An Investigation of the Presence, Types and Sensitivity to Different Antimicrobial Medications of the Communal Bacteria in the Children Dental Clinic Service
Fowziya M Ali1* and Miftah S M Najm1, Noor Alhooda Milood Alawaklli2 and Ahmed Abouserwel3
1 Oral Science Department, University of Benghazi, Libya
2 Ministry of Health, Libya
3 Birmingham Community Health Foundation Trust, UK
Submission: July 30, 2019; Published: August 13, 2019
*Corresponding author:Fowziya M Ali, BDS, M Dent Sc., PhD, Paediatric Dentistry Department, Faculty of Dentistry, University of Benghazi, Libya
How to cite this article:Fowziya M Ali, Miftah S M Najm. An Investigation of the Presence, Types and Sensitivity to Different Antimicrobial Medications of the Communal Bacteria in the Children Dental Clinic Service. Adv Dent & Oral Health. 2019; 11(1): 555805. DOI: 10.19080/ADOH.2019.11.555805
Abstract
a) To investigate the presence of airborne and splatter microbial contamination in a children dental clinic practice inside the children dental clinic service after standard procedure of infection control and safety practice in controlling cross-contamination before the start of the dental clinical work. b) To identify the type of microbial contamination and its sensitivity to mostly used antimicrobial medications in dentistry. c) To explore the presence of resistant bacteria in different selected place and surfaces in the dental clinical practice. d) To verify the effectiveness of the cross-contamination control in the area.
Keywords: Communal bacteria; Dental chair contamination; Resistant bacteria; Staphylococcus; Micrococcus gram positive; Antimicrobial; Susceptibility; Sensitivity test
Introduction
The mouth contains tremendous diversity of microorganisms which constitutes the normal oral flora. The dental clinical practice room is predominantly the source of largely multiple dental procedures that exhibit occupational exposure to blood and saliva borne contamination and other potentially infectious dental materials, (such as the periodontal crevice and pockets, tongue dorsum and other mucosal surfaces) [1]. Therefore, requires a high standard of infection control and safety practice in controlling cross-contamination and occupational exposures to blood- and saliva-borne diseases. The microbial flora of the oral cavity is rich and extremely diverse and has an important role in initiation of oral disease [2]. Temperature and availability of multiple surfaces on which microbial inhabitants can develop. Most of these organisms carry no significant risk to dental professionals; however, several them cause infections that may be difficult to cure [3,4].
It was revealed that there are in excess of 700 bacterial species inhabiting the mouth. Moreover, the latest developing based methods have further exceeded the extent of bacterial diversity in oral niches [5]. Dental health care workers may prone to the risk of infection from blood or saliva borne pathogens as result of their work [6].
Materials and Methods
Ten swabs were taken from different selected apparatus and surfaces inside the dental clinic room and culture-based identification was considered for the detection of most bacterial presence, type and bacterial sensitivity and resistance. Every tube swab was filled with 5ml of 10% dextrous solution. Then each tube was identified by number, date of examination and name of the selected surface to be examined. The 10 selected apparatus and surfaces were: 1. the wall of the dental room, 2. hand wash basin, 3. the handle of the autoclave, 4. sterilised handpiece by an autoclave method, 5. the surface dental chair, 6. The sides of the dental chair, 7. The dentist’s setting tool, 8. the working table surface of the dental chair, 9. The roof of the dental room, 10. the suction tubes. All tube swabs were taken immediately to Alakeed medical laboratory for culture- based identification.
Results
Positive bacterial culture results were found in three places as follows:
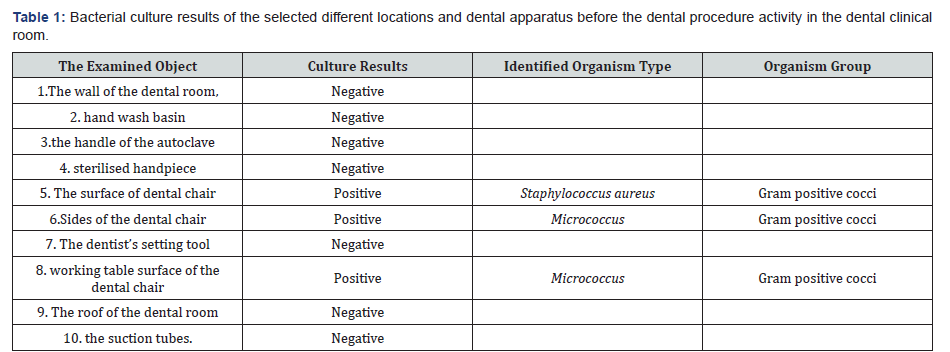
a) The surface and sides of the dental chair and the working surface table of the dental chair. Contamination with staphylococcus aureus identified on the surface of the dental chair while micrococcus gram positive bacteria species found on the sides and the working surface table of the dental chair. Whereas, the other investigated locations and dental apparatus showed negative bacterial culture results (Table 1).
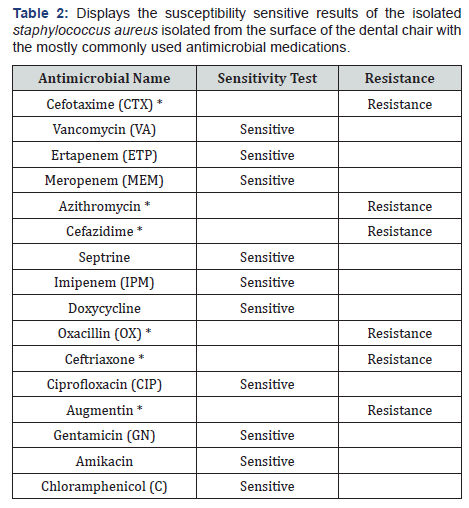
b) The susceptibility sensitive results of staphylococcus aureus (SA) isolated from the surface of the dental chair to the various most common antimicrobial medications show resistance to six most commonly used antimicrobials such as Cefotaxime, Azithromycin, Cefazidim, Oxacillin, Ceftriaxone and Augmentin (Table 2).
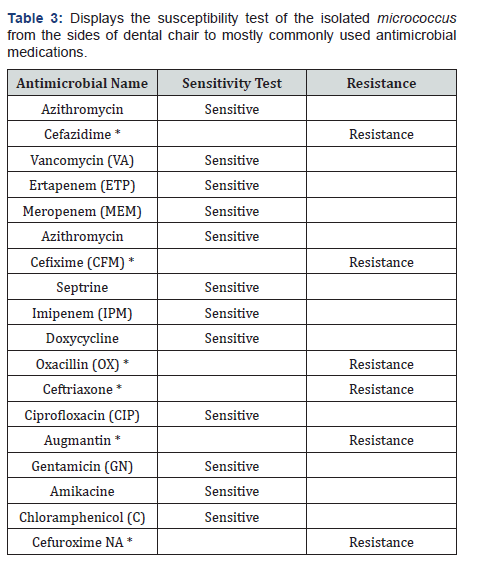
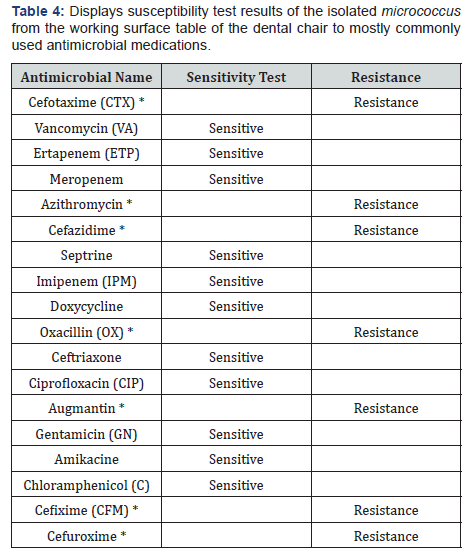
* Resistant antimicrobial medications. c) The susceptibility test results of micrococcus gram positive which isolated from the sides of the dental chair to the various most common antimicrobial medications show resistance to six most commonly used antimicrobials such as Cefixime (CFM), Cefuroxime NA, Cefazidime, Oxacillin, Ceftriaxone and Augmentin (Table 3). In Table 4, the results show six antimicrobials resist micrococcus gram positive bacteria which taken from the working surface table of the dental chair. The resistant antimicrobials are as Cefixime (CFM), Cefazidime, Oxacillin, Azithromycin and Augmentin
*Resistant antimicrobial medications
Discussion
This is the first study in our dental faculty to investigate microbial contamination in the dental clinical room and little is known about the presence of bacterial resistance to commonly used antimicrobial medications. The study was conducted before the beginning of dental procedure activity on the morning time after conventional infection control. There was no air-conditioning or central ventilation system, so ventilation was achieved by opening windows. The cleaning procedure for the surfaces implicates using 70% Ethanol and autoclave sterilisation for the handpiece instrument. Furthermore, to explore the Susceptibility test of the isolated bacteria species to different commonly used antimicrobial medications in dentistry. In this study, we detect the most bacterial contamination was with staphylococcus aureus and micrococcus gram positive by culture methods and determining the antimicrobial sensitivity and the resistant strains. These bacterial species were considered normal bacterial flora in the mouth. Staphylococcus and Micrococcus species were found on some areas of human skin [7]. Staphylococcus aureus and micrococcus species were also detected in orthopaedic health worker surgical scrubs room It has also been shown that the bacterial burden of scrubs in the operating room that may influence the contamination of wounds intraoperatively and the risk for postoperative infection of those wound Krueger et al. [8].
Micrococcus leutus and staphylococcus species were also isolated from dental clinic Decraene et al. [9] Aerosols or airborne bacteria mostly gram positive cocci such as staphylococci and streptococcus viridians spread during dental treatment was found when using modern high-speed rotating instruments Rautemaa et al. [10].
Our findings revealed that the dental chair and its working surfaces table are the most common areas for bacterial reservoir. Negative culture results were obtained from other selected areas for microbial investigations. The normal microbiota of the oral cavity consists mainly of bacteria. Therefore, saliva must be treated as potentially infectious as blood or other body fluids with respect to HIV and other blood-borne diseases. Resistant form of methicillin resistant-strain staphylococci aureus bacteria can survive in the hospital environment despite use of infection control protocols, Duckworth et al. [11]. It was suggested to be responsible for staphylococcal infection in hospitals and patients Beachey [12], McKeown-Longo [13].
Streptococci constitute 60 to 90% of the bacteria that colonize the teeth in the oral cavity [14]. Micrococcus leutus bacteria was isolated from the tongue, and supra- and subgingival plaque in the mouths of volunteers and patients with periodontitis Anesti et al. [15]. Caries-associated bacteria were detected in samples of dental plaque and bacterial profiles change with caries lesion progression and differ between primary and secondary dentition Aas et al. [16]. It was believed that a gram of dental plaque (about one quarter of a teaspoonful contains about 200 billion bacteria, and saliva contains 10 to 100 million bacteria per millilitre [17].
Culture-based microorganism’s identification has been considered the ‘gold standard’ for the detection of most bacterial and fungal infections [18]. Another novel metagenomic approach has increased numbers of bacterial species Parahitiyawa et al. [5]. Various bloods borne bacteria, airborne bacteria saliva borne bacteria and aerosol streptococcal produced by dental turbines remains in air up to 24 hours. Samples of bacteria isolate from environmental hospital Formica blocks found to be survived up to 24 hours Duckworth et al. [11].
The importance of determination of antimicrobial sensitivity highlights the detection of the resistant species of bacteria to the commonly prescribed antibiotic in dentistry. This is the first study presented antibiotic susceptibility testing against different commonly used antimicrobial in dentistry against staphylococcus aureus and micrococcus leutus. Our finding showed both staphylococcus and micrococcus positive cocci found to have resistance to Augmentin and oxacillin and those commonly prescribed among dental practitioners.
Staphylococcus aureus isolated from the surface of the dental chair showed resistance to six most commonly used antimicrobials such as Cefotaxime, Azithromycin, Cefazidim, Oxacillin, Ceftriaxone and Augmentin. While, micrococcus gram positive which isolated from the sides of the dental chair to the various most common antimicrobial medications show resistance to six most commonly used antimicrobials such as Cefixime (CFM), Cefuroxime NA, Cefazidime, Oxacillin, Ceftriaxone and Augmentin. Micrococcus gram positive that isolated from the working surface table of the dental chair demonstrated resistant to 7 antimicrobials Cefixime (CFM), Cefazidime, Oxacillin, Azithromycin and Augmentin Cefuroxime, Cefotaxime resistance.
Thus, most dental infections can be treated local operative procedures without antibiotic prescriptions and dentists still prescribing antibiotic unnecessarily Oberoi et al. [19].
Conclusion
a) Dental chair and its apparatus were more contaminated than other investigated areas and harbouring resistant staphylococcus and micrococcus bacteria even after conventional disinfection procedures. b) Negative culture results were obtained from the wall and the roof of the dental clinical room, dentist mobile setting tool. c) No bacterial growths were found from the hand washing sink inside the dental room and the handle of the door of the commonly used autoclave for sterilisation. d) No bacterial growth was found from the sterilised handpiece drilling instrument that indicates the autoclaving system is effective. e) No bacterial growth from the suction tube line apparatus that attached to the dental chair. f) Emerging of resistant species of staphylococcus and micrococcus leutus to most commonly used antimicrobials. g) Augmentin and oxacillin which are commonly used antibiotic showed ineffectiveness in gram positive cocci. h) Vancomycin, gentamycin and doxycycline are more sensitive to staphylococcus and micrococcus leutus. i) Chloramphenicol, septrine, Ertapenem (ETP), and Meropenem are less prescribed among dental practitioners, for that reason proved to be sensitive to staphylococcus and micrococcus leutus.
References
- Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE (2005) Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 43(11): 5721-5732.
- Tanzer JM, Livingston J, Thompson AM (2001) The microbiology of primary dental caries in humans. J Dent Educ 65(10): 1028-1037.
- Porter RS (1991) Infection control in dentistry. Current opinion in dentistry 1: 429-435.
- Osorio R, Toledano M, Liébana J, Rosales JI, Lozano JA (1995) Environmental microbial contamination. Pilot study in a dental surgery. Int Dent J 45(6): 352-357.
- Parahitiyawa NB, Scully C, Leung WK, Yam WC, Jin LJ, et al. (2010) Exploring the oral bacterial flora: current status and future directions. Oral Dis 16(2): 136-145.
- Lee SA, Yoo SY, Kay KS, Kook JK (2004) Detection of hepatitis B virus and Mycobacterium tuberculosis in Korean dental patients. J Microbiol 42(3): 239-242.
- Kloos WE, Musselwhite MS (1975) Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol 30(3): 381-395.
- Krueger CA, Murray CK, Mende K, Guymon CH, Gerlinger TL (2012) The bacterial contamination of surgical scrubs. Am J Orthop (Belle Mead NJ) 41(5): 69-73.
- Decraene V, Ready D, Pattern J (2008) Air borne microbial contamination of surfaces in a UK dental clinic. J Gen Appl Microbiol 54(4): 195-203.
- Rautemaa R, Nordberg A, Wuolijoki-Saaristo K, Meurman JH (2006) Bacterial aerosols in dental practice - a potential hospital infection problem? J Hosp Infect 54(1): 75-81.
- Duckworth GJ, Jordens JZ (1990) Adherence and survival properties of an epidemic methicillin- resistant strain of Staphylococcus aureus compared with those of methicillin-sensitive strains. J Med Microbiol 32(3): 195-200.
- Beachey EH (1981) Bacterial adherence: adhesin-receptor inter-actions mediating the attachment of bacteria to mucosal surface. J Infect Dis 143(3): 325-345.
- McKeown-Longo PJ (1987) Fibronectin-cells surface interactions. Rev Infect Dis 9(Suppl 4): 322-334.
- Nyvad B, Kilian M (1987) Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand J Dent Res 95(5): 369-380.
- Anesti V, McDonald IR, Ramaswamy M, Wade WG, Kelly DP, et al. (2005) Isolation and molecular detection of methylotrophic bacteria occurring in the human mouth. Environ Microbiol 7(8): 1227-1238.
- Aas JA, Griffen AL, Dardis SR, Lee AM, Olsen I, et al. (2008) Bacteria of Dental Caries in Primary and Permanent Teeth in Children and Young Adult. J Clin Microbiol 46(4): 1407-1417.
- Miller CH, Palenik C J (1998) Infection control and management of hazardous materials for the dental team. (2nd edn), V. Mosby Company, Maryland Heights, Missouri, USA.
- D’Ercole S, Catamo G, Piccolomini R (2008) Diagnosis in periodontology: a further aid through microbiological tests. Crit Rev Microbiol 34(1): 33-41.
- Oberoi SU, Dhingra C, Sharma G, Sardana D (2015) Antibiotics in dental practice: how justified are we. International Dental Journal 65(1): 4-10.






























