Safety and Efficacy of a Novel Toothbrush Utilizing RF Energy for Teeth Shade Whitening and the Reduction of Teeth Stains
Kimberly R Milleman1, Tori L Grahovac1, Abigale L Yoder1, Liora Levi2* and Jeffery L Milleman1
1Clinical Investigator, Salus Research, 1220-4, Medical Park Drive, Fort Wayne, USA
¹Director, Salus Research, 1220-4, Medical Park Drive, Fort Wayne, USA
¹Clinical Investigator, Salus Research, 1220-4, Medical Park Drive, Fort Wayne, USA
1Clinical Coordinator, Salus Research, 1220-4, Medical Park Drive, Fort Wayne, USA
Submission: February 19, 2020 Published: March 02, 2020
*Corresponding author: Liora Levi, Director of Clinical Affairs, Home Skinovations LTD, Tavor Building, Shaar Yokneam, Israel
How to cite this article: Liora Levi, Kimberly R Milleman, Jeffery L Milleman, et al. Safety and Efficacy of a Novel Toothbrush Utilizing RF Energy for Teeth Shade Whitening and the Reduction of Teeth Stains. Adv Dent & Oral Health. 2020; 12(3): 555831. DOI::10.19080/ADOH.2019.11.555831
Abstract
Purpose: To evaluate the effect of a novel RF-utilizing toothbrush on teeth stains and shade as compared to a control standard ADA-approved powered toothbrush.
Methods: This was a single-blind double arm prospective study, including 4 clinical visits that were conducted every two weeks. Subjects were randomized to one of two study groups, receiving either the ToothWave™ or control toothbrush, and performed twice daily brushing during a test period of 6 weeks. Teeth stains and shade were assessed using the Lobene stain index and Vita Bleached guide 3D Master, at baseline and after 4 and 6 weeks of brushing. Results were compared within each group and between the groups; delta values (reduction from baseline) were also compared between the groups. Statistical analyses were conducted using the Mann Whitney non-parametric model.
Results: Eighty-four (84) subjects (41 and 43 in the treatment and control groups respectively) completed the study, having fully evaluable data. At baseline, the test groups did not differ significantly in the efficacy measurements mean scores (p≥0.667). Following 6 weeks of brushing the test group showed significant reductions in all the tested measures as compared to the control. In addition, the delta values of the measured scores were significantly greater in the treatment group as compared to the control (p≤0.001). Both toothbrushes were well-tolerated, and no device related adverse events were reported during the study.
Conclusion: The ToothWave™ RF-utilizing toothbrush produced significant benefits in reduction of teeth stains and whitening of teeth shade as compared to a control, ADA-approved powered toothbrush.
Keywords: Teeth whitening; Teeth stains; Power Toothbrush
Abbreviations: OST: Oral Soft Tissue; ADA: American Dental Association; LSI: Lobene Stain Index; SGU: Shade Guide Units; ITT: Intent-to-treat; PP: Per-Protocol
Introduction
Over the past two decades, tooth bleaching or whitening has become one of the most popular aesthetic dental treatments [1]. Current tooth bleaching materials are based primarily on either hydrogen peroxide or carbamide peroxide, both found to be effective, but raise concerns about the long-term safety of bleaching procedures, referring to the tooth structure, pulp tissues, and the mucosal tissues of the mouth, as well as systemic ingestion [2-6]. Recently, products containing chlorine dioxide were introduced, but with no evidence of equivalent efficacy and with additional safety concerns due to the low pH of the material [7]. At-home bleaching methods include gels, chewing gums, rinses, toothpastes, paint-on films, and whitening strips [8]. Toothpastes and mouth washes which are advertised as “whitening” rarely contain peroxide derivatives in low concentrations [8]. These toothpastes are abrasive (usually containing alumina or silica) for the purpose of removing surface stains from the tooth surface [8], can cause thinning of the enamel layer, and contributes to a gradual darkening in the appearance of the tooth as the dentin layer becomes more noticeable [9]. Peroxide derivatives were shown to promote the loss of mineral structure from enamel, and changes in the enamel structure resulting in a rough and porous enamel surface [10,11]. This molecular process may explain the transient mild to moderate tooth sensitivity that occurs in up to two-thirds of users during the early stages of bleaching treatment [12,1]. In addition, hydrogen peroxide produces free radicals, which are known to be capable of reacting with proteins, lipids and nucleic acids, causing cellular damage and having a potential toxicological effect in the mucosal tissues [1,10]. Clinical studies have shown that hydrogen peroxide exhibits a higher prevalence of gingival irritation in patients using bleaching materials with higher peroxide concentrations [13,14,10].
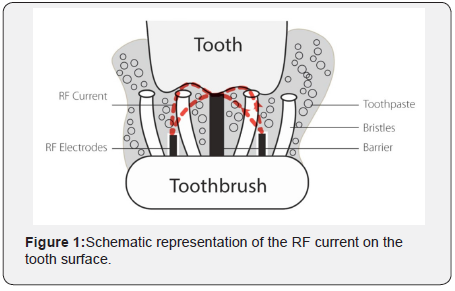
At concentrations of 10% hydrogen peroxide or higher, the chemical was found to be potentially corrosive to mucous membranes or skin and can cause a burning sensation and tissue damage [15-17]. Because of the hydrogen peroxide potential to interact with DNA, concerns with carcinogenicity and cocarcinogenicity of hydrogen peroxide have been raised, although these concerns so far have not been substantiated through research [5,6,18,19]. With an aim to provide safe and efficient reduction of extrinsic stains at home and teeth whitening, and without changing the daily dental hygiene routine, Home Skinovations LTD. (Yokneam, Israel) has developed the ToothWave™ powered toothbrush. It is a novel toothbrush intended to remove effectively the impurities that are attached to the tooth surface, to significantly improve the dental hygiene and thus to promote the reduction of gingival inflammation. ToothWave™ works in a non-abrasive way; it utilizes low-power RF energy that streams between two electrodes and over a silicon barrier and reaches the tooth surface during brushing see Figure 1. RF is an alternating electric current that oscillates at radio frequencies in the range of 3kHz-300GHz. It has been used in medicine for several decades for many different applications, from surgical to aesthetic, providing various effects, depending on the specific parameters of the device in use [20]. Specifically, the ToothWave™ RF technology is proposed to bring charged molecules that originate from the toothpaste, to the tooth surface, in order to destabilize the electrostatic bonds between the tooth and the impurities (calculus, stains, plaque) that are attached to it. In the current clinical study, the RF technology utilized by the ToothWave™ was evaluated for patient safety and efficacy in relation to extrinsic stains and teeth shade, compared to a standard powered toothbrush (control brush) that has been accepted by the American Dental Association (ADA).
Materials and Methods
A randomized single-blind double arm prospective study was conducted, in order to evaluate the safety and efficiency of the RF-utilizing toothbrush (ToothWave™, Manufactured by Home Skinovations) to remove extrinsic dental stains as compared to a reference ADA accepted powered toothbrush. The protocol and consent form were approved by the U.S. Institutional Review Board (U.S. IRB2019SRI/02) before study initiation, and verbal and written consent were obtained from all subjects.
Participants
Screened subjects received an oral soft tissue (OST) examination. Extrinsic dental stains and tooth shades of the 12 anterior teeth were evaluated using the Lobene Stain Index (LSI) [21,22] and the VITA Bleached guide 3D-Master. Recruited subjects were 18-70 years of age, with a total extrinsic facial tooth stain mean score of at least 1.80, according to LSI. Exclusion criteria were composed of current or history of oral cavity cancer or oropharyngeal cancer, any active electrical implant anywhere in the body, pregnant or nursing, and any active condition or surgery in the oral cavity within 3 months prior to treatment. Regular tobacco smokers and subjects who did not practice daily oral hygiene were also excluded.
Study Procedures
Eligible study participants were provided with regular, marketed Crest® Cavity Protection Cool Mint Gel (0.243% Sodium Fluoride, Procter & Gamble, Cincinnati, OH 45202), and a toothbrush (either the ToothWave™ or control brush). Participants were randomized and assigned to study group in accordance with a randomization schedule generated by an independent statistical consultant prior to the start of the study, using validated software (SPSS Version 25.0). Participants were stratified according to their age and ethnicity. Randomization numbers within each stratum were assigned in ascending numerical order according to the amount of extrinsic stain they presented at Visit 1. Participating subjects were instructed to brush at home twice-daily (morning and evening) with a full brush head of toothpaste for two timed minutes. The study included 6 weeks of test phase, during which 4 study visits were performed at 2-week intervals. Each use was recorded in a provided diary. The first brushing session was carried out under supervision at the study site. Participants brushed for 2 timed minutes in their usual manner with the standard fluoride toothpaste (Crest® Cavity Protection Cool Mint Gel). Participants continued to use their assigned study treatment twice-daily (morning and evening) for the next 6 weeks, recording each brushing in the diary card provided. Participants returned to the study site every two weeks over the 6-week study period, bringing their study kit so that the toothpaste could be weighed to verify study compliance. Diaries were checked to assess compliance. The participants undertook a supervised brushing during the second visit as was conducted at the first visit in order to make sure brushing was conducted according to the instructions.
Assessments
Clinical efficacy was evaluated at visits 3 and 4 (following 4 and 6 weeks of brushing, respectively). The Lobene stain index was used to measure the intensity and area of extrinsic stain on the facial surfaces of the 12 anterior teeth. Using a provided soft toothbrush, subjects brushed their anterior teeth (with water) for 15 seconds to remove plaque debris and enhance the visibility of stains. The facial surface of the selected teeth was divided into two regions, each scored separately:
a) The gingival region which is a crescent-shaped 3 mm wide band adjacent to the gingival margin and extending to the crest of the interdental papillae of the adjacent teeth.
b) The body region which constitutes the remainder of the labial surface of the tooth.
Intensity was scored from 0 (no stain) to 3 (heavy stain; dark brown to black) and area was scored from 0 (no stain) to 3 (stain covering over two-thirds of the region). For each site, stain intensity (I) was multiplied by stain area (A). The average Lobene index was calculated for each subject by summing the multiplication scores (IxA) of all sites and dividing by the number of sites (sum scores/all site graded). A single examiner performed the visual tooth color assessments under color-corrected lighting conditions (5000K fluorescent lights). Tooth shade was scored on the selected anterior teeth using the VITA Bleached guide 3D-Master Shade Guide. The tab marking system includes linear shade guide units (SGU), ranging from 1 to 29 SGU. The lightest tab, 0M1, is assigned a rank of 1 and the darkest tab, 5M3 is assigned a rank of 29. Subject shade rank scores were determined for each exam period by calculating the mean across the selected anterior teeth scores.
Safety
For safety, a thorough evaluation of the oral soft tissues was conducted at each visit, by way of a visual examination of the oral cavity, including the gingiva (free and attached), hard and soft palate, oropharynx/uvula, buccal mucosa, tongue, floor of the mouth, labial mucosa, mucobuccal/mucolabial folds, lips, and perioral area. A trained dental evaluator performed intraoral examinations at each study visit. In case an adverse event occurred (AE) it was recorded and monitored throughout the study. Any observed abnormalities noted during the OST examination were transcribed beginning at the screening visit until 5 days after the final use of study product. The investigator determined the causal relationship of each AE using their clinical experience and selected the appropriate severity descriptor as mild, moderate, or severe. Treatment-emergent AEs were reported for the safety population, which included all randomized participants who received study product.
Data Analysis
A sufficient number of participants were to be screened in order to randomize at least 90 participants (approximately 45 to the Test, and 45 to the Control groups) to ensure 84 evaluable participants completed the entire study. The sample size in this study provided 80% power to detect a significant difference in the score’s improvements, with type 1 error of 5%. Safety analysis was carried out on a modified intent-to-treat (ITT) population, defined as all randomized participants who conducted at least one treatment. Efficacy analysis was conducted on the perprotocol (PP) population including all participants in the ITT population who had no protocol deviations deemed to affect efficacy. Summary statistics (e.g., count, mean & SD, Median, 25th and 75th percentile) of the demographic characteristics and the efficacy measurements were calculated for each group and study visit. Normality distribution of measures was evaluated using Shapiro and Wilk test; as the majority of measures deviate from normal distribution, non-parametric approach was implemented. Friedman’s test followed by Dunn’s test was used to evaluate the change over time (the time effect) within each group. The Mann Whitney test was used to compare the improvement after 4 and 6 weeks between the groups; the improvement was calculated as
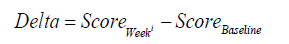
Significance level was defined as α=0.05. Analyses were carried out using SPSS version 25.0.
Results
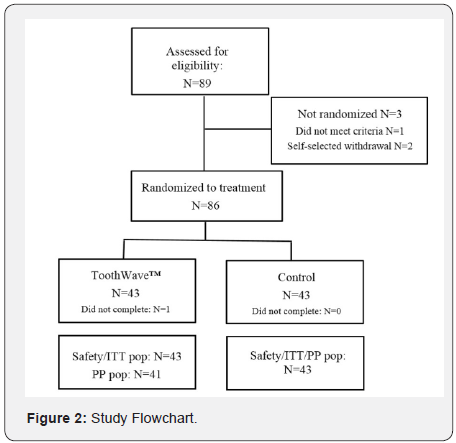
A total of 89 subjects provided informed consent and were enrolled to this study, and 88 of these met the entrance criteria. Two subjects self-selected to withdraw during the first visit, 86 subjects were randomized at baseline to receive either the ToothWave™ or the control powered toothbrush. Two subjects in the treatment group discontinued study participation prior to study end, with 84 subjects (94.38%) completing and deemed fully evaluable at the trial’s conclusion (Figure 2). As shown in Table 1, the mean age of the randomized study population was 48 years, with a range of 18 to 70 years; 59 (68.6%) of the subjects were female. Table 1 exemplifies the demographic baseline values, indicating that the study population was well- balanced with respect to all baseline demographic variables (p ≥ 0.245).
Efficacy
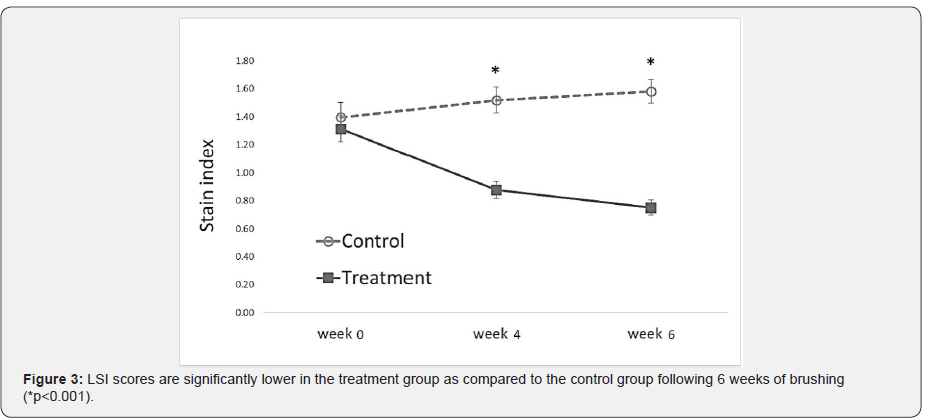
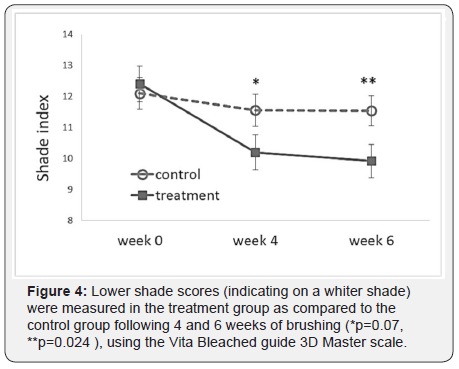
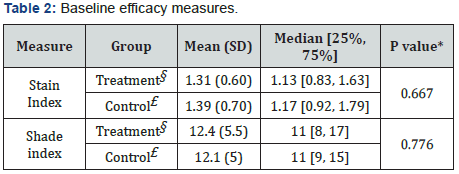
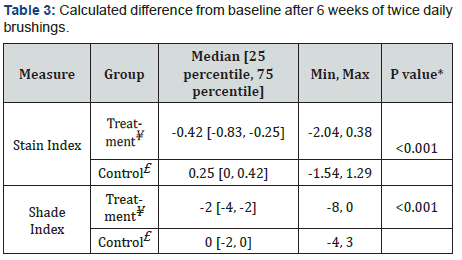
The treatment and control groups’ average baseline scores are shown in Table 2. The test groups did not differ significantly in the efficacy measurements mean scores (p≥0.667). Figure 3 summarizes the changes in the LSI scores of the treatment and control groups over time, indicating a statistically significant reduction in extrinsic dental stain of the treatment group (p<0.001), as compared to a significant increase in extrinsic stain in the control (p<0.001). Moreover, the final LSI scores following 4 and 6 weeks of brushing were significantly lower in the treatment group as compared to the control (p<0.001). Figure 4 exemplifies the changes in teeth shade index (VITA Bleached guide 3DMaster) scores over time, indicating a significantly lower score (increase in tooth whiteness) in the treatment group as compared to the control following 6 weeks of brushing (p=0.024). Table 3 summarizes the median delta values, (difference from Baseline) of the efficacy measures, following 6 weeks test phase. Negative delta values represent an improvement in the measured score, having scores that are decreased with time; a greater negative value represents a greater improvement. Statistical analysis indicates that the delta values achieved in the treatment group are significantly lower than those of the control (p<0.001), showing a significantly greater improvement in extrinsic stain removal and shade tooth color (i.e. whiteness) in the treatment group as compared to the control. Moreover, while the treatment group exhibited a significant decrease in stains (delta<0, p<0.001), the control group showed an increase in stains score (delta>0, p<0.001) during the test phase of the study.
§n=41; £n=43
*representing significance level, comparing the mean scores of treatment and control at baseline.
¥n=41, £n=43
*representing significance level comparing the difference from BL in treatment vs control.
Safety
Both toothbrushes were well-tolerated and no device-related adverse events or any side effects were reported during the study. There were no serious AEs, no medical devise incidents, and no participants with AEs that led to discontinuation of treatment or withdrawal from the study.
Discussion
Extrinsic stains and impurities that are attached to the teeth surface can rarely be removed by regular brushing (including both manual and powered toothbrushes). However, in the current study, we demonstrated that the ToothWave™ toothbrush provided a significant reduction in extrinsic dental stains and whitening of teeth shade as measured by the LSI and VITA bleached guide scales. The improvement seen in the efficacy measures was found to be significantly greater in the ToothWave™ group as compared to the control. The results of this study provide evidence for the unique technological feature of the ToothWave™, which utilizes RF energy that streams on the teeth surface during brushing. There are several references in the literature to “electronic toothbrushes”, which are also referred to as “ionic toothbrushes.” These toothbrushes produce a low-level direct electrical current that streams from the brush head into the oral cavity, using a power source (battery or solar) and a metal rod conductor [23-31]. The scientific data available on these electronic toothbrushes is highly inconsistent. Although in vitro studies indicate their potential effect on removal of bacterial biofilms, mixed results were reported on reduction of microbial activity [27,28]. Moreover, while some clinical studies indicate that a significant benefit on plaque [26,31] or gingival inflammation [29] was observed, most of the clinical evidence concludes that the performance of an electronic toothbrush does not differ from that of a conventional one [23-25,30] and no beneficial effect on stains was reported in any of the studies.
The unique beneficial effect observed in this study may be explained by technological differences between the ToothWave™ and the electronic toothbrushes described in the literature. The electronic toothbrush utilizes a direct electrical current (DC), which runs from the brush into the oral cavity through the body and arm back to the brush handle [25]. Instead, the Tooth wave™ utilizes RF energy, which is an alternating electrical current (AC) that streams back and forth between two electrodes, providing a localized effect that is limited to the teeth surface. The high frequency of the alternating current that is set by the RF parameters allows to safely increase the electrical power as compared to DC current, and thus achieve more significant results [32]. The difference in the effect of the ToothWave when compared to the electronic toothbrushes, results from the type of current that is utilized (alternating vs direct) and its intensity. Moreover, the RF current tends to flow along the surfaces of electrical conductors, what is known as the “skin effect” [33], and thus directing the current towards the teeth surface. The electro-mechanical silicon barrier, which is located between the ToothWave™ electrodes additionally contributes to the ToothWave™ increased efficacy. Furthermore, when compared to a standard powered toothbrush, the electric current is theorized to reach hard-to-reach areas (i.e. between the teeth) as these areas and surfaces would otherwise be chronically missed using traditional mechanical means (i.e. bristles).
Despite their technological differences, both the ToothWave and the electronic toothbrushes share the same mechanism of action, which is based on the principle of polarity that every element in nature has a positive or negative charge [31]. The electronic toothbrushes induce an electric charge, which is postulated to damage electrostatic bonding of plaque proteins to tooth surfaces; thus, enhancing plaque removal [26]. Similarly, since the RF alternating current streams close to the tooth, it brings the charged molecules that are present in the toothpaste close to the tooth surface and changes the chemical environment around it. Once these molecules accumulate near the tooth surface the chemical balance is shifted towards the removal of compounds that are electrostatically attached, replacing them by other, nonstaining charged substances, which might have greater affinity to the surface area (for instance fluoride). We hypothesize that by changing the local charges around the tooth the alternating electrical current is able to disturb the electro-chemical balance on the tooth surface and remove substances (i.e., stains) that are otherwise attached strongly to the enamel layer. We assume that the electrically charged toothpaste ingredients take part in the process that occurs on the teeth surface. Toothpastes are water-based complex mixtures of abrasives and surfactants, humectants, binders, and other active ingredients. All available toothpastes contain charged molecular compounds that once the RF is activated, act as electrolytes in the medium, carry the charges along the tooth surface, and achieve the desired effect.
Conclusion
The ToothWave™ toothbrush is shown to produce a significant reduction in extrinsic dental stains and to achieve improved (and statistically significant) whitening of the teeth shade following 6 weeks of brushing. These benefits were shown to be significantly greater as compared to a control powered toothbrush and are attributed to the RF feature that is uniquely utilized by the ToothWave™ brush.
Acknowledgement
This study was funded by Home Skinovations LTD. The authors would like to thank Zmira Silman, Biostatistician, for performing the statistical analyses and Tsuria Pelet for assistance with manuscript preparation.
References
- Sulieman MA (2008) An overview of tooth-bleaching techniques: chemistry, safety and efficacy. Periodontol 2000 48:148-69.
- Dahl JE, Pallesen U (2003) Tooth bleaching-a critical review of the biological aspects. Crit Rev Oral Biol Med 14(4): 292-304.
- Schallreuter KU, Elwary S (2007) Hydrogen peroxide regulates the cholinergic signal in a concentration dependent manner. Life Sci 80(24-25): 2221-2226.
- Minoux M, Serfaty R (2008) Vital tooth bleaching: biologic adverse effects-a review. Quintessence Int 39(8): 645-659.
- Goldberg M, Grootveld M, Lynch E (2010) Undesirable and adverse effects of tooth-whitening products: A Review. Clin Oral Invest 14(1): 1-10.
- Mahaseth A, Kuzminov T (2017) Potentiation of Hydrogen Peroxide Toxicity: From Catalase Inhibition to Stable DNA-iron Complexes. Mutat Res 773: 274-281.
- Greenwall L (2008) The dangers of chlorine dioxide tooth bleaching. Aesthetic Dentistry Today May 2: 20-22.
- Clifton MC (2014) Tooth whitening: what we now know. J Evid Based Dent Pract 14 Suppl: 70-76.
- Hara AT, Turssi CP (2017) Baking soda as an abrasive in toothpastes: mechanism of action and safety and effectiveness considerations. J Am Dent Assoc 148(11S): S27-S33.
- Soares DG, Dias Riberio AP, Sacono NT, Loguercio AD, Hebling J, et al. (2013) Mineral loss and morphosogical changes in dental enamel induced by a 16% carbanide peroxide bleaching gel. Bras Dent J 24(5): 517-521.
- Hilgenberg SP, Souza Pinto SC, Farago PV, Santos FA, Wambier DS (2011) Physical-chemical characteristics of whitening toothpaste and evaluation of its effects on enamel roughness. Braz Oral Res 25(4): 288-294.
- Hasson H, Ismail AI, Neiva G (2006) Home-based chemically induced whitening of teeth in adults. Cochrane Database of Systematic Reviews (4): CD006202.
- Gerlach RW, Zhou X (2002) Comparative clinical efficacy of two professional bleaching systems. Compend Contin Educ Dent23(1A): 35-41.
- Kugel G, Aboushala A, Zhou X, Gerlach RW (2009) Daily use of whitening strips on tetracycline- stained teeth: comparative results after 2 months. Compend Contin Educ Dent 23(1A): 29-34.
- Li Y (1996) Biological properties of peroxide-containing tooth whiteners. Food Chem Toxicol 34(9): 887-904.
- (2007) Agency for Toxic Substances and Disease Registry. Medical Management Guidelines for Hydrogen Peroxide (H2O2).
- (2005) Scientific Committee on Consumer Products (European Commission). Opinion on hydrogen peroxide in tooth whitening products.
- Munro IC, Williams GA, Heymann HO, Kroes R (2006) Tooth whitening products and the risk of oral cancer. Food Chem Toxicol 44(3): 301-315.
- Munro IC, Williams GA, Heymann HO, Kroes R (2006) Use of hydrogen peroxide-based tooth whitening products and the relationship to oral cancer. J Esthet and Rest Dent 18(3): 119-125.
- Belenky I, Margulis A, Elman M, Bar-Yosef U, Paun SD (2012) Exploring channeling optimized radiofrequency energy: a review of radiofrequency history and applications in esthetic fields. Adv Ther 29(3): 249-266.
- Terézhalmy GT, Walters PA, Bartizek RD, Grender JM, Biesbrock AR (2008) A clinical evaluation of extrinsic stain removal: a rotation-oscillation power toothbrush versus a dental prophylaxis. J Contemp Dent Pract 9(5): 1-8.
- Lobene, RR (1968) Effect of Dentifrices on Tooth Stains with Controlled Brushing. J Am Dent Assoc 77(4): 849-855.
- Van der Weijden GA, Timmerman MF, Piscaer M, Snoek I, van der Velden U, et al (2002) Effectiveness of an electrically active brush in the removal of overnight plaque and treatment of gingivitis. J Clin Periodontol 29(8): 699-704.
- Hotta M, Aono M (1992) A clinical study on the control of dental plaque using an electronic toothbrush with piezo-electric element. Clin Prev Dent 14(4): 16-18.
- Van der Weijden GA, Timmerman MF, Reijerse E, Mantel MS, Van der Velden U (1995) The effectiveness of an electronic toothbrush in the removal of established plaque and treatment of gingivitis. J Clin Periodontol 22(2): 179-182.
- Galgut PN (1996) Efficacy of a new electronic toothbrush in removing bacterial dental plaque in young adults. Gen Dent 44(5): 441-445.
- Sato T, Hirai N, Oishi Y, Uswak G, Komiyama K, et al. (2015) Efficacy of a solar-powered TiO2 semiconductor electric toothbrush on P. Gingivalis biofilm. Am J Dent 28(2): 81-84.
- Perry CN, Beard RD, Lolley RJ, Saunders LEB, Quest D, et al. (2017) Energy output and in vitro biologic effects of an ionic toothbrush. Tex Dent J 134(4): 236-245.
- Van Swol RL, Van Scotter DE, Pucher JJ, Dentino AR (1996) Clinical evaluation of an ionic toothbrush in the removal of established plaque and reduction of gingivitis. Quintessence Int 27(6): 389-394.
- A Moreira CH, Luz PB, Villarinho EA, Petri LC, Rösing CK (2008) Efficacy of an ionic toothbrush on gingival crevicular fluid--a pilot study. Acta Odontol Latinoam 21(1): 17-20.
- Deshmukh J, Vandana KL, Chandrashekar KT, Savitha B (2006) Clinical evaluation of an ionic toothbrush on oral hygiene status, gingival status, and microbial parameter. Ind J Dent Res 17(2): 74-77.
- Dalziel CF (1972) Electric shock hazard. IEEE Spectrum 9(2): 41-50.
- Popovic, Zoya; Popovic, Branko (1999), Chapter 20, The Skin Effect, Introductory Electromagnetics, Prentice-Hall.






























