Regeneration of Permanent Tooth Enamel (Alternative Solution of Nano-hydroxyapatite) after Exposure to Beer (Laboratory Study at the Dental Center, Moscow, Russia)
Hamed Nabahat1*, Melika Tahan2, Faezeh AzaditalabDavoudabadi3, Leonid sergeevich Kolomeitsev4, Kapralova Valentina Vasilevna4
1Yevdokimov Moscow State University of Medicine and Dentistry, Russian Federation, Moscow, Russia
2Sechenov First Moscow State Medical University, Russian Federation, Moscow, Russia
3Evdokimov Moscow State University of Medicine and Dentistry( MSMSU ), Russian Federation, Moscow, Russia
4Federal State Budgetary Educational Institution of Higher Education Moscow State University of Food Production, Russia
Submission: June 26, 2021; Published: July 07, 2021
*Corresponding author: Hamed Nabahat, Yevdokimov Moscow State University of Medicine and Dentistry, Moscow, Russian Federation, Russia
How to cite this article: Hamed Nabahat, Melika Tahan, Faezeh AzaditalabDavoudabadi, Leonid sergeevich Kolomeitsev, Kapralova Valentina Vasilevna, et al. Regeneration of Permanent Tooth Enamel (Alternative Solution of Nano-hydroxyapatite) after Exposure to Beer (Laboratory Study at the Dental Center, Moscow, Russia). Adv Dent & Oral Health. 2021; 14(4): 555891. DOI: 10.19080/ADOH.2021.14.555891
Abstract
Hydroxyapatite is an important mineral component of tooth enamel, bioactive and tissue-compatible materials. Nano-hydroxyapatite is more similar to enamel apatite crystals, in this respect Nano-hydroxyapatite seems to have the potential to remineralize decay lesions. In terms of restorative and preventive dentistry, nano-hydroxyapatite has significant remineralizing effects on initial enamel lesions, certainly superior to conventional fluoride, and good results on the sensitivity of the teeth. The nano-hydroxyapatite has also been used as an additive material, in order to improve already existing and widely used dental materials, in the restorative field (experimental addition to conventional glass ionomer cements, which has led to significant improvements in their mechanical properties). This study was performed due to the role of nano-hydroxyapatite in remineralization of enamel lesions and due to the fact that this role has not been studied in the in-situ study. The aim of this study was to investigate the effect of nano-hydroxyapatite on tooth remineralization following its demineralization with beer in Russia.
Keywords: Nano-hydroxyapatite tooth; Dentistry; Dental; Beer; Remineralization
Introduction
Tooth decay remineralization is caused by acids from non-bacterial sources. These can be extrinsic in source, such as carbonated drinks or intrinsic acids, usually from stomach acid coming into the mouth. Dental caries is a multifactorial disease induced by the oral environment's interplay of food sugars, dental biofilm, and the host's dental tissue. It is the product of multiple rounds of remineralization at the biofilm-to-tooth surface interaction. After ingesting sugar, oral bacteria excrete acid, which causes remineralization. The hydroxyapatite crystals in the subsoil disintegrate when exposed to acid. For non-cavitated lesions, the normal repair mechanism is remineralization. It uses calcium and phosphate ions, along with fluoride, to create a new surface on the sub surface’s existing crystal remains [1]. The original crystals are less acid soluble than the remineralized crystals. Saliva is supersaturated with calcium and phosphate ions under normal physiological conditions (pH=7), slowing caries progression. Plaque pH decreases to 4.5-5.5 as bacteria in the biofilm continue to create acid in response to sugar ingestion. This causes mineral disintegration to become the driving force within the tooth. The saturation point of the minerals in the surrounding fluid changes as the pH is reduced [2]. The higher the calcium and phosphate concentrations required to reach saturation about hydroxyapatite, the lower the pH. The point where equilibrium exists is known as the "critical pH." Mineral dissolution and mineral precipitation are not present. Fluorapatite has a critical pH of 4.5, while hydroxyapatite has a critical pH=5.5.
This change depending on the patient. Demineralization occurs below critical pH, while remineralization occurs above critical pH. Tooth decay is mostly caused by erosion, especially in adolescents who eat high volumes of non-alcoholic beverages or sugary drinks with a low ph. The deterioration of enamel in children and teenagers can be reduced by limiting the use of these drinks [3]. Following the decline of caries in children and adolescents, other dental disorders such as erosions have increased dramatically in recent decades, particularly in developed countries. There have been numerous studies on tooth wear, particularly erosion, since 1980, and there is agreement on the prevalence of dental erosion, and the prevalence of erosion is mentioned between 15 and 70% in various sources. However, most articles state that the prevalence of erosion is on the rise. Acid exposure is usually the primary cause of erosion. Researchers have connected non-alcoholic beverage consumption to tooth erosion, particularly in children and teenagers [4]. A considerable increase in non-alcoholic beverages, diet drinks, and juices is the primary cause of dental erosion.
It's crucial to consider the negative effects of erosion and the impact of various treatment medicines on enhancing enamel hardness after consuming erogenous beverages. The influence of various treatment elements on remineralization has been studied in various ways. Various investigations of Nano-hydroxyapatite on remineralization of erosive lesions and dental caries have been conducted on erosive waste. The effect of different concentrations of Nano-hydroxyapatite (1%, 5%, 10%, 15%) and sodium fluoride on the remineralization of primary caries lesions was investigated in a study, for remineralization of these pollutants, a concentration of 10% Nano-hydroxyapatite was determined to be optimal [5]. The effect of combining nano-hydroxylapatite and sodium fluoride mouthwash in the remineralization of primary caries lesions was investigated, and it was discovered that the combination can remineralize primary caries lesions. The effects of toothpaste including:1: nanohydroxy and fluoride,2: and toothpaste containing simply nano-hydroxylapatite were tested in another study, and it was discovered that while both can demineralize primary lesions, there is no difference between them [6,7]. The purpose of this study was to evaluate the impact of nano-hydroxylapatite in the remineralization of teeth after remineralization with (Baltica Zero) non-alcoholic beers. The results showed that the purpose of this study was to evaluate the impact of nano-hydroxylapatite in the remineralization of teeth.
Material and Methods
This study was performed experimentally in vitro on 18 permanent third incisors that were surgically removed and did not have any caries on clinical examination (according to WHO criteria) (wear, cracks and hypo calcification). Teeth during collection (1 month) in containers new glasses that were purchased for this purpose without any abrasive and interfering substances were kept. Containers of water in the pipes in Moscow were filled and placed at room temperature. In the Duration to prevent surface changes and contamination 2 times a week the water inside the bottles changed. The surface of the teeth by mechanical method, with use prophylactic paste containing pumice and free of fluoride, parasites and W&H air motor with the low speed with a certain rotation range between 800 to 2000 rpm and a special brush for mass and debris was cleared. The teeth are then magnified with a stereomicroscope at 40 equivalents to the presence of any enamel disorders, caries lesions microscopy, and cracks were examined, and 18 of them. A square-shaped label with dimensions of 5 × 5 was selected for the study Mm was glued to the distal surface of the teeth and all surfaces.
The rest of the teeth were covered with clear polyester. For correct measurement of microhardness, the surface of the samples was polished in the presence of water with 5000 sandpaper up to smooth surface created with Vickers microhardness tester be checked. After polishing, the surface of the samples was dried and used Vickers microhardness tester (Shimadzu, M-g5037, Japan). The initial microhardness of the teeth was measured. Then a force of 50 grams in three L-shaped points was applied on each sample and the amount of microhardness of each effect was calculated and recorded.
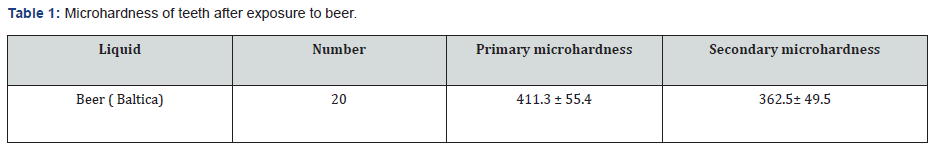
After measuring the initial microhardness of the studied teeth in (Baltica Zero) non-alcoholic beers in the Russian federation were immersed for 5 minutes. Baltica most popular nonalcoholic beer brand in Russia which holds a 60% market share. According to the daily consumption of non-alcoholic beverages per day and the duration of storage of beverages in the mouth (for 20 seconds) (before cleansing with saliva), 5 minutes is the duration of daily consumption of non-alcoholic carbonated beverages. To simulate the conditions consumers of solutions should be refrigerated before testing and its temperature was 10 degrees Celsius at the time of use. During this time the solutions were stirred gently. After immersion, the teeth are removed and again with the Vickers device secondary microhardness of teeth was measured (Table 1 & 2).
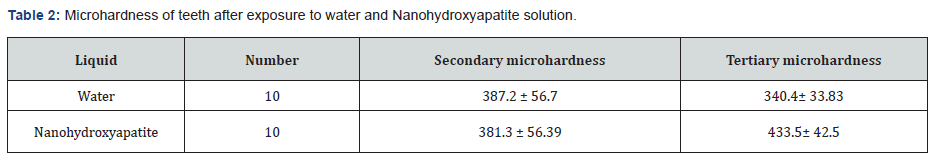
After secondary hardness measurement, teeth into 2 groups of 9 piped water in Moscow (control) and Nano-hydroxyapatite solution (experimentally) were divided. Nine pairs of teeth were placed in 9 plaques and each plaque was placed in the mouth of 1 volunteer. Samples (volunteers) were selected from students who were aware of the project and had given written consent. Volunteers were trained to wash teeth for 5 minutes on one side in the water and 5 minutes on the opposite side nano- hydroxyapatite solution 6 times a day for 10 days. The results were statistically analyzed, changes in the microhardness of teeth inside and between each group by Aired T-test were judged. Due to the inclination created by the sinking tip of the device in place the effect of secondary microhardness measurement at the same point of effect is not possible, but based on a previous study, it was concluded that the measured secondary and tertiary stiffness is not related to the first point but due to the lack of statistically significant differences between the microhardness of the effect points of each group. The hardness of each point can be considered a symbol of surface hardness.
Results
In this study, the effect of Nano-hydroxyapatite as a treatment solution and urban water of Moscow region (Russia) as a control solution on microhardness 20 Permanent impacted third molar teeth due to delusional exposure demineralized were examined, and mean microhardness enamel before and after immersion in beer and then after immersion in Nano-hydroxyapatite and municipal water was measured. The mean initial hardness of 20 existing samples was 411.3 ± 55.4. Hardship Primary teeth of the two groups before exposure to beer using test independent t-test were compared, the result of this test showed a significant difference there was no difference between the hardness of the teeth in the two groups. (P = 0/85). It was found that this decrease was significant (P = 0.03) following exposure to Nano-hydroxyapatite, average microhardness. After immersion in Baltica non-alcoholic beer, this level reached an average of 362.5± 49.5 and this level of hardness was determined by independent t-test, which was a significant reduction). The results showed a significant difference in hardness changes between the two groups. There was (P = 0/001) in fact, the hardness decreased in the control group and increased in the nano group. The average hardness increased by 38.15 in the nano group and the group control was 45.21. To evaluate the significant increase in stiffness in each group of t-test pairs were used in each group. In the nano-hardness group significantly increased, the results of paired t-test showed this (P = 0.001) had a significant decrease in hardness in the control group.
Discussion
Erosion is the destruction of the tooth structure without interference and activity of microorganisms. The most common cause of erosion is exposure to acid. Causes in adolescents include acidic beverages such as juices containing citric acid, non-alcoholic beverages, or drinks containing carbonate [8,9]. Beer is a beverage containing citric acid. In this study, its effect on the enamel microhardness of permanent teeth was investigated. Based on the results of this study, the microhardness of tooth enamel after exposure decreased significantly with malt. These results are consistent with the results of studies by (Haghgoo and Abbasi) [4,6]. Separate studies on the effect of malt and cola on tooth microhardness checked for milk and permanence. The results of studies showed that the effect of malt on reducing the microhardness of deciduous and permanent enamel significantly Fanta cola is less. Of course, in these studies, only the effect of these two drinks on the microhardness of deciduous and permanent teeth has been investigated, while the present study also examined the effect of a therapeutic agent (Nano-hydroxyapatite) has been reviewed.
Also that Elnaz Shafiq, 2021 study results in the role of pH of Iranian and foreign beverages on the amount of tooth erosion by method examined for calcium ion analysis, showed that calcium from the enamel surface removed after exposure to the tested beverages, it is taken with the present study is indirectly coordinated. In this study, the teeth were kept in tap water and no substance no disinfectants were used [1,10]. Our reason for not using chemical materials was that chemical agents may affect the microhardness of the teeth. In this study, completely healthy teeth were used implanted teeth that were surgically removed rather than differences due to the oral habits and diets of people who have had their teeth extracted do not interfere with the study results and conditions for all samples be the same. In the study by Lippert et al. the pulp of the crown and the root of the tooth were first extracted and then disinfected in 15% hypochlorite solution for 24 hours and 3 diamond enamel blocks were prepared from each tooth using a diamond blade. Removal of dental pulp by conventional methods causes trauma to the tooth and remove the chamber pulp chamber against the strength of the tooth reduces shear forces. In the present study, the teeth were not cut because of the heat, and the pressure caused by the cut reduces the strength of the enamel and the sensitivity of enamel increases against the formation of erosion. Also, sandpaper 8000 was used to polish teeth. Application of sandpaper with a high degree of roughness due to higher yield of hyper mineralized enamel is undesirable.
Therefore, in this study, the softest sandpaper was used. In the present study, the exposure time to beverages was 5 minutes. In some studies, the study time is from 1-20 hours, and in some studies, it was 15 minutes and in some, it was 5 minutes according to the average daily consumption of non-alcoholic beverages and time of keeping the beverage in the mouth 20 seconds (5 minutes) seems reasonable. In different studies for quantitative evaluation of erosion by different methods used. From the practice of Lippert 2004, calcium ion analysis was used by the spectrometric method. The study assumed that the amount of calcium in the enamel was 4.37% by weight. However, the amount of calcium varies from person to person [8]. In Marchan et al. study the solubility of hydroxyl crystals apatite was measured by spectrophotometry. Hydroxyapatite powder alone is not a good representative of tooth enamel and the reaction of hydroxyapatite powder can be easily generalized to the teeth. Also, in this study, a part of each tooth that 2 half-divided was placed in experimental solutions and treated with SEM the surface roughness of the tested enamel was compared with the other half. The surface roughness of the two halves of a tooth is probably not the same. Kislyakov, 2020 in their study by profiling investigate the effect of regular drinks on the microhardness of enamel and the result found that the enamel microhardness decreased after consuming beverages [4,11,12].
This study was performed by profiling. In this method, the surface of the samples is scanned by a diamond probe and the roughness and heights of this level were measured. With this method changes, slight surface roughness due to loss of tooth tissue before and after immersion is compared. In the present study, Vickers microhardness measurement method for evaluation dental erosion was used. Given that after contact with dental tissue with the acidic solution initially reduced microhardness and loss of tissue the surface, which is part of the erogenous process, appears to subsequently occur it turns out that this method is a more accurate method in assessing dental erosion. Hydroxyapatite is an important mineral component of tooth enamel. It is bioactive and compatible with tissue. Nano hydroxyapatite similarity more to the enamel apatite crystals of the tooth in this regard Nano-hydroxyapatite appears to have remineralization potential have caries lesions. Nano-hydroxyapatite has hydrophilic properties and these crystals have a superficial moisturizing property when applied on the tooth surface produce a thin but strong layer on the enamel surface that with the dental crown is banded.
Nano-hydroxyapatite causes remineralization. In this study, the effect of Nano-hydroxyapatite solution on waste erosion resulting from the consumption of beer is examined in situ. The results of the present study showed that the microhardness of tooth enamel to reason for exposure to reduced malt after exposure to Nano-hydroxyapatite solution was not measurable. It seems that after the application of Nano-hydroxyapatite due to the penetration of Nano-hydroxyapatite in the porosity caused by the dexterity of the irregular surface, it was not possible to measure the microhardness of the enamel. In the present study, we used Nano-hydroxyapatite solution in pure form without the use of any other substance. Various studies have been performed in the field of remineralization of primary caries and erosive lesions and different results have been obtained according to the method and material studied.
The results of the Demito et al. study showed that Nano-hydroxyapatite in mouthwash can remineralize primary caries lesions. However, in the study of Demi et al. The effect of a mouthwash containing Nano-hydroxyapatite and sodium fluoride on primary caries lesions was investigated and the presence of fluoride can have a synergistic effect in the remineralization of these wastes [9,13], but in the present study, Nano-hydroxyapatite was used purely and evaluation of its effects alone existed in the remineralization of erosive lesions. The results of a study by Haghgoo et al. showed that enamel microhardness which was reduced after exposure to beer after treatment with the solution Nano-hydroxyapatite increased. In this study, after induction of erosive lesions by malaria, the lesions are exposed to Nano-hydroxyapatite solution and the results of this study showed that Nano-hydroxyapatite significantly increases the microhardness of permanent enamel that was reduced after exposure to malt. However, the present study was performed in situ where plaque containing teeth worn by malt in the mouth was exposed to Nano-hydroxyapatite [6]. In this study, demineralized enamel in the presence of saliva and under normal oral conditions exposed to Nano-hydroxy apatite was placed. In this study, a concentration of 10% Nano-hydroxyapatite was selected. In a study, Haung et al. examined concentrations of 1, 5, 10, and 15% Nano-hydroxyapatite in the remineralization of decay lesions [7,8,14]. The results of this study showed that the remineralization potential of Nano -hydroxyapatite in concentrations of 10 and 15% is not different, the choice of this concentration of 10% in the present study was for this reason.
Conclusion
The results of this study, 10% soluble nano-hydroxyapatite can be erosive lesions repair.
References
- Pepla E, Besharat LK, Palaia G, Tenore G, Migliau G (2014) Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Ann Stomatol 5(3): 108114.
- Pajor K, Pajchel L, Kolmas J (2019) Hydroxyapatite and fluorapatite in conservative dentistry and oral implantology-a review. Materials 12(17): 2683.
- Singh S, Pal A, Mohanty S (2019) Nano Structure of Hydroxyapatite and its modern approach in Pharmaceutical Science. Research Journal of Pharmacy and Technology 12(3): 1463-1472.
- Zupancic S, Kocbek P, Baumgartne S, Kristl J (2015) Contribution of nanotechnology to improved treatment of periodontal disease. Curr Pharm Des 21(22): 3257-3271.
- Naserzadeh Y, Mahmoudi N, Pakina E, Marie Wase IA, Heydari M, et al. (2019) Parameters Affecting the Biosynthesis of Gold Nanoparticles Using the Aquatic Extract of Scrophularia striata and their Antibacterial Properties. Journal of Nanoanalysis 6(2): 105-114.
- Kona S, Wadajkar AS, Nguyen KT (2011) Tissue engineering applications of injectable biomaterials. In Injectable biomaterials, Woodhead Publishing pp. 142-182.
- Haghgoo R, Mehran M, Ahmadvand M, Ahmadvand MJ (2016) Remineralization effect of eggshell versus nano-hydroxyapatite on caries-like lesions in permanent teeth (in vitro). Journal of International Oral Health 8(4): 435-439.
- Huang S, Gao S, Cheng L, Yu H (2010) Combined effects of nano-hydroxyapatite and Galla chinensis on remineralisation of initial enamel lesion in vitro. J Dent 38(10): 811-819.
- Lippert F, Parker DM, Jandt KD (2004) Susceptibility of deciduous and permanent enamel to dietary acid‐induced erosion studied with atomic force microscopy nanoindentation. Eur J Oral Sci 112(1): 61-66.
- Daas IN (2017) Comparison Between Fluoride and Nano-Hydroxyapatite in Remineralizing Initial Enamel Lesion: In-Vitro Study. J Contemp Dent Pract 19(3): 306-312.
- Demito CF, Costa JVD, Fracasso MDLC, Ramos AL (2020) Efficacy of fluoride associated with nano-hydroxyapatite in reducing enamel demineralization adjacent to orthodontic brackets: in situ study. Dental press journal of orthodontics 24: 48-55.
- ElnazShafiq RF, Shafikhani M (2021) Investigating the Effect of 45S5 Bioactive Glasses and Calcium Oxalate (Gluma) Covering the Dentinal Tubules in Dental Sensitivity. Annals of the Romanian Society for Cell Biology 25(6): 3559-3570.
- Kislyakov EA, Karotkiyan RV, Sadyrin EV, Mitrin BI, Yogina DV, et al. (2020) Nanoindentation Derived Mechanical Properties of Human Enamel and Dentine Subjected to Etching with Different Concentrations of Citric Acid. In Modeling, Synthesis and Fracture of Advanced Materials for Industrial and Medical Applications. Springer, Cham p. 75-83.
- Marchan S, Hector T, Bascombe K (2021) The pH and Titratable Acidity of Still and Sparkling Flavored Waters: The Effects of Temperature and Storage Time. Open Journal of Stomatology 11(3): 148-158.






























