Dentinal Tubules Occluding by Nanohydroxyapatite Obtained from Eggshell in Colloidal System
Alban Maria Judith1, Bonilla Pablo1* and Jiménez Paola2
1Laboratory of Nanostructures, Faculty of Chemical Sciences, Central University of Ecuador, University Avenue, Ecuador
2Faculty of Odontology, Central University of Ecuador, Ecuador
Submission: December 16, 2019 Published: January 24, 2020
*Corresponding author: Pablo Bonilla, Laboratory of Nanostructures, Faculty of Chemical Sciences, Central University of Ecuador, University Avenue, Ecuador
How to cite this article: Alban Maria Judith, Bonilla Pablo, Jiménez Paola. Dentinal Tubules Occluding by Nanohydroxyapatite Obtained from Eggshell in Colloidal System. Adv Dent & Oral Health. 2020; 12(1): 555826. DOI:10.19080/ADOH.2019.11.555826
Abstract
Dental hypersensitivity is a condition of pain in the teeth produced by the contact of the tooth with cold, hot, sweet or acidic food, and even touch because of the exposure of dentine and disocclusion of dentinal tubules. Searching for solutions for this condition, nanohydroxyapatite showed up promising to be an effective active agent to remineralizer dentin and occlude dentinal tubules. In order to create a simple and comparable product with a commercial product, this investigation was carried out to propose a nanohydroxyapatite synthesis method and the formulation of a colloidal system with such active ingredient. The synthesis of nanohydroxyapatite was made using clean and ground eggshells. In synthesis method, pH, temperature, stirring was kept constant likewise sintering temperature and drip rate of addition of diammonium phosphate were varied. The statistics showed that these factors with the proposed levels do not represent a significant difference in particle size analyzed by DLS. Hydroxyapatite was characterized through infrared spectrophotometry, X-ray diffraction, and determination of calcium and phosphorus. On the other hand, three dentifrices were formulated, varying the particle size of the incorporated hydroxyapatite. The abrasion capacity, pH, viscosity, rheological behavior, and effectiveness in the occlusion of the dentinal tubules of the formulated dentifrices and the commercial dentifrice were analyzed. Through the Dunnet statistical method, it was seen that the smaller the particles size of the hydroxyapatite the greater occlusion of the dentinal tubules
Keywords: Occlusion; Dentinal tubules; Nanohydroxyapatite; Eggshell; Dentrifice
Abbreviations: EDTA: Ethylene Dinitrilo Tetraacetic Acid; CTAB: Cetyltrimethylammonium Bromide; Nano HAP: Nanohidroxyapatite; DLS: Dynamic Light Scattering; AFM: Atomic Force Microscope; Ca/P: Calcium- phosphorus ratio; PDI: Polydispersity Index
Introduction
Hypersensitivity is a clinic condition defined as a dental pain due to the exposition of the dentin, and it appears when there is contact with external stimulus such as hot, cold, sweet or acidic food, and even touch. Statistically, that condition is suffered by 3-57% of the world population especially at the age of 30-40 years old American Dental Association [1]. There are plenty of causes for hypersensitivity: tooth decay, aggressive tooth brushing, etc. Medina [2]. Throughout the years, it has been developed some products with desentizing properties. In general, those products contain potassium salts, calcium, phosphates, or arginine Colgate [3]. In 2014, Dentaid Expertise launched to the market the first toothpaste with nanohydroxyapatite Dentaid [4]. Hydroxyapatite is a mineral formed by calcium, phosphorus, and oxygen. It is the mineral which gives the hardness to teeth and bones. Since the hydroxyapatite and hypersensitivity importance, there are a few previous studies about the topic. Bardhan, Mahata & Mondal [5] published an article in Advances in Applied Ceramics called “Processing of natural resourced hydroxyapatite from eggshell waste by wet precipitation method”. The authors developed a synthesis method of hydroxyapatite from eggshells controlling aspects as pH, temperature, stirring time, etc. They synthetized sub micrometric hydroxyapatite.
“Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature” was written by Pepla [6]. They analyzed all of the bibliography around that topic and concluded that nanohydroxyapatite is a revolutionary material with a great remineralizing capacity even better that fluoride. Amaechi, Mathews, Ramalingam Mensinkai (2015) carried out a study in Texas University, which was published in American Journal of Dentistry. The research was “Evaluation of nanohydroxyapatite-containing toothpaste for occluding dentin tubules”. The objective was comparing some dentifrices: with 10% of nanohydroxyapatite, with 15% of nanohydroxyapatite, with sodium monophosphate, and with Nova Min. The occlusion was analyzed trough SEM, and the results showed that the dentifrices with nanohydroxyapatite and Nova Min are more effective than the one with sodium monophosphate. The present study demonstrates effectiveness in dentinal occlusion using different nanoparticles size of hydroxyapatite (nano HAP). In Latin-American, there is little research about the topic. Besides, there is not a commercial product with hydroxyapatite especially in countries like Ecuador, Peru, Colombia, and Venezuela. Taking into account the statistics for this region of the world, it is important to develop a formulation and made some investigation around this topic. The use of eggshell as a source of calcium to synthesize nanohydroxyapatite is a valid and useful method for the preparation of toothpastes that can help improve oral health in places where this raw material is scarce.
Materials and Methods
Nanohydroxyapatite synthesis
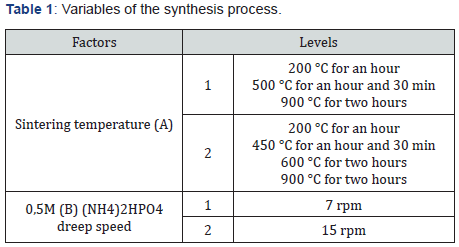
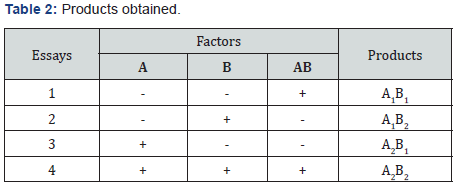
Nanohydroxyapatite was synthesized from eggshells applying the precipitation method by Bardhan R, Mahata, S, Mondal B [5] with some modifications. The eggshells were cleaned, and the calcium content was determined by complexometric titration with EDTA by previously doing an acid treatment of the sample. The hydroxyapatite was synthesized by weighing 5g of clean and crushes eggshells was subjected to an acid treatment and filtration. Ammonium hydroxide was added to the solution adjusting pH value 9 to 9,5. Then 0,0728 g of CTAB was added at 60° C with magnetic stirrer at 1000 rpm and 0.5M diammonium phosphate solution was added by a peristaltic pump. After the addition, the precipitate obtained was filtered and dried in oven at 80° C. Finally, it underwent a sintering process in a muffle and ground in a vibrating mill. The variables of the process are detailed in the Table 1 and the products obtained in the Table 2. In addition, hydroxyapatite larger than 2000 nm was synthesized to analyze effect of size and improves the effect on the dentinal tubules. The synthesis process was the same, varying some conditions: the addition of 0.5M diammonium phosphate was at 40rpm, no drying of the product before sintering process, and sintering made directly at 900° C. This product was named as HAP. The products obtained were characterized through DLS (for particle size) in a 0,3M sodium lauryl sulfate suspension, infrared spectroscopy, X-Ray diffraction, and the Ca/P ratio by analyzing calcium percentage through atomic absorption and total phosphorus percentage through UV-Vis spectrophotometry with vanate-molybdate reagent.
Formulation of the Colloidal System
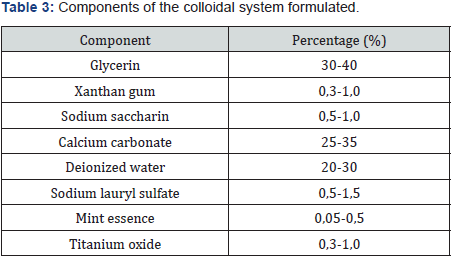
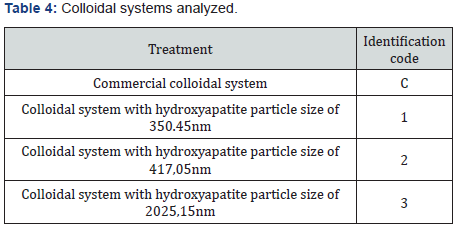
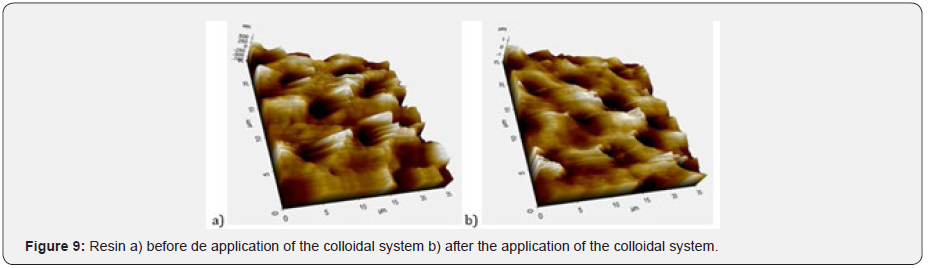
Table 3 details the percentages of the components used in the formulation of the colloidal system. Hydroxyapatite was added at a percentage of 0,45%. The colloidal system was characterized according to its abrasion effect with the methodology proposed by González G, Reyes RN [7] using Filtek P60 3M ESPE dental curing resin as well as pH, rheological behavior, and effect on dentinal tubules. For the last part, sixteen human dental pieces with a cross-section in the dentin were applied 35% phosphoric acid for 15 seconds. Then, they were washed, dried, and analyzed by AFM in order to measure the diameter of the dentinal tubules. After that, it was applied the colloidal system formulated with an electric toothbrush for two minutes. Finally, the dental parts were analyzed again by AFM. Details of colloidal systems analyzed can be found in Table 4.
Results
Nanohydroxyapatite
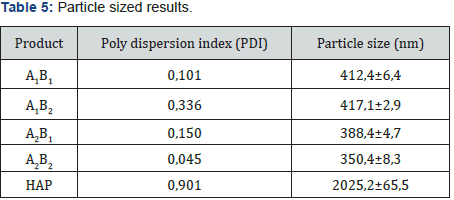
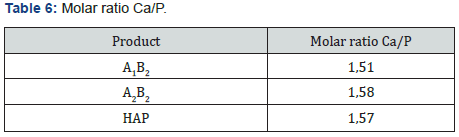
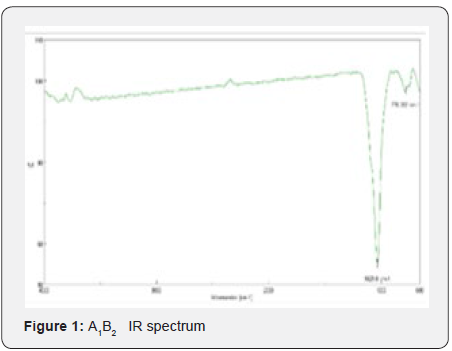
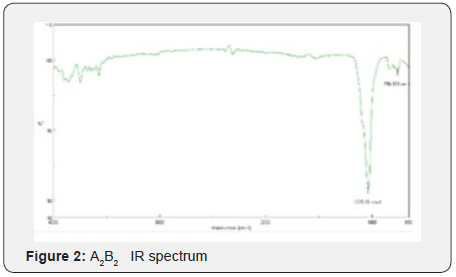
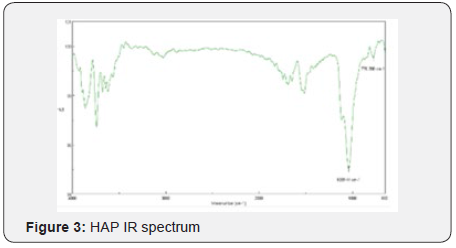
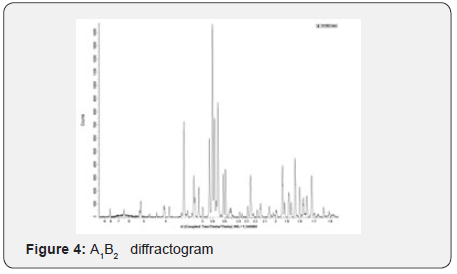
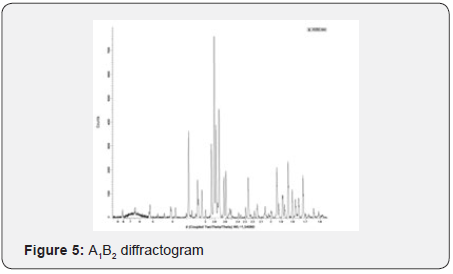
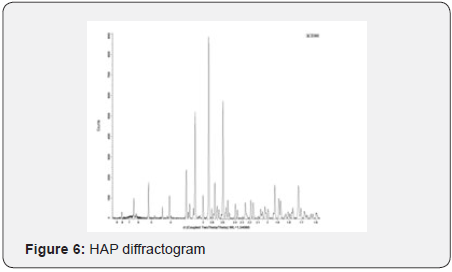
Yields obtained in all hydroxyapatite synthesis processes were more than 77%. In terms of particle size, Table 5 contains the results. The PDI values are between 0 and 1 for all of the products which means the systems are monodisperse Seymour [8]. The highest PDI value belongs to HAP because of the synthesis conditions for this product. With the results obtained, three of the five products were chosen for the colloidal system formulations. Besides, the data were statistical processed by Yates Algorithm identifying A factor, B factor and their interaction were not meaningful during the synthesis process. Infrared spectroscopy helps to identify the most important functional groups in a molecule. Three spectrums (Figure 1-3) are similar to each other. The hydroxyapatite formula is Ca10(PO4)6(OH)2, so the bands between 3500 y 4000 cm-1 correspond to a high-energy elongation absorption of the hydroxyapatite -OH group. The spectrum of HAP (Figure 3) contains more intense bands in that region due to higher degree of hydration of the sample. In the same spectrum, there are bands between 1500 y 2000 cm-1, those bands show the presence of a small amount of CO32. The most intense band (1000 cm-1) and the band in 775 cm-1 are very characteristic of the phosphate group (–PO4) Torres [9]. The diffractograms (Figure 4-6) show characteristic peaks for hydroxyapatite at d: 3,5; 3,2; 3,1; 2,8; 2,7; 2,6; 2,2; 1,9; 1,8 y 1,7. Table 6 details the molar ratio Ca/P for the products. Stoichiometric hydroxyapatite has a molar ratio Ca/P of 1,67, nevertheless the biologically active hydroxyapatite has a molar ratio Ca/P less than 1,67. That means the synthetized hydroxyapatite can be better assimilated than the stoichiometric hydroxyapatite Okada & Matsumoto [10].
Colloidal System
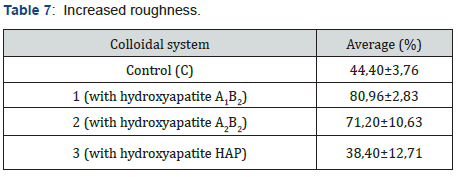
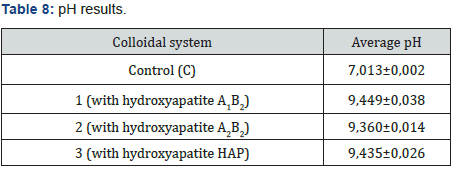
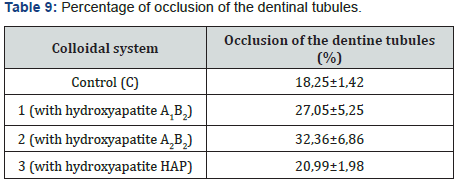
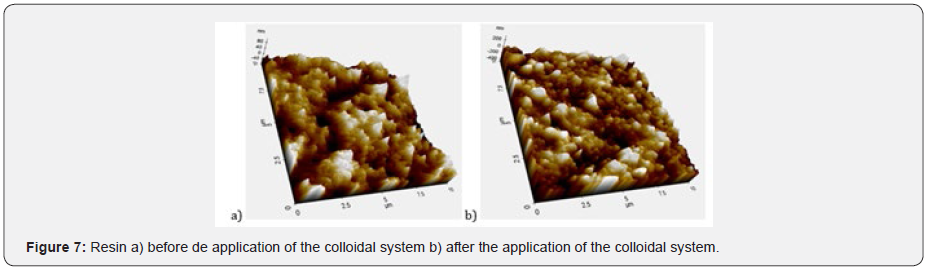
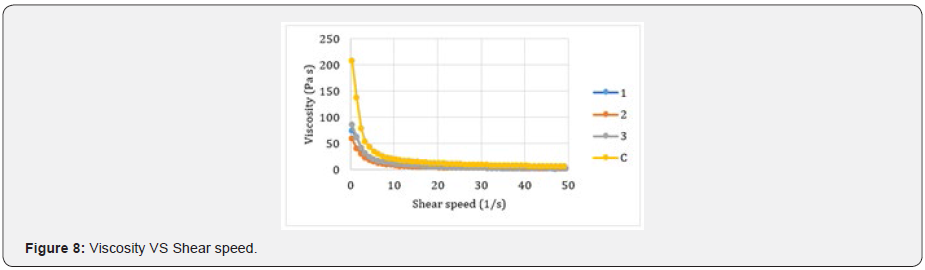
The abrasive effect of the colloidal systems is directly proportional to the increased roughness in the dental curing resin analyzed González & Reyes [7]. Table 7 shows the increased roughness obtained for the colloidal systems formulated. Based on those data, the smaller the particle size, the greater the abrasive effect. Figure 7 shows an example of the images obtained by AFM before and after the application of the colloidal system 2. pH was measured applying the procedure detailed in Standard Procedure INEN 1596 for toothpastes. Standard methods point out pH can vary from 4,5 to 10,5. Table 8 contains the results for pH analysis. In reference at rheological analysis the Figure 8 show a typical behavior of a pseudo-plastic fluid (viscosity decreases dramatically as shear speed increases). The viscosity at the starting point shows the biggest difference between the colloidal systems analyzed. As a result, the commercial product has the best stand up properties, which means that its consistency on the bristles of the toothbrush is ideal Picó [11]. The Table 9 shows the percentage of occlusion of the dentine tubules after applying the colloidal systems on the dental pieces. The results were analyzed through Dunnet statistical model comparing the formulated colloidal systems with the commercial one. Statistics shows that there is no difference between the 1 and 3 colloidal systems and the commercial one, but there is significant difference between 2 colloidal system and the commercial one. That proves that particle sized of hydroxyapatite used to formulate the system influences the effect on the occlusion of dentinal tubules. Figure 9 shows an example of the images obtained by AFM before and after the application of the colloidal system 2.
Discussion
The equation 1 shows the process of formation of hydroxyapatite in two steps, a) Formation of CaCO3 and b) synthesis of HAP
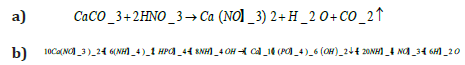
Equation 1 a) calcium carbonate and nitric acid reaction; b) hydroxyapatite precipitation
The pH is important due to a pH value between 9 and 11 promotes the formation of hydroxyapatite with better size distribution and crystallinity. A pH value less to 9 can improve the production of dicalcium phosphate Sjahan & Ibrahim [12]. There are plenty of variables during the synthesis process such as concentration of the reagents, synthesis temperature, drip rate of precipitating reagent, and sintering temperature. Based on some preliminary tests and previous studies, it was decided to work with drip rate of precipitating reagent and sintering temperature as variables and keep the others as constants. If sintering process is not made with temperature ramps, there is a very high degree of agglomeration Okada & Matsumoto [10]. For the measure of the particle size was necessary to add sodium lauryl sulfate (surfactant) to avoid the formation of clusters because of the Brownian Movement Singer, Barakat, & Mohapatra [13]. Infrared and X-Ray spectroscopy allow defining the functional groups and crystallinity of hydroxyapatite. In the same way, molar ratio Ca/P values determinates hydroxyapatite synthetized is bioactive. It is important to notice that the precipitation process can lead molar ratio Ca/P is less than theoretical. It was necessary to look up all of the components of the colloidal system in the Handbook of Pharmaceutical Excipients for avoiding incompatibilities between them. The results for abrasive effects show that the colloidal systems with the smallest particle size hydroxyapatite has effect that is more abrasive.
This can be considered a negative aspect due to abrasion produces accumulation of plaque and bacteria González & Reyes [7]. In terms of pH, there is a big difference between the formulated colloidal systems and commercial colloidal system. The phenomenon happens because of calcium carbonate is used in the formulation which increases the pH. Nevertheless, the pH values are within the norm. The rheological analysis tries to simulate the tooth brushing process. Look how the viscosity decreases while the shear speed increases. That is a typical behavior of a pseudo-plastic fluid, which is the classification of this kind of dental products. The effect on the dentine tubules results allow showing that hydroxyapatite is an occlude of the dentine tubules, so it can help to avoid dental sensibility processes. It has not been accurately defined the mechanism trough hydroxyapatite has the mentioned effect, however it has been seen that hydroxyapatite has the ability to cling to the tooth surface and then it forms kind of micro clusters Swarup & Rao [14]. It can be because hydroxyapatite has high level of biomimetics. In addition, it was proved that the smaller particle size hydroxyapatite, the better adhesion process. The contact surface is also better with small particle sizes [15].
Conclusion
Three colloidal systems were formulated, and they have similar characteristics to the commercial colloidal system. The colloidal systems had different particle size hydroxyapatite, so the effect on the dentin tubules varied between colloidal system 2 and the others. Colloidal system 2 had the smallest particle size hydroxyapatite. As a conclusion, the effect of a colloidal system on the dentin tubules depends on the particle size of the hydroxyapatite, and the smaller particle size, the better effect.
References
- (2003) American Dental Association.
- Medina A (2009) Dentine hypersensitivity: A review of its aetiology, pathogenesis and management. Av Odontoestomatol p. 25.
- Colgate (2016) Colgate Sensitive PRO-Alivio.
- Dentaid (2015) Dentaid. Salud Bucal.
- Bardhan R, Mahata S, Mondal B (2011) Processing of natural resourced hydroxyapatite from eggshell waste by wet precipitation method. India: Advances in Applied Ceramics. 110(2): 80-86.
- Pepla E, Kostantinos L, Palaia G, Tenore G, Migliau G (2014) Nano-hydroxyapatite and its applications in preventive, restorative and regenerative dentistry: a review of literature. Italia: Annali Di Stomatologia. Ann Stomatol 20(3): 108-114.
- González G, Reyes R, Ramón Fernando, Gabriela Jasmin (2017) Determination of the pH and abrasion of dentifrices based on natural products, compared to a conventional toothpaste. México: Universidad Autónoma de Mé
- Seymour R (2012) An introduction to polymer chemistry. United States: M. Dekker.
- Torres J (2010) Obtención y caracterización de hidroxiapatita porosa a partir de cáscara de huevo y tunicina. Chile: Universidad de Chile.
- Okada M, Matsumoto T (2015) Synthesis and modification of apatite nanoparticles for use in dental and medical applications. Japan: Elsevier 51(4): 85-95.
- Picó J (2016) Cosmetotecnia de los dentí Relevancia del comportamiento reológico. España: Universidad de Valencia.
- Sjahan N, Ibrahim W (2014) Microwave Irradiation of Nanohydroxyapatite from chicken eggshells and duck eggshells. Malaysia: The Scientific World Journal 275984: 7.
- Singer A, Barakat Z, Mohapatra S (2018) Nanoscale Drug-Delivery systems. Estados Unidos: Science Direct.
- Swarup J, Rao A (2013) Enamel surface remineralization: using synthetic nanohydroxyapatite. India: Universidad de Manipal.
- Standard Method NTE INEN (2013) Pasta dental. Determinación del pH. Ecuador.






























