. Personal Computer - ONE IBM-compatible PC with an operating system for Microsoft Windows (version 4.20.2222A, Microsoft. Co., Seattle., WA, USA). High-speed phones with high-speed burs Low-speed phones with Disc fine sandpaper transmissing lights.
Results
In this study, the number of valid molars collected was 332, including 52 molars and 32 DRGs, and the overall incidence rate was 61.5% (32/52). The number of the maxillary first molars (16&26) was 52, and the number of DRGs was 32, and the overall incidence was 61.5% (32/55).There were 91 maxillary second molars (17&27), and the number of DRGs was 77, and the overall incidence was 84.6% (77/91). The number of the mandibular first molars (36&46) was 58, and the number of DRGs was 82.8% (48/58). The number of mandibular second molars (37&47) was 131, and the number of DRGs was 112, and the overall incidence was 85.5% (112/131). Among the total of 332 molars of the four categories, 269 molars were obtained with DRGs, accounting for 81.0% (269/332). Therefore, in order of incidence, the maxillary first molars (16&26) (32/91; 61.5%), the maxillary second molars (17&27) (77/91; 84.6%), the mandibular first (36&46) (48/131; 82.8%) and second molars (37&47) of (112/131; 85.5%) (Table 1).

Table 2 showed the Incidence and distribution of DRGs on the maxillary first and second molars. According to molar with and without DRG on the right and left sides as a distinction, it can be obtained that the incidence of on the maxillary first molars (16&26) with DRG is 34.6% (18/52) on the right side and 26.9% (14/52) on the left side, respectively. The total 52 teeth of maxillary first molars (16&26) with 32 DRG given an incidence of 61.5%. In addition, the incidence was 38.5% in a total 20 molar without DRG. The incidence of the right and left sides of the maxillary second molars (17&27) is 48.4% (44/91) on the right side and 36.2% (33/91) on the left side. The total 84.6% incidence was located on the 91 maxillary second molars (37&47) with a 48.4% of 44 DRGs on the right side and a 36.2% of 33 DRGs in a total 91 maxillary second molars (17&27). In addition, only both of 4 (4.4%) and 10(11.0%) molars without DRG showed a total incidence15.4% (14/91), respectively.

Table 3 illustrated that the incidence of the right and left sides of the mandibular first molars (36&46) was 39.7% (23/58) on the right side and 43.1% (25/58) on the left side. The total incidence of 58 teeth of mandibular first molars (36&46) with 48 DRG was 82.8%. In addition, the incidence was 17.2% in a total 10 molar without DRG. The incidence of the right and left sides of the mandibular second molars (37&47) was 45.0% (59/131) on the right side and 40.5% (53/131) on the left side. The total 85.5% incidence was located on the 112 mandibular second molars (37&47) with DRGs. In addition, the incidence was 14.5% in a total 19 molar without DRG, respectively.
Discussion
According to the definition of DRGs, the root grooves are deep grooves formed by the incomplete separation or even fusion of two roots when the root canal development center is not completely separated or too close together during the growth and development of the root canal; [15] Root concavities, on the other hand, are the depressions on the root surface that are formed by changes in the direction of root development or when the root trunk enters the furcation [13]. Therefore, the DRG usually occurs in a single root with two root canals or in a fused root.

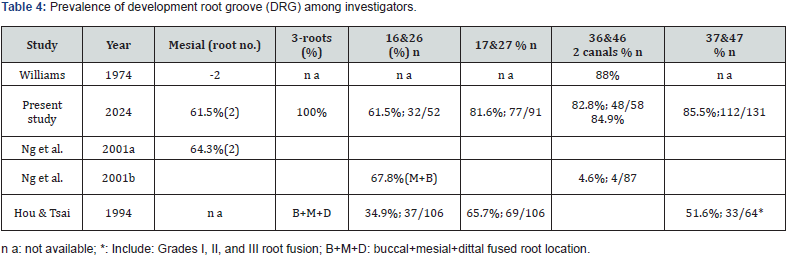
Ng et al. [18] in their study of the relationship between the root canal and the root of the mandibular molars in Burmese showed that 84.9% of the first molars of the double root (proximal and distal) had a double root canal. Among the molars with three roots in the first molar, 64.3% of the mesial roots had double canals. Williams [19] showed that 88% of the mesial roots of the mandibular first molars (36&46) had double canals. In this study, 82.8% of the mesial roots of the first molars had double canals and root grooves, which was similar but low compared with the above-mentioned scholars. The reason for this can be explained by the fact that although there are two root canals in a single root, there is no obvious DRG between the two root canals, i.e., between the two developmental centers, such molars are not taken as experimental samples. Therefore, the proportion is relatively low. In this study, 100% of the molars with three roots in the mandibualr first molars, and the proportion of proximal roots with double canals. The reason for this can be explained by the small sample size, which does not reflect the true distribution of the parent population.
Ng et al. [20] reported that the maxillary first molars in Burmese showed that the disto-buccal root and palatal root were single root canal; The proportion of single root and double root canal of mesiobuccal root was 67.8%. In addition, from 1973 to 1999, different scholars in different countries and places used different methods to study the mesio-buccal root of the maxillary first molar, and the proportion of single root and double root canal ranged from 27.3% to 90.5%, but most of them were between 50% and 70%. In this study, the mesial root of the maxillary first molar had a single root and a double root canal and the proportion of root grooves was 61.5%, which was similar to the results of other scholars [19-21]. However, this method cannot further distinguish between the direction of the root canal and the presence or absence of the communication branch within the root canal.
Ng et al. [20] reported that the distal buccal root and buccal root were single root and single canal in the maxillary first molars of Burmese. The proportion of single root and double root canal of mesiobuccal root was 67.8%. In addition, from 1973 to 1999, different scholars, in different countries and places, used different methods to study the mesiobuccal root of the first molar, with a single root and double canal. The proportion ranged from 27.3% to 90.5%, but most of them were between 50% and 70%. In this study, the mesial root of the first molar had a single root and a double root canal and the proportion of root grooves was 61.5% (Table 2 and Table 4), which was similar to the results of other scholars [18, 19, 21] However, this method cannot further distinguish between the direction of the root canal and the presence or absence of the communication branch within the root canal.
In this study, it was found that there was no fusion in the root morphology of the maxillary and mandibular first molars, which was similar to that of Hou & Tsai et al. [21] showed that the incidence of fusion of the first molars of the maxillary and mandibular molars was different, and the results showed that the proportion of root fusion of the maxillary first molars (16&26) was 27.1%, and mandibular molars (36&46) was 4.6%, respectively. Among them, the grade I to grade III of the maxillary first molars were 15.3%, 8.2% and 3.5%, respectively. The grades of grade I to grade III of the mandibular first molars were 3.5%, 0% and 1.2%, respectively, and the possible reasons for this difference were as follows: 1. The sample size was relatively small, which may not reflect the true distribution of the parent population; 2. In the study of Hou et al. [21], the criterion for determining root fusion is the true fusion of normal roots, but this study can only be used as a sample if there is a significant DRG under the premise of fusion. Therefore, if the root is fused but there is no obvious root groove, it is not included in the sample of this study, so if the incidence of root groove development is studied according to this standard, the incidence rate should be lower than that of root fusion alone. The above reasons may be the main reason for the lower incidence of DRG than root fusion.
In this study, the incidence of DRG of the second molar was 84.6%, and the results were similar to those of Hou & Tsai [21] or Gulabivala & Ng [20] had a high proportion of 48.1% of the single root with double root canals without root fusion, and this result was due to the fact that in the study of Hou &Tsai, the second molars without root fusion were excluded first, and only the fused roots were used as the sample of the study, so the incidence rate was low, and if the teeth without root fusion were excluded in this study, the incidence rate was 67.7%. Such results are consistent with the study of Hou & Tsai [21] However, if only the maxillary second molars were counted without root fusion, the result was 16.9%, which was lower than that of Gulabivala & Ng [20] or other scholars have a double root canal at the proximal buccal root of the second largest molar. The reasons for this may be as follows: (1) although there are two root canals in a single tooth root, but there is no obvious developmental groove between the two root canals, that is, between the two developmental centers, this kind of molars will not be taken as experimental samples, so its proportion is lower than that of other scholars, (2) the upper molars are three-tooth roots, and the growth mode between each tooth root is relatively inward, so when the transmitted light is examined, it is easy to be hindered by other tooth roots, so the transmitted light transmission degree is low, and the observation results are lower than the actual situation.
Kerms & Kerm reported that the incidence of root groove was 53.3% in the first molar, 89.5% in the second molar, 61.2% in the first molar, and 98.1% in the second molar [22]. The incidence of the disease is quite different from that in this study, and the reason for this is that Kerm refers to the concavities before the root trunk enters the furcation entrance [22] which is inconsistent with the definition of root developmental groove in this study. In terms of developmental relationship, such a depression can only be regarded as the traces left by the DRG on the root surface when the root development is not completed.
Marlin and Arthur [8] illustrated the method of studying the surface area of the root of the first molar of the maxilla was to make a cross section every 1 mm below the CEJ, and then scan it into the computer, and use the interpretation of the image to determine the surface area of the root of the first molar of the upper molar. Robert and Marlin [23] also employed the same method, to study the root surface area of the first molar of the lower jaw. Roussa [24] used the method of studying the root concavities of the first and second molars of the maxilla and mandible was to do a cross section every 70 μm from CEJ or below, and then scan it into a computer by means of image scanning, and then study the surface area of the root depression on the root surface. Brook and Dan [25] used a dissecting microscope to study the proximal depression of the first molar. Although this method can observe the amount of root surface area, or the surface area or extent of the root depression at a very fine distance, it cannot provide an ideal observation method for the direction of the root development groove and the depth of the deepest point in the root surface development groove.
In this study, corss-section Figure 1 and Figure 2(a) and longitudinal section Figure 2(b) were used to observe the development of root groove (DRG). In the longitudinal cutting, a high-speed handpiece and a drill needle are used to cut the deepest part of the DRG, and once the deepest part of the DRG is approached, grinding paper is used instead. Try to deal with the DRG in a way that is close to the DRG and does not destroy the deepest point and direction of the DRG. When cutting at an equal distance, the deepest point of the DRG is first anchored, and the low speed and disc are used to cut horizontally at a distance of about 0.5 to 1 mm from the anchor point, and then ground to the anchor point with fine sandpaper to observe the deepest point of the DRG. Such a longitudinal incision method can faithfully restore the direction of the DRG, and clearly present the starting point, the position of the deepest point and the end point of the DRG, as well as the distance relationship with CEJ. In terms of cross-cutting, the deepest point will not be cut out or left between the tangent point and the tangent point of the isometric cut. The method of observation is to place the cut tooth root in a stereomicroscope for direct observation. The stereomicroscope used in this experiment is attached with a scale with a minimum actual value of 0.1 mm and an estimated value of 0.05 mm.:




References
- Hou GL, Tsai CC (1993) Relationship between palato-radicular groove and localized periodontitis.J Clin Periodontol 20(9): 678-682.
- Leknes KN, Lie T, Selvig KA (1994) Root grooves: A risk factors in periodontal attachment loss. J Periodontol 65: 859-863.
- Hou GL, Tsai CC (1997) Clinical significance of tooth morphology correlated with periodontal disease. Kaohsiung J Med Science 13(4): 200-212.
- Franklin I, Evanchick PA (1981) Root fusion in molars - incidence and sex linkage. J Periodontol 52(11): 663-667.
- Hou GL, Tsai CC (1997b) Relationship between molar root fusion and loca periodontitis. J Periodontol 68(4): 313-319.
- Shiloah J, Kopczyk RA (1979) Development variations of tooth morphology and periodontal disease. J Am Dent Assoc 99(4): 627-630.
- Formin A (1990) Glickman’s Clinical Periodontology. ed. 4. Philadelphia W.B. Sounder Company.
- Marlin EG, Arthur RV (1980) Root morphology - Clinical significance in pathogene- sis and treatment of periodontal disease. J AM Dent Assoc 101(4): 627-633.
- Hou GL, Tsai CC (1997c) Enamel projection and intermediate bifurcational ridge correlated with molar furcation involvements. J Periodontol 68(7): 687-693.
- Donald H (1998) The diagnosis and treatment of molar furcation invasion. Dent Clin North American 42(2): 301-307.
- Arthur B, Tamani JP (2001) Root trunk concavities as a risk factor for regenerative procedures of class II furcation lesions. J Periodontol 72(5): 612-619.
- Ong G, Neo J (1990) A survey of approximal root concavities in an ethnic Chinese population. Archs Oral Biol 35(11): 925-928.
- Tencate AR (1989) Development of the tooth. ed. 2. Oral Histology: Development, Structure, and Function. St Louis CV Mosby Company.
- James K, Avery (1994) Essentials of oral histology and embryology - A clinical approach. ed. 2: 51-69. St. Louis CV. Mosby Company.
- Butler PM (1956) The odongenesis of molar pattern. Biol Rev 31: 30-70.
- Carlsen O (1990) Radix entomolaris: Identification and morphology. Scand J Dent Res 98(5): 363-373.
- Scott GR, Turner (1997) The anthropology of modern human teeth. Dental morphology and its variation in recent human population. Cambridge University Press.
- Ng YL, Aung TH, Alavi A, Gulabivala K (2001a) Root and canal morphology of Burmese mandibular molars. Inter Endodontic J 34(5): 359-370.
- Williams R, Vertucci FJ (1974) Root canal anatomy of the mandibular first molar. J NJ Dent Assoc 45(3): 27-28.
- Ng YL, Aung TH, Gulabivala K (2001b) Root and canal morphology of Burmese maxillary molars. Inter Endodontic J 34(8): 620-630.
- Hou GL, Tsai CC (1994) The morphology of root fusion in Chinese adult. J Clin Periodontol 21(4): 260-264.
- Kerms DG, Kerm LL (1999) Root trunk dimemsions of five different tooth types. Inter J Periodontics Rest Dent 19(1): 83-91.
- Robert MD, Marlin EG (1984) Root surface measurements of mandibular first molar. J Periodontol 56(4): 234-238.
- Roussa E (1998) Anatomic characteristics of the furcation and root surfaces of molar teeth and their significance in the clinical management of marginal periodontitis. Clin Anat 11(3): 177-186.
- Brooks WB, Dan ML (1985) A morphologic study of the mesial root surface of the adolescent maxillary first bicuspid. J Periodontol 56(11): 666-670.






























