TYPE-I Collagen as an Enhancer of Strontium Acetate
Leonardo de Pádua Andrade Almeida1*, Tatiane Cristina Dotta1, Raisa Castelo1, Mayara Manfrim Arnez1, Carla Roberta de Oliveira Maciel1, Ana Paula Ramos2 and Alma Blasida Concepcion Elizaur Catirse1
1Department of Dental Materials and Prosthodontics, Ribeirão Preto School of Dentistry, University of São Paulo – Ribeirão Preto, São Paulo, Brazil
2Department of Chemistry, Ribeirão Preto Faculty of Philosophy, Sciences and Letters at Ribeirão Preto, University of São Paulo – Ribeirão Preto, Brazil
Submission: January 04, 2024; Published: January 18, 2024
*Corresponding author: Leonardo de Pádua Andrade Almeida, Department of Dental Materials and Prosthodontics, Ribeirão Preto School of Dentistry, University of São Paulo – Ribeirão Preto, São Paulo, Brazil Adv Dent
How to cite this article: Leonardo de Pádua Andrade Almeida*, Tatiane Cristina Dotta, Raisa Castelo, Mayara Manfrim Arnez, Carla Roberta de Oliveira Maciel, et al. TYPE-I Collagen as an Enhancer of Strontium Acetate. Adv Dent & Oral Health. 2024; 17(2): 555957. DOI: 10.19080/ADOH.2024.17.555957
Abstract
This study evaluated the effect of collagen-strontum acetate (SrAc) composites on dentin tubular obliteration. Methods: Type-I collagen was isolated from mouse tail tendons, solubilized in acetic acid and purified by dialysis. Then, SrAc was dispersed in the collagen solution. Dentin discs were used for the application of SrAc-collagen by the topical method, for 1 minute. The samples were submitted to acid challenge by immersion in Coca® Cola under stirring for 2 minutes. To analyze the chemical composition of the dentin surfaces before and after application, ATR-FTIR and EDS were used. Dentin structure, tubular obliteration and deposit resistance to acid challenge were analyzed by SEM. Results: Changes in the FTIR spectra were observed, mainly in the 1070 cm-1 and 1250 cm-1 bands, referring to the phosphate and amine groups, respectively. The images obtained by SEM show changes in the dentin morphology after applicaton of G2 and G3. SEM revealed that immersion in acidic drink was not enough to remove the deposited material. The EDS results revealed an increase in the amount of Sr in the samples proportional to the increase in SrAc-collagen application cycles and confirmed the presence of the mineral deposit even after acid challenge. Was also observed the penetration of the product into the dentinal tubules in all modified samples of G2 and G3. Conclusion: The use of products inspired in natural compounds found in dentin, associated with particles used in commercial dentifrices can have a positive and significant effect on the structural conditions of sensitive teeth.
Keywords: Dentin sensitivity; Strontium acetate; Collagen Type-I; Phosphoric acid; Dentinal tubules
Abbreviations: EDS: X-ray Dispersion Spectroscopy; FTIR: Fourier Transform Infrared Spectroscopy; PBS: Phosphate-Buffered Saline; SEM: Scanning Electron Microscope; SRAC: Strontum Acetate; Sr: Strontum
Introduction
Defined as an acute, sudden and painful reaction, dentinal hypersensitivity occurs when teeth are exposed to certain stimuli, whether hot, cold, chemical, tactile or mechanical, which are not related to any other pathology or dental anormaly [1-3]. Although the literature reports a well stablished knowledge about dentinal hypersensitivity, the etiology is not clear [2]. The most used treatment for dentinal hypersensitivity is the use of dentifrices, since it consists of a cheap, non-invasive, convenient and routine method, with high availability and easy acquisition in the market [4,5]. However, there are several other methods for treating hypersensitivity [6-8]. Since the active ingredients differ in the formulations, these products have different mechanisms of action [7,9,10]. Calcium ions can be replaced by strontium, changing the chemical structure of hydroxyapatite [11-14]. Strontium has high affinity for dentin and apatite, similarly to other substances such as zinc and tin, which are used for the treatment of sensitivity. The use of 8% strontium acetate-based dentifrices promotes the formation of a deposit on the dentin surface, resistant to rinsing with water, in addition to the enhanced occlusion of the canaliculi compared to the other substances present in another dentifrices [5,7,11,15-18]. As a result of the formation and deposition of mineral based on apatite, there is a significant increase in radiodensity after immersion of conditioned dentin in strontium chloride solution [11,12]. Although present in several toothpastes and still being a significant component in certain restorative dental materials, the biological effects of strontium in the dentin-pulp complex need to be explored [19,20].
In addition to the minerals present in human teeth, organic components are also found [9,15]. The mechanisms that lead to the synthesis of collagen (predominantly type I) by fibroblasts, odontoblasts and osteoblasts are essentially the same [21]. However, in the dental matrix the non-collagen proteins tend to occupy the space between the collagen fibers, accumulating over the peripheral area of the dentinal tubules [3,9]. The exposure of collagen on the surface dentin may enhance the binding of the fibers with exogeneous compounds as bioadhesive polymers and inorganic particles [9]. Thus, it is theoretically acceptable that compounds of opposite charges in relation to the surface of the dentinal tubules may have greater affinity to the dentinal surface [9,22,23]. In this study we explored the possibility of enhancing the action of strontium acetate on the obliteration of dentinal tubules by using typo-I collagen as a carrier and bonding agent following a biomimetic approach. Since strontium contributes to the precipitation of protective coatings on dentin surface also promoting the obliteration of dentinal tubules and type I collagen predominant present a positive surface charge, opposed to the negatively charged dentinal surface, there is a probability that dentifrices containing both substances have a greater deepness of tubule obliteration over a longer period. The resistance of deposits to acidic drinks was also evaluated.
Materials and Methods
Materials
Dentin samples were prepared from third molar teeth stored at the teeth-bank of the Dentristy School (University of São Paulo- Ribeirão Preto, Brazil) after the approval of the Ethics in Research Committee of the same institute (06245218.2.0000.5419). The strontium acetate was purchased from Sigma Aldrich. Type I collagen was isolated from mouse tail tendons using the protocol described elsewhere [14].
Methods
Preparation of the dentin samples: The third molars were sectioned in the cement-enamel region, with the aid of a manufactured acrylic matrix (Dencor®, Methyl Methacrylate - Artigos Odontológicos Clássico Ltda- Brazil), to obtain dentin discs with approximately 1mm thickness. Integral dentin discs were selected with the aid of a magnifying glass to eliminate samples with caries, cracks and reactive dentin [19]. The disks were cleaned in ultrasound (ALT Sonic Clean) bath with deionized water for 30s and placed in a 6wt % aqueous citric acid solution for 2 minutes to remove the smear layer [19]. Then, the samples were immersed again in ultrasound bath with deionized water for 10 minutes. Finally, the dentin discs were stored in 0.5 ml of artificial saliva [20] at a controlled temperature of 37+1ºC.
Type I Collagen solution preparation: Before use, the mouse tails were stored at -20°C. The procedure used to prepare the solutions is described elsewhere [24]. Briefly, the tails were conditioned in a Petri dish containing absolute ethanol to accelerate the thawing. After skin remotion, the tendon bundle near the thinnest end was cut and immersed in Phosphate-Buffered Saline (PBS) pH 7.2. Then, they were immersed in acetone for 5 min and transferred to a 70% isopropanol solution for 5 min more. Then, the tendons were transferred to recipient containing acetic acid and 2.5 mol/L NaCl under constant agitation at 4ºC for at least 48 hours, until complete dissolution. The solution was then subjected to centrifugation at 10000g, for 30 minutes at 4ºC. The precipitate was redissolved in the smallest possible volume of 0.50 mol / mL acetic acid, followed by stirring for 24 hours. Finally, the soolutin was dialysed against 0.10 mol / mL acetic acid for 4 days, changing the medium each day. The dialyzed solution was stored in a glass bottle in a refrigerator at 4°C.
Synthesis of SrAc-Collagen composites: 8wt.% of SrAc (Sigma-Aldrich) were manually mixed in an aqueous solution containing 1wt% of type I collagen, until a pasty product was obtained.
Application of SrAc-Collagen on the dentin surface: Dentin discs were randomly divided into 3 groups according to the number of applications: G1 - 1 single application, G2 - 2 daily applications (morning and afternoon) for 7 days, G3 - 2 daily applications (morning and afternoon) for 14 days. The discs were subjected to topical application of SrAc-collagen (62mg) for 1 minute, according to the recommendation of products with similar effects found on the market (Sensodyne, Brentfor, United Kingdom), using procedure gloves and performing circular movements on the samples surface. The interval between daily applications was 4.5 hours. The treated dentin discs were stored in artificial saliva with controlled temperature, at 37ºC.
Resistance of deposited material to acid attack: The resistance of the material deposited in the dentin structure was evaluated 1h after application of SrAc-Collagen by immersing the samples in 3 mL of Coca-Cola® for 2 min on a stirring table (Cintec, Ribeirão Preto, Brazil) according to the procedure described by Olley et al7. At the end, the samples were washed for 10 s with deionized water and stored in artificial saliva at 37ºC.
Characterization of the dentin samples: Changes in the chemical composition of the dentin surfaces before and after SrAc-Collagen application were performed by Fourier transform infrared spectroscopy (FTIR), using an attenuated total reflectance device (IRPrestige-21, Shimadzu). The concentration of Sr, Ca and P on the samples was semi-quantitatively analyzed by X-ray dispersion spectroscopy (EDS) (500 Digital Processing; IXRF Systems), coupled to a scanning electron microscope (SEM) (Zeiss EVO 50 - Zeiss).
Percentage and deepness of dentinal tubules obliteration: The images obtained by SEM were used to count the percentage of tubule obliteration after the treatment of the dentil discs with SrAc-Collagen. Two evaluators observed the images and divided the tubules according to the groups: partially obliterated (P), totally obliterated (O) and not obliterated (A). The percentage of obliteration (n) was calculated using the following equation [25]:

The average of the values of the two observers was calculated at the end of the count. The deepness of obliteration was analysed by the SEM imagens of transversely sectioned discs by measuring the distance (μm) between the top of the dentinal tubule to the end of the coating deposited [11].
Results
Chemical analysis of the dentin surface before and after the topical application of SrAc-Collagen
Figure 1 shows the ATR-FTIR spectra of the dentin surface after different application periods. The spectrum of the untreated dentin disc (Figure1-black line) depicts an intense band at 1070 cm-1, corresponding to the asymmetric stretching of the phosphate group, present in the hydroxyapatite chemical structure. Other less intense bands in the 1400 to 1700 cm-1 range attributed to the presence of carbonyl and amine groups from the organic dental matrix as well as B-type substitution of phosphate by carbonate in the apatite structure can be noted. The low intensity band at 1250 cm-1 is characteristic of the amide I group, present in type-I collagen [26,27]. No significative differences were observed in the position or in the intensity of these bands after a single application of the experimental desensitizing agent (Figure 1-red line), except for the bands close to 1400 cm-1, assigned to the vibrations of amino groups, which may be indicative of the collagen adsorption on the surface [26,27]. Significant changes were observed in the FTIR spectrum of the samples treated twice a day for 7 days (Figure 1-green line) when compared to the untreated sample (Figure 1-black line). Displacement of the band related to the phosphate groups at 1070 cm-1 as well as the change in the intensity and the profile of the bands related to the organic matrix attest the changes in the chemical structure of the surfaces after treatment with SrAc-collagen. This trend was maintained for the samples treated twice a day for 14 days of application (Figure 1-blue line) indicating that maximum surface coverage was reached.

Morphological characterization, percentage, and deepness of the dentinal tubules obliteration after SrAc-Collagen application
The morphology of the dentin surfaces after the topic application of SrAc-collagen was analyzed by SEM (Figure 2). The deposition of a coating that resulted in the decrease in the diameter of the dentinal tubules was observed after 7 days of application (Figure 2B). This trend was maintained after 14 days in which the nozzle of the dentinal tubules is almost non-visible (Figure 2C). Partial removal of the coatings in the intertubular region was observed after immersion of the samples in acidic beverage (Figure 2E & F). However, the coating was maintained in the top of the tubules. SEM cross-sectional images of the dentin samples were also analyzed (Figure 3). There were no significant changes in the transversal cuts of the sample treated with SrAc-collagen once when compared to the untreated sample. The fibrillar structures identified inside the tubules of the samples treated for 7 and 14 days may be related to the adsoption of collagen from the SrAccollagen composite (Figure 3B & C). Moreover, the morphology of the tubules and the profile of the mineral deposits were changed after treatment. After immersion in the acid drink, was not enough to remove the mineral particles as well as the fibers from inside the tubules (Figure 3 E and F). From these images it was possible to calculate the mean values of obliteration deepness for the samples treated with SrAc-collagen (Tables 1 & 2). The deepness of penetration was calculated for the samples treated with Sr-collagen for 7 and 14 days, after immersion in acidic drink (Tables 1 & 2). Significant amounts of the deposited material were observed only in G2 and G3. The average deepness values of both groups were calculated using a millimeter ruler.



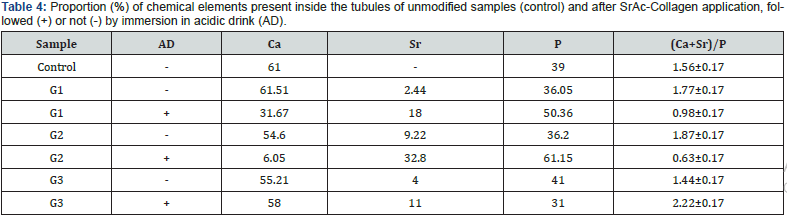
Mapping and quantification of Ca, P and Sr in dentinal structures
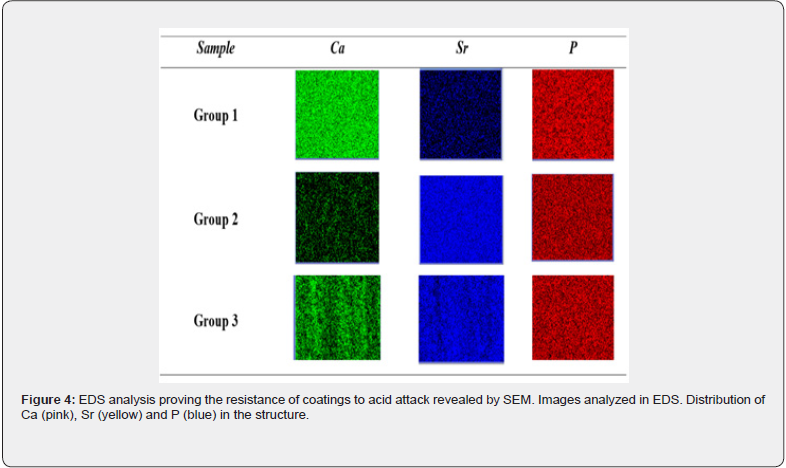
The mapping and semi-quantitative analysis of the chemical elements present in surface of the dentin discs, before and after SrAc-Collagen application were obtained by EDS analysis. For this, the Ca + Sr / P molar ratio was calculated for the surfaces (Table 3) and inside the tubules (Table 4) and compared with the 1.67 ratio obtained from the chemical structure of hydroxyapatite [Ca10(PO4)6(OH)2]. The sample treated only once with SrAccollagen exihibited Ca + Sr / P molar ratio close to 1.63 like the untreated samples. Values lower than 1.66 and the increased relative amount of Sr for the samples treated for 7 and 14 days corroborates the full coverage of the samples by the coatings promoted by the SrAc-collagen application. The EDS analysis also corroborates the resistance of the coatings under acid attack revealed by SEM (Figure 4). The reduction in the relative amount of Sr deposited on the samples after immersion in the acid drink was close to 10% and 20% for the samples treated for 7 and 14 days, respectively. The Ca, Sr, and P mapping of the surface and side-view was also performed before and after treatment with SrAc-collagen (Figure 4 & 6) The increased intensity of the blue dots, related to the presence of Sr on the surfaces and inside the tubules was increased proportionally to the the number of SrAccollagen applications. In Figures 5 & 7, the decrease in Ca particles and Sr evidence, resulting from the agitation in acidic drinks, agree with the relative amount of the elements presented in Tables 2 & 3, where a higher amount of Sr remained in the dentin structure even after contact with the acidic environment. Circumscriptions of Sr on the surface of G3 samples after treatment with acidic drinks (Figure 5) confirm the previous idea.


Discussion
For centuries, dentin hypersensitivity has affected a large part of the population, independently of the ethnic group. Erosion has been considered as the main etiological factor of hypersensitivity, responsible for open and enlarge the dentinal tubules present [25]. The collagen type I obtained from the tail of rats can be extracted in large amounts. It has been widely studied as a natural material in the field of tissue engineering, dressing and medication administration [24]. However, the administration in the dentin is not yet consolidated in the literature. Wang et al. [18] suggested that collagen has an impact on the hydration and structure of the apatite phosphate group, corroborating with other results that mention the importance of protein in the mediation of ionic bonds, nucleation, orientation, and alignment of the mineral [28]. They observed that groups loaded with collagen provided sites that induced the nucleation of apatite in the remineralization process. In the FTIR equipment, the specimens that received more frequency of applications (G2 and G3) showed a variation in the shape of the band comprised by the phosphate and amide group. The results are characterized by the crystallization and mineralization of strontium molecules together with the hydroxyapatite of the dental structure and protein adsorption (collagen), respectively. In general, the strontium particle weight and diameter are higher than calcium, which justifies the alteration of the vibrations in the region of the phosphate group, observed in the FTIR spectra. However, in the G1 spectrum, the difference between the linear values before and after application is practically null, implying that it was not enough to promote any changes.
The identification of the elements started initially from the samples of the control group. Even though Sr values are absent, Wang et al. [18] report that strontium can be found in food and drinking water, and its daily intake is about 2.4mg, stating that in natural samples they can be found even at low levels amount. Even just submitted to an application of strontium acetate and collagen, G1 showed a small percentage of Sr, a much lower value compared to G2 and G3, also observed in compositional maps. Nevertheless, it becomes evident the need for repeated applications and a greater extension of the treatment to obtain a greater concentration of the new particle in the dentin. On the other hand, even after a longer application time, the percentage of Sr present in G2 and G3 analyzed by the cross-section is considerably lower in relation to the superficial, showing a challenging character of penetration of the product inside the tubules, which is relatively normal based on previous studies. Observing the values obtained after submission to the acid challenge, it is notable for the numbers that there was a considerable decrease in the amount of calcium, which results from the demineralization promoted by phosphoric acid. While the Ca concentration was reduced, Sr was presented with a higher percentage. In the SEM images corresponding to groups 2 and 3 after being submitted to the acid challenge, decalcification is characterized by partial surface wear in specific regions, together with circumscriptions in the vicinity of the tubules. Comparing the compositional maps for group 1 with groups 2 and 3, it appears that the loss of Ca due to demineralization was greater than that of Sr under the same experimental condition. Due to the collagen coating on Sr molecules that promote a greater chemical union between the natural structures of the samples and the product, strontium did not suffer such interference and demineralization provided by phosphoric acid when compared to calcium.
According to researchers’ analysis, phosphoric acid, found mainly in carbonated drinks, together with carbonic acid has an excess of phosphate, impairing calcium metabolism, favoring greater loss of the mineral [29]. Authors [30] emphasize that acidic substances can open dentinal tubules, increasing clinical permeability and increasing hypersensitivity. After analyzing the influence of different acids and dentifrices on the market, Seong et al. [31] noted that in the presence of an acid challenge in the diet, the obliteration with strontium-containing toothpaste was clearly superior to the others used. In the SEM photomicrographs, the groups with the highest frequency of application showed micrometric penetration of collagen-coated estrogen acetate into the dentinal tubules. According to previous studies [11], penetration of up to 20μm deep inside the dentinal canaliculi, they are easily rinsed with water. Observing the surface of the specimens, the modification and decrease in the diameter of the dentinal tubules was significant, mainly in G2 and G3. It is possible to observe the presence of fibrillar structures in the images obtained, determinants for confirming the maintenance of the collagen integrity at the end of the applications. Even after the acid challenge, the continued decrease in the diameter of the tubules was noticeable, despite a marked demineralization of the structures in certain regions, showing a satisfactory initial result when compared with similar studies already mentioned. The results achieved are compatible with what was expected. However, further studies are needed, as well as new experiments to confirm the efficiency of Strontium and Collagen Acetate. Clinical studies may, in the future, provide guidance on the effectiveness and effects of using the product.
Conclusion
According to the methodology used, it was possible to conclude that:
i) After the application of strontium acetate coated with collagen in the period of 7 and 14 days, it promoted changes in the vibrational frequency related to hydroxyapatite and in the amine group (FTIR) and changes in the percentage of calcium and strontium (EDS).
ii) There was a decrease in the diameter of the dentinal tubules.
iii) The depth of dentinal tubule obliteration after 14 days of treatment was greater than that of 7 days.
iv) The acid reduced calcium particles: however, strontium particles were maintained in the dentin structure.
References
- Gallob J, Sufi F, Amini P, Siddiqi M, Mason S (2017) A randomised exploratory clinical evaluation of dentifrices used as controls in dentinal hypersensitivity studies. J Dent 64: 80-87.
- Hu ML, Zheng G, Zhang YD, Yan X, Li XC, et al. (2018) Effect of desensitizing toothpastes on dentine hypersensitivity: A systematic review and meta-analysis. J Dent 7: 12-21.
- Oh S, Jung HS, Kim HJ, Jang JH, Kim DS, et al. (2018) Effect of zinc on the collagen degradation in acid-etched dentin. J Dent Sci 13(2): 97-102.
- Farooq I, Moheet IA, Alshwaimi E (2015) In vitro dentin tubule occlusion and remineralization competence of various toothpastes. Arch Oral Biol 60(9): 1246-1253.
- Olley RC, Pilecki P, Hughes N, Jeffery P, Austin RS, et al. (2012) An in situ study investigating dentine tubule occlusion of dentifrices following acid challenge. J Dent 40(7): 585-593.
- Shiau HJ (2012) Dentin hypersensitivity. J Evid Based Dent Pract 12(3): 220-228.
- Montoya C, Arola D, Ossa EA (2016) Importance of tubule density to the fracture toughness of dentin. Arch Oral Biol 67: 9-14.
- Wang Q, Kang Y, Barnes V, DeVizio W, Kashi A, Ren YF (2013) Dentin tubule occlusion and erosion protection effects of dentifrice containing bioadhesive PVM/MA copolymers. Clin Oral Investig 17(3): 775-783.
- Hall C, Mason S, Cooke J (2017) Exploratory randomised controlled clinical study to evaluate the comparative efficacy of two occluding toothpastes - a 5% calcium sodium phosphosilicate toothpaste and an 8% arginine/calcium carbonate toothpaste – for the longer-term relief of dentine hypersensitivity. J Dent 60: 36-43.
- Huang M, Hill RG, Rawlinson SCF (2016) Strontium (Sr) elicits odontogenic differentiation of human dental pulp stem cells (hDPSCs): A therapeutic role for Sr in dentine repair? Acta Biomater 38: 201-211.
- Saeki K, Marshall GW, Gansky SA, Parkinson CR, Marshall SJ (2016) Strontium effects on root dentin tubule occlusion and nanomechanical properties. Dent Mater 32(2): 240-251.
- Medvecky L, Stulajterova R, Giretova M, Mincik J, Vojtko M, et al. (2018) Effect of tetracalcium phosphate/monetite toothpaste on dentin remineralization and tubule occlusion in vitro. Dent Mater 34(3): 442-451.
- Wang YL, Chang HH, Chiang YC, Lin CH, Lin CP (2019) Strontium ion can significantly decrease enamel demineralization and prevent the enamel surface hardness loss in acidic environment. J Formos Med Assoc 118(1 Pt 1): 39-49.
- Laure Rittié (2017) Type I Collagen Purification from Rat Tail Tendons. Fibros Methods Protoc Methods Mol Biol 1627: 287-308.
- Arnold WH, Prange M, Naumova EA (2015) Effectiveness of various toothpastes on dentine tubule occlusion. J Dent 43(4): 440-449.
- Parkinson CR, Butler A, Willson RJ (2010) Development of an acid challenge-based in vitro dentin disc occlusion model. J Clin Dent 21(2): 31-36.
- Rajan N, Habermehl J, Coté M-F, Doillon CJ, Mantovani D (2006) Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Protoc 1(6): 2753-2758.
- Wang Y, Azaïs T, Robin M, Vallée A, Catania C, et al. (2012) The predominant role of collagen in the nucleation, growth, structure and orientation of bone apatite. Nat Mater 11: 724-733.
- Gillam DG, Tang JY, Mordan NJ, Newman HN (2002) The effects of a novel Bioglass® dentifrice on dentine sensitivity: A scanning electron microscopy investigation. J Oral Rehabil 29(4): 305-313.
- Toledano-Osorio M, Osorio E, Aguilera FS, Medina-Castillo AL, Toledano M, et al. (2018) Improved reactive nanoparticles to treat dentin hypersensitivity. Acta Biomater 72: 371-380.
- Cohen A (1961) Preliminary Sudy of the Effects of aStrontium Chloride Dentifrice for the Control of Hypersensitivie Teeth. Oral Surgery, Oral Med Oral Pathol 14: 1046-1052.
- Hannas AR, Kato MT, Cardoso C De AB, Magalhães AC, Pereira JC, et al. (2016) Preventive effect of toothpastes with MMP inhibitors on human dentine erosion and abrasion in vitro. J Appl Oral Sci 24(1): 61-66.
- Krishnan V, Bhatia A, Varma H (2016) Development, characterization and comparison of two strontium doped nano hydroxyapatite molecules for enamel repair/regeneration. Dent Mater 32(5): 646-659.
- Tirapelli C, Panzeri H, Soares RG, Peitl O, Zanotto ED (2010) A novel bioactive glass-ceramic for treating dentin hypersensitivity. Braz Oral Res 24(4): 381-387.
- Escalante-Otárola W, Castro-Núñez G, Jordão-Basso KCF, Lima SL, Kuga MC, et al. (2017) Treatment protocol for dentin hypersensitivity. World J Dent 8(1): 1-4.
- Hughes N, Mason S, Jeffery P, Welton H, Tobin M, et al. (2010) A comparative clinical study investigating the efficacy of a test dentifrice containing 8% strontium acetate and 1040 ppm sodium fluoride versus a marketed control dentifrice containing 8% arginine, calcium carbonate, and 1450 ppm sodium monofluorophosphate. J Clin Dent 21(2): 49-55.
- Layer T, Hughes N (2010) Evidence for the efficacy of an 8% strontium acetate dentifrice for instant and lasting relief of dentin hypersensitivity. J Clin Dent 21(2): 56-58.
- Pinto SCS, Batitucci RG, Pinheiro MC, Zandim DL, Spin-Neto R, et al. (2010) Effect of an Acid Diet Allied to Sonic Toothbrushing on Root Dentin Permeability- An in vitro study. Braz Dent J 21(5): 390-395.
- He L, Hao Y, Zhen L, Liu H, Shao M, et al. (2022) Biomineralization of dentin. J Struct Biol 207(2): 115-222.
- Banfield N, Addy M (2004) Dentine hypersensitivity: Development and evaluation of a model in situ to study tubule patency. J Clin Periodontol 31(5):325-335.
- Seong J, Macdonald E, Newcombe RG, Davies M, Jones SB, et al. (2013) In situ randomised trial to investigate the occluding properties of two desensitising toothpastes on dentine after subsequent acid challenge. Clin Oral Investig 17(1): 195-203.






























