The Anti-Inflammatory Effects of Phyto Cannabinoids on IL-1β-induced PGE2 Production in Gingival Fibroblasts
Vinay Jain1, Undral Munkhsaikhan1,2, Amal Sahyoun2, Mustafa Kh Dabbous1, Vrushali Abhyankar1, Karima Ait-Aissa2, Modar Kassan2, Saad Sarraj1,2* and Ammaar H Abidi1
1University of Tennessee Health Science Center (UTHSC), College of Dentistry, Memphis, Tennessee, Unites States
2Lincoln Memorial University (LMU), College of Dental Medicine, Knoxville, Tennessee, Unites States
Submission: September 12, 2023; Published: September 25, 2023
*Corresponding author: Modar Kassan, Lincoln Memorial University (LMU), College of Dental Medicine, Knoxville, Tennessee, Unites States
How to cite this article: Vinay J, Undral M, Amal S, Mustafa Kh D, Vrushali A. The Anti-Inflammatory Effects of Phyto Cannabinoids on IL-1β-induced PGE2 Production in Gingival Fibroblasts. Adv Dent & Oral Health. 2023; 16(4): 555944. DOI: 10.19080/ADOH.2023.16.555944
Abstract
Objectives: Periodontal disease (PD), which is commonly associated with inflammatory pain, can eventually lead to tooth and bone loss. Nonsteroid anti-inflammatory drugs (NSAIDs) are commonly prescribed to manage acute inflammatory pain by inhibiting prostaglandin production. The endocannabinoid system (ECS), such as anandamide (AEA), 2-arachidonoyl glycerol (2-AG) and phytocannabinoides (pCBs) also plays a role in the modulation of prostaglandins and inflammation. This study aims to build on a previous publication by identifying the dose-dependent response of non-psychotropic phytocannabinoids (pCBs) on prostaglandin E2 (PGE2) levels in primary human gingival fibroblasts (HGFs) stimulated with interleukin-1β (IL-1β). The study hypothesizes that the pharmacological properties of phytocannabinoids (pCBs) are dose-dependent and may produce different outcomes on prostaglandin activity.
Methods: HGFs were cultured and the conditioned media was treated with IL-1β (1ng/ml) and varying doses of cannabidivarin (CBVN or CBDV, dose), cannabigerol (CBG), and cannabidiol (CBD) or a nonselective cox inhibitor, Indomethacin (10μM) on HGFs. At the end of the treatment PEG2 was determined using The CisBio HTRF PGE2 assay kit
Results: CBVN, CBG, and CBD all showed significant suppression of IL-1β stimulated PGE2 in HGFs at a lower concentration. CBG significantly suppressed PGE2 levels at 0.3-1.0 μg/ml. CBD exhibited suppression of PGE2 at 0.1-0.75 μg/ml, while significantly increasing PGE2 levels at 1.0-5.0 μg/ml. CBVN exhibited the most profound suppression of PGE2 at 0.1-1.0 μg/ml, but significantly elevated PGE2 at 2.0-5.0 μg/ml. The treatment with indomethacin (10μM) resulted in suppression of PGE2 significantly back to control levels.
Conclusion: The crosstalk between prostaglandins and the ECS in the modulation of inflammation provides a potential therapeutic option for the management of inflammatory pain. However, it is important to understand the limitations of each pCBs, as at lower doses pCBs reduce PGE2 levels, while showing increased PGE2 at higher doses promoting inflammatory effects. The pCBs alone or in combination may benefit the development of new therapeutic strategies for pain management during periodontal therapy aiding in improving public oral health.
Keywords: Cannabinoids; Cannabis; Dentistry; Oral Pain; Periodontal Disease
Abbreviations: PD: Periodontal Disease; ECS: Endocannabinoid System; NSAIDs: Nonsteroid Anti-Inflammatory Drugs; 2-AG: Arachidonoyl Glycerol; PCB: Phytocannabinoides; PGE2: Prostaglandin E2; HGFs: Human Gingival Fibroblasts; CBVN: Cannabidivarin; CBG: Cannabigerol ; LPS: Lipopolysaccharide; PMNs: Polymorphonuclear Neutrophils; GCF: Gingival Crevicular Fluid; CBD: Cannabidiol; PGE2: Prostaglandin E2; ECS: Endocannabinoid System; ATCC: American Type Culture Collection; FBS: Fetal Bovine Serum; BFM: Basal Fibroblast Medium; COX: Cyclo-Oxygenase
Introduction
Periodontal disease (PD) is one of the most common inflammatory diseases affecting the hard and soft tissues surrounding teeth [1], which increases the risk of systemic diseases like cardiovascular disease and rheumatoid arthritis [2-5]. Oral biofilms, a diverse set of microbial communities, are etiological factors for PD. Gram-negative bacteria cell wall endotoxin lipopolysaccharide (LPS), pathogen-associated molecular pattern (PAMP) [6,7] and polymorphonuclear neutrophils (PMN’s) increase in gingival crevicular fluid (GCF) with active inflammation-causing local tissue damage. These stimuli shift active biochemical and metabolic processes altering the profile of eicosanoids (prostaglandins), endocannabinoids, cytokines, and chemokines. Cannabis Sativa has been well documented for its use in medicine for pain relief and anti-spasmodic activity [8- 11]. The non-psychotropic/non-intoxicating phytocannabinoid cannabidiol (CBD) is derived from the cannabis sativa or hemp and has gained recent attraction due to FDA approval of Epidiolex (CBD oral solution) to treat seizures associated with rare epileptic conditions [12]. The considerable growth of CBD/ hemp oil products and their off-label use has been considered for a variety of conditions including pain control [13], migraines, and other inflammatory conditions [14]. The endocannabinoids and eicosanoids [15,16] regulate several important processes of inflammation by modulation of cyclooxygenase enzyme-2 (COX- 2) which plays an important role in inflammation and pain. The upregulation of prostaglandin E 2 (PGE2) in PD via COX-2 in fibroblasts [17] is well known to cause pain by vasodilation and vascular permeability affecting somatosensory nerves [18]. Furthermore, COX enzymes and cytokine IL-1β have been welldocumented in PD development and/or progression.
In our previous study, we observed that regulation of the endocannabinoid system (ECS) exhibited suppression of cytokine, chemokines, inflammatory lipid mediators, and angiogenic and vascular markers that were increased by the proinflammatory stimulus IL-1β [19,20]. The canonical receptors of the ECS are cannabinoid type 1 (CB1R) and cannabinoid type 2 (CB2R) G-protein coupled receptors, along with other putative receptors. In addition, several off-target effects have also been reported which include the modulation of COX enzymes [16]. There is evidence that the endocannabinoids anandamide (AEA) and 2-archidonylglycerol (2-AG) are substrates for the COX-2 enzyme and play an important role during inflammation [21,22]. The analgesic effects of cannabinoids mainly are attributed to the psychotropic compound delta 9-tetrahydrocannabinol (Delta 9-THC) and cannabidiol CBD (non-psychotropic) [23]. The modulation of the endocannabinoid system (i.e., CBD) holds considerable promise for oral and topical analgesic drug development [12]. Physiologically relevant doses (1.0 μg/ml) of CBD, cannabinol (CBN), and THC at exhibit suppression of P. gingivalisinduced cytokines while enhancing IL-10 (an anti-inflammatory cytokine) while doses 5.0 μg/ml or greater compromised cell viability [24]. Therefore, different concentrations due to routes of administration can have differential responses. To verify this, we aimed to assess the reduction of prostaglandin E 2 (PGE2) in vitro by various cannabinoids that would translate to a reduction in inflammatory pain in clinical settings. This study examines the concentration or dose-dependent effects of cannabinoids, CBD, cannabidivarin (CBDV), and cannabigerol (CBG) in human gingival fibroblasts (HGF) and assesses the inhibition of PGE2.
Material and Methods
Cell Culture of Human Gingival Fibroblasts
Human gingival fibroblasts from the American Type Culture Collection (ATCC) (HGF-1 ATCC CRL2014) [19] cultured according to the manufacturer’s recommendation in the Basal Fibroblast Medium (BFM, ATCC PCS-201-030) supplemented with 2 % fetal bovine serum (FBS) (Atlanta Biologicals, Flowery Branch, GA). The experiments were performed at 70-80% confluent cells within passages [3-5].
Preparation of Conditioned Media
HGFs were plated in 96-well polystyrene flat-bottom plates (Corning, NY) at cell densities of 10,000-20,000 cells/well. Cells were maintained in the full growth-BFM medium for 24 hours followed by incubation with serum-free BFM containing 1% Penicillin-Streptomycin (P/S) for another 24 hours at 37˚C, 5% CO2 to synchronize cell activity. CBD, CBG, and CBVN (Figure 1) were purchased from Cayman Chemicals (Anne Arbor, MI). We used concentrations of CBD, CBG, and CBVN ranging from 0-5μg/ ml with or without IL-1β (Invitrogen, CA) at a final concentration of 1 ng/ml. Indomethacin (10μM) was used as a positive control with or without IL-1β (1ng/ml).
Detection of Prostaglandin E 2 (PGE2)
The HGFs were seeded in Corning Biostar 96-well polystyrene flat-bottom plates at a density of 20,000 cells/well in a full-growth BFM medium. This medium was removed after 24 hours and replaced with 100 μl of serum-free BFM containing P/S for another 24 hours to synchronize the cells. On the third day, wells received 100 μl of serum-free BFM with 1% P/S containing CBD, CBG, or CBVN. As a positive control, final concentration of indomethacin (Caymen Chemicals, MI) was 10μM with or without IL-1β (1 ng/ ml) The conditioned medium was removed after 24 hours and added to CisBio HTRF PGE2 assay that was used per manufacturer recommendation to measure prostaglandin E2 levels (Cisbio PGE2 kit).
Data Analysis
All data represent an average of 6 replicates per determinant. Data were analyzed using GraphPad Prism 6.0 using the One-way ANOVA test with Bonferroni’s correction. Statistical significance values were set at P ≤ 0.05.
Results
Evaluation of Effects of CBD on Prostaglandin Activity
The endocannabinoids like arachidonic acid are substrates for the cyclo-oxygenase (COX) enzymes [25]. To assess the effects of pCB CBD on PGE2 production, we treated HGF with 1 ng/ml of IL-1β leading to higher levels of PGE2 (p<0.0001) after 24 hours of treatment (Figure 2). The pCB treatment was preceded by stimulation with IL-1β for an hour before the addition of the CBD. CBD exhibited suppression of PGE2 at 0.1-0.75 μg/ml (p<0.01), while significantly increasing PGE2 levels at 1.0-5.0 μg/ ml (p<0.0001). To validate our results, we used a positive control by stimulating the cells with 1 ng/ml IL-1β and indomethacin (10 μM). IL-1β significantly increased PGE2 levels, while indomethacin, which is a nonselective-inhibitor of COX enzyme, decreased PGE2 levels significantly under IL-1β-stimulation in HGFs (Figure 2-4).


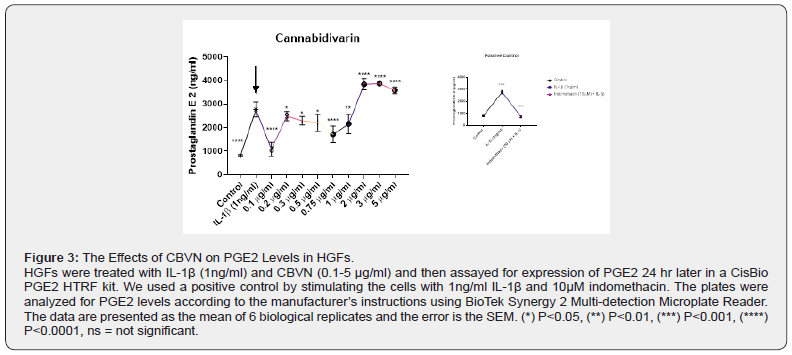
Evaluation of Effects of CBVN on Prostaglandin Activity
To evaluate the effects of pCB CBVN on PGE2 production, we treated HGF with 1 ng/ml IL-1β which led to a significant increase in PGE2 levels following 24 hours of treatment (Figure 3). The pCB treatment was preceded by stimulation with IL-1β for an hour before the addition of the CBVN. While CBVN exhibited significant decrease in PGE2 levels at 0.1-1.0 μg/ml (P<0.0001, p<0.05, p<0.05, p<0.05, p<0.0001 respectively) it induced higher levels of PGE2 at 2.0-5.0 μg/ml (p<0.0001)
Evaluation of Effects of CBG on Prostaglandin Activity
To observe the effects of pCB CBG on PGE2 production, we treated HGF with 1 ng/ml of IL-1β which induced an increase in PGE2 levels (p<0.0001) after 24 hours of treatment (Figure 4). The cells were pre-stimulated with IL-1β for an hour before the addition of the CBG. The data showed that CBG significantly suppressed PGE2 levels at 0.3-1.0 μg/ml (p<0.0001).

Discussion
During periodontal inflammation, upregulation of proinflammatory cytokines, prostaglandins, and other inflammatory markers are produced by fibroblasts [26,27] and can be clinically measured in the gingival crevicular fluids. Pathogens breach the mucosal epithelial barrier, gaining access to the underlying connective tissue in which gingival fibroblasts are the most abundant cell type. These fibroblasts become the gatekeepers of inflammatory pathway signaling and help produce extracellular matrix proteins, cytokines, and enzyme-like MMPs, which play an important role in the homeostasis of the gingiva. Fibroblasts are known to upregulate PGE2 [17], and the effects of PGE2 cause vasodilation and vascular permeability affecting the somatosensory nerve in inflamed areas consequently perceived as pain 18. The modulation of fibroblasts can be regulated by macrophages, which are involved in promoting and resolving inflammation, wound healing, and host defense [28]. Therefore, human gingival fibroblasts (principal cells of the periodontium) serve as a good model to study inflammatory response. A wide range of effects are attributed to the ECS playing a role in cognition, memory, neurotransmission, modulation of cytokines, immune cell migration, and wound healing [29]. The endogenous cannabinoid, anandamide (AEA), enhances fibroblastic proliferation and tissue healing [30]. Owing to these benefits exhibited by cannabinoids, CBD, CBVN, and CBG concentrationdependent responses were examined as part of this study. Oral fluids can vary in concentrations of pCBs after smoking and can reach levels near 1μg/ml (1,338 and 1,041 μg/L) [31]. Smoking or oral administration of cannabis (CBD and THC levels) can increase 10-20-fold upon continuous smoking, and ranges can vary within the population [32]. There is a grave concern in dentistry about the misuse of cannabinoids that could potentially cause bacterial dysbiosis, immunomodulation, and compromise cell vitality [24].
However, if used appropriately they serve to hold a great therapeutic potential. The results of the present study demonstrated the ability of pCBs to inhibit PGE2 which is associated with inflammatory pain. We previously explored CBD, CBG, and CBVN, and only CBG and CBVN showed significant suppression of IL-1β stimulated PGE2. CBD was responsible for elevating PGE2 levels at the concentration of 1μg/ml 19. In the current study, all three of the pCBs, CBVN, CBG, and CBD all showed significant suppression of IL-1β stimulated PGE2 in HGFs at lower concentrations under IL-1β stimulation, while proinflammatory activities were seen at higher concentrations. CBVN exhibited the most significant decrease of PGE2 levels at lower concentrations, but significantly elevated PGE2 levels at 2.0-5.0 μg/ml. Meanwhile, CBG also showed a similar trend and significantly suppressed PGE2 levels at 0.3-1.0 μg/ml. CBD exhibited suppression of PGE2 at 0.1-0.75 μg/ml and exhibited a significant increase in PGE2 levels at higher concentrations in HGFs. A study shows that pCBs have the ability to modulate the COX enzyme activity, in which cannabidiolic acid (CBDA), a chemical precursor of CBD [16] stimulates prostaglandin production, while in the same study, CBD and CBG exhibit reduction of prostaglandins at a high concentration (2.0·10⁻5 M). Within the parameters of this study, a higher concentration of pCBs exhibits an increase in PGE2 within the HGF. It is plausible if we further increased the concentration of pCBs, there may be a reduction in inflammation and a similar pattern may be exhibited as the aforementioned study. Furthermore, Qi X et al., [33] have demonstrated that the CBD oral spray on acid- or trauma-induced oral ulcers on mice tongue inhibits inflammation, relieves pain, and accelerates wound closure.
The authors also reported an intriguing pathway whereby CBD decreases the expression of cytidine/uridine monophosphate (CMP), which in turn inhibits the generation of oxidized mitochondrial DNA and suppresses inflammasome activation. This suppression is mediated mostly by PPARγ in the nucleus and partially by the CB1 receptor [33]. This study also reported that the therapeutic effects were not restricted to a lower (1mg/ ml) or higher dose (10mg/ml) of the CBD oral spray application. The higher dose of CBD topical administration however did show better benefits on oral ulcer healing without toxicity. The animal model used for oral ulcers complements our design as CBD topical application significantly alleviated the release of inflammatory cytokines such as IL-1β [33], which is the proinflammatory stimulus used in our study. In addition, it is noteworthy that pCBs not only have the capacity to reduce prostaglandins to alleviate pain but can affect the signal transduction of chemical and physical stimuli (i.e., tooth pain) [34] through transient receptor potential (TRP) channels including TRP vanilloid, TRP ankyrin, and TRP mela statin subfamilies [35], thereby alleviating pain stimuli in more than one way. The overall anti-inflammatory effects of pCBs on IL-1β-stimulated HGFs suggest that specific concentrations of pCBs may be useful in treating inflammatory pain and could be used therapeutically as an analgesic for the oral cavity. More studies are needed to expand our current knowledge of pCBs and their effects on oral tissues to evaluate their potential in treating or preventing oral pathologies and management of pain.
Conclusion
The crosstalk between the eicosanoid and endocannabinoid systems has the potential to be a therapeutic option for the management of inflammation and pain. However, it is important to understand the limitations of each pCBs, at lower doses pCBs show a reduction of PGE2 levels, while increasing PGE2 at higher doses. These effects may be tissue or cell-specific; therefore, more study needs to be performed to understand the full potential of pCBs for oral treatment or management. The pCBs alone or in combination may benefit the development of new therapeutic strategies for pain management and periodontal therapy aiding in improving public oral health.
References
- Loesche WJ, Grossman NS (2001) Periodontal disease as a specific, albeit chronic, infection: diagnosis and treatment. Clin Microbiol Rev 14(4): 727-752.
- DeStefano F, Anda RF, Kahn HS, Williamson DF, Russell CM (1993) Dental disease and risk of coronary heart disease and mortality. BMJ 306(6879): 688-691.
- Khader YS, Taani Q (2005) Periodontal diseases and the risk of preterm birth and low birth weight: a meta-analysis. J Periodontol 76(2): 161-165.
- Koziel J, Mydel P, Potempa J (2014) The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep 16(3): 408.
- Mealey BL, Ocampo GL (2007) Diabetes mellitus and periodontal disease. Periodontology 2000 44: 127-153.
- Genco RJ (1992) Host responses in periodontal diseases: current concepts. J Periodontol 63(4 Suppl): 338-355.
- Holt SC, Kesavalu L, Walker S, Genco CA (1999) Virulence factors of Porphyromonas gingivalis. Periodontology 2000 20: 168-238.
- Mikuriya TH (1969) Marijuana in medicine: past, present and future. Calif Med 110(1): 34-40.
- Mechoulam R, Carlini EA (1978) Toward drugs derived from cannabis. Naturwissenschaften 65(4): 174-179.
- Dabbagh A, Elyasi H, Rajaei S (2010) Anesthesia in ancient Iran. Anesth Analg 111(2): 584.
- Dabbagh A, Rajaei S, Golzari SE (2014) History of anesthesia and pain in old Iranian texts. Anesth Pain Med 4(3): e15363.
- Franco V, Perucca E (2019) Pharmacological and Therapeutic Properties of Cannabidiol for Epilepsy. Drugs 79(13): 1435-1454.
- Good P, Haywood A, Gogna G (2019) Oral medicinal cannabinoids to relieve symptom burden in the palliative care of patients with advanced cancer: a double-blind, placebo controlled, randomised clinical trial of efficacy and safety of cannabidiol (CBD). BMC Palliat Care 18(1): 110.
- Burstein S (2015) Cannabidiol (CBD) and its analogs: a review of their effects on inflammation. Bioorg Med Chem 23(7): 1377-1385.
- Tsuboi K, Uyama T, Okamoto Y, Ueda N (2018) Endocannabinoids and related N-acylethanolamines: biological activities and metabolism. Inflamm Regen 38(1): 28.
- Ruhaak LR, Felth J, Karlsson PC, Rafter JJ, Verpoorte R (2011) Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol Pharm Bull 34(5): 774-778.
- Kang W, Hu Z, Ge S (2016) Healthy and Inflamed Gingival Fibroblasts Differ in Their Inflammatory Response to Porphyromonas gingivalis Lipopolysaccharide. Inflammation 39(5): 1842-1852.
- Jang Y, Kim M, Hwang SW (2020) Molecular mechanisms underlying the actions of arachidonic acid-derived prostaglandins on peripheral nociception. J Neuroinflammation 17(1): 30-30.
- Abidi AH, Abhyankar V, Alghamdi SS, Tipton DA, Dabbous M (2022) Phyto cannabinoids regulate inflammation in IL-1β-stimulated human gingival fibroblasts. J Periodontal Res 57(6): 1127-1138.
- Abidi AH, Alghamdi SS, Dabbous MK, Tipton DA, Mustafa SM (2020) Cannabinoid type-2 receptor agonist, inverse agonist, and anandamide regulation of inflammatory responses in IL-1β stimulated primary human periodontal ligament fibroblasts. J Periodontal Res 55(5): 762-783.
- Kim J, Watkins BA (2014) Cannabinoid receptor antagonists and fatty acids alter endocannabinoid system gene expression and COX activity. J Nutr Biochem 25(8): 815-823.
- Fowler CJ (2007) The contribution of cyclooxygenase-2 to endocannabinoid metabolism and action. Br J Pharmacol 152(5): 594-601.
- Henson JD, Vitetta L, Hall S (2022) Tetrahydrocannabinol and cannabidiol medicines for chronic pain and mental health conditions. Inflammopharmacology 30(4): 1167-1178.
- Gu Z, Singh S, Niyogi RG (2019) Marijuana-Derived Cannabinoids Trigger a CB2/PI3K Axis of Suppression of the Innate Response to Oral Pathogens. Front Immunol 10(2288).
- Rouzer CA, Marnett LJ (2011) Endocannabinoid Oxygenation by Cyclooxygenases, Lipoxygenases, and Cytochromes P450: Cross-Talk between the Eicosanoid and Endocannabinoid Signaling Pathways. Chem Rev 111(10): 5899-5921.
- Elias JA, Reynolds MM, Kotloff RM, Kern JA (1989) Fibroblast interleukin 1 beta: synergistic stimulation by recombinant interleukin 1 and tumor necrosis factor and posttranscriptional regulation. Proc Natl Acad Sci 86(16): 6171-6175.
- Ozaki K, Hanazawa S, Takeshita A (1996) Interleukin-1 beta and tumor necrosis factor-alpha stimulate synergistically the expression of monocyte chemoattractant protein-1 in fibroblastic cells derived from human periodontal ligament. Oral Microbiol immunology 11(2): 109-114.
- Koh TJ, Dipietro LA (2011) Inflammation and wound healing: The role of the macrophage. Expert Rev Mol Med 13: e23-e23.
- Joshi N, Onaivi ES (2019) Endocannabinoid System Components: Overview and Tissue Distribution. Adv Exp Med Biol 1162: 1-12.
- Kozono S, Matsuyama T, Biwasa KK (2010) Involvement of the endocannabinoid system in periodontal healing. Biochem Biophysical research communications 394(4): 928-933.
- Fabritius M, Chtioui H, Battistella G (2013) Comparison of cannabinoid concentrations in oral fluid and whole blood between occasional and regular cannabis smokers prior to and after smoking a cannabis joint. Anal Bioanal Chem 405(30): 9791-9803.
- Cone EJ, Bigelow GE, Herrmann ES (2015) Nonsmoker Exposure to Secondhand Cannabis Smoke. III. Oral Fluid and Blood Drug Concentrations and Corresponding Subjective Effects. J Anal Toxicol 39(7): 497-509.
- Qi X, Lin W, Wu Y (2021) CBD Promotes Oral Ulcer Healing via Inhibiting CMPK2-Mediated Inflammasome. J Dent Res 101(2): 206-215.
- Hossain MZ, Bakri MM, Yahya F, Ando H, Unno S (2019) The Role of Transient Receptor Potential (TRP) Channels in the Transduction of Dental Pain. Int J Mol Sci 20(3): 526.
- Muller C, Morales P, Reggio PH (2019) Cannabinoid Ligands Targeting TRP Channels. Front Mol Neurosci 11: 487-487.






























