Evaluating the Effect of Low Concentrations of Common Chemical Solutions on Disinfecting Heat-Cured Acrylic-Resin
Sara Mogharrabi1, Safoura Ghodsi2, Maryam Jarahzadeh3, Alireza Moshaverinia4, Ahmadreza Homayouni5, Maryam Baghizadeh Fini6*
1 1DDS, MSc, Assistant professor, Department of Prosthodontics, School of dentistry, Ahvaz Jundishapur University of Medical Sciences, Ahvaz, Iran
2 2DDS, MSc, Assistant professor, Dental research center, Dentistry research institute, Department of prosthetics, School of dentistry, Tehran University of Medical Sciences, Tehran, Iran
3 3Dentist, Private practice, Tehran, Iran
4 4DDS, MS, PHD, FACP, Assistant Professor, Department of prosthetics, School of dentistry, University of California , Los Angeles, US
5 5Ph.D. student, Industrial Engineering and Management department, Oklahoma State University, US.
66DDS, School of Dentistry, Tehran University of Medical Sciences, Tehran, Iran, Tehran, Iran
Submission: July 17, 2019; Published: August 07, 2019
*Corresponding author:Maryam Baghizadeh, Tehran University of Medical Sciences, 1208 N. Knoblock, Stillwater, Oklahoma, US, Iran
How to cite this article: Sara M, Safoura G, Maryam J, Alireza M, Ahmadreza H, et al. Evaluating the Effect of Low Concentrations of Common Chemical Solutions on Disinfecting Heat-Cured Acrylic-Resin. Adv Dent & Oral Health. 2019; 11(1): 555804. DOI: 10.19080/ADOH.2019.11.555804
Abstract
Purpose: Dental prostheses are exposed to different microbial contaminant that potentially leads to prosthesis-related infections. This study aimed to evaluate the effectiveness of low concentrations of antimicrobial solutions on disinfecting heat-cured acrylic resin. Materials and Methods: 188 acrylic (KAVO EWL 5518, Automatic curing device, Germany) specimens were fabricated (17×6×1mm), and sterilized. Two specimens (Brain-Heart Infusion Broth, Merk, type 110493, Germany) were kept ensuring the process validation while the remaining specimens were randomly divided in to 3groups (n=62). Each group was immersed in fresh colony suspensions of either Candida-albicans, Staphylococcus-aureus, or Streptococcus-mutans. Two specimens were kept in sterilized culture media (Brain- Heart Infusion Broth, Merk, type 110493, Germany) for ensuring the process validation while the remaining specimens were divided to 4 groups according to subsequent disinfecting procedure (n=15). Each group was immersed either in 0.5% sodium-hypochlorite solution, 0.2% chlorhexidine solution, vinegar, or water. The turbidity measurement of culture media was evaluated at interval time of 30 minutes, 2, and 4 hours. Student T-test, Chi-square and Fisher-exact test were used for results comparison (P<0.05). Results: Chlorhexidine showed maximum effectiveness on eliminating candida-albicans at all time intervals. For Staph aureus, chlorhexidine was completely effective after two hours. 0.5% Hypochlorite solution eliminated 40% of Staph aureus colonies after 2 hours and retained this effect for 4 hours. All disinfectants were equally effective in eliminating Strep mutans. Conclusion: Using chlorhexidine, disinfection of acrylic resin from Strep-mutans and Candida-albicans requires less time compare to Staph-aureus. Hypochlorite and vinegar were only effective in complete elimination of Strep-mutans.
Keywords: Acrylic resins; Disinfection; Prosthodontics; Solutions
Introduction
Dental prosthesis could be highly contaminated as it is exposed to different infected sources and conditions [1]. Biofilms formation in oral environment may cause different health complications [2], one of the most important of which is denture stomatitis that affects about two-thirds of full denture wearers [3,4]. Infectious agents can also lead to meningitis, pneumonia, and even heart diseases if swallowed or enter oral mucosal lesions [5]. Different microorganisms may play role in the formation of such infections, the most important of which are Candida albicans, Staphylococcus aureus, and Streptococcus mutans [5,6]. Several procedures have been proposed to eliminate these microorganisms; the most prevalent have been reported to be mechanical hygienic procedures, and use of solutions like vinegar, Castrol oil, glutaraldehyde, sodium hypochlorite and chlorohexidine [7-16].
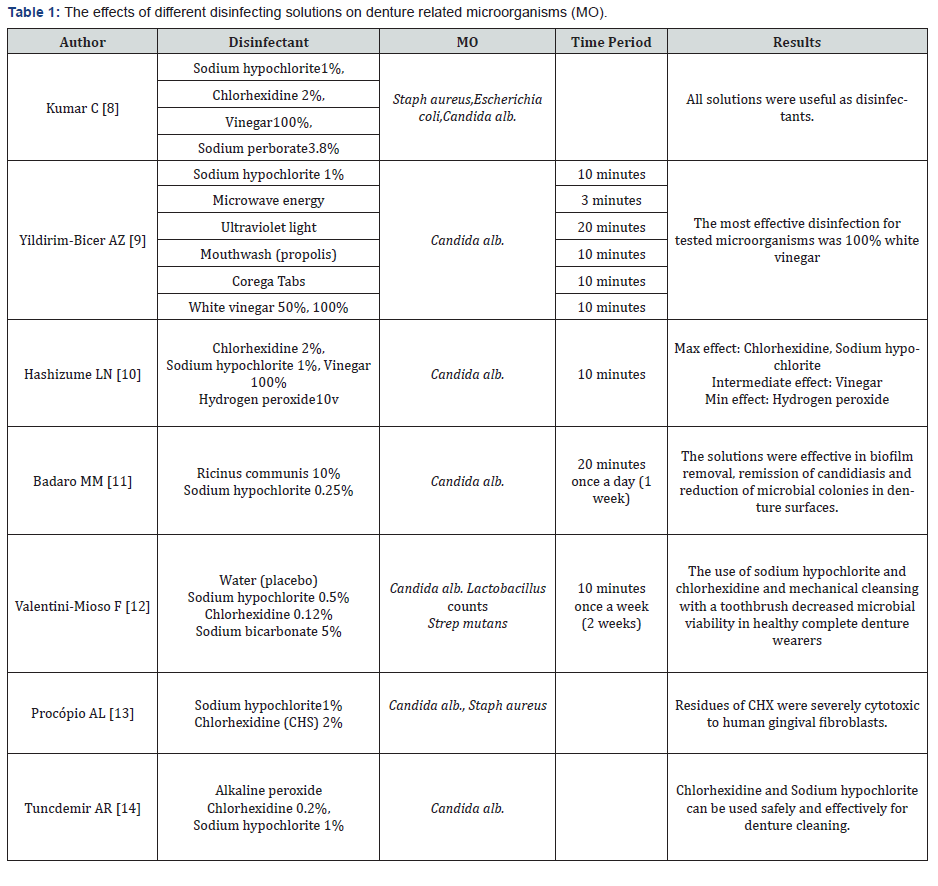
Each suggested material has its own advantages and problems. Among the most commonly used solutions, the benefits of organic vinegar are its lower price, availability, and lower toxicity [8,9]. Chlorhexidine 0.2% is an antimicrobial agent, commonly used in dentistry. It has low systemic toxicity and price; however, the adverse effects include mucosal erosion, unilateral or bilateral parotid swelling, and bitter taste that cannot be eliminated [8,9]. Hypochlorite alkaline solution has also shown good results for disinfecting the denture. It has anti-bacterial and antifungal properties, but in long-term use, may change the color of the denture base and corrode the denture metallic elements [15,17]. Several studies have evaluated the effect of different concentrations of various solutions on eliminating denture microorganisms; the results of studies are summarized in Table 1. [8-14]
Increased concentrations of disinfecting solutions could increase the effectiveness of antiseptic agents, but at the same time, increase the material side effects. The present study aimed to evaluate the effectiveness of lower concentrations of easily accessible materials commonly used for denture disinfection, on most common microorganisms contaminate acrylic denture bases. The null hypothesis was that these materials could effectively eliminate the microorganisms without any significant difference.
Materials and Methods
188 rectangular acrylic specimens were made in the same shape, size, and thickness (17×6×1 mm), and processed (KAVO EWL 5518, Automatic curing device, Germany) at 70°C for 9 hours using standard procedure recommended by manufacturer. The acrylic specimens were immersed in a sterile distilled water container; and autoclaved at 121°C for 15 minutes. Two specimens were transferred to sterilized culture media (Brain-Heart Infusion Broth, Merk, type 110493, Germany) to validate sterilization accuracy. After 24 hours incubation at 37 °C the specimens were evaluated for turbidity and cultivate in blood agar. Turbidity is the quality of being cloudy or hazy caused by particles basically invisible to naked eyes. Turbidity was used as a criterion to assess the microbial infection [18]. Any growth at this stage, called for revision and repeating the sterilization procedure.
The remaining 186 specimens were divided into three groups (n=62); and each group was transferred to one of three microorganism suspension to form an experimental biofilm. After 24 hours, the specimens were removed from the suspension and washed with sterile water. Two specimens from each group were transferred to the Brain-Heart Infusion (BHI) medium to ensure the expected contamination; two samples from each mold were needed to demonstrate the solutions’ efficiency and confirm whether the molds had been disinfected [19]. After 24 hours incubation at 37 °C and cultivation in a blood agar, the turbidity was evaluated. Each group was redistributed into four subgroups (n=15), that were immersed in sterile containers containing either sodium hypochlorite 0.5%, chlorhexidine 0.2%, vinegar 100%, or water (as control). The reasons of using these disinfecting materials are the prevalence and availability for patients [20].
After validation of sterilizing procedure, fresh colonies of standard strain of 3 microorganisms were prepared with the concentration of 0.5 McFarland (1.5×108cells/mL): Staphylococcus aureus (ATCC29213), Streptococcus mutans (ATCC1683), and Candida albicans (ATCC10231).
Five specimens were randomly selected from each subgroup at 30 minutes, 2 hours, and 4 hours, rinsed, and immersed in 10cc of BHI liquid culture medium. The randomly selected specimens were incubated (Brinsea OvaScope Egg Viewer, Titusville, FL, USA) at 37°C for 24 hours, and the turbidity results were recorded. In order to ensure the accuracy of results, the specimens were also cultured in blood agar solid medium. The procedure was performed using loop, next to the flame, observed the preventive principles. The media were incubated at 37 °C for 24 hours and the results were recorded (finding colonies on the crop line indicated the presence of microorganisms while the absence was an indicator for acceptable disinfection). The results were recorded as growth (+) or non-growth (-) of three microorganisms (Staph aureus, Strep mutans, and Candida albicans) in the culture medium
SPSS 23 was used for data analysis (P<0.05), and Chi square and Fisher exact test were applied to compare the results.
Results
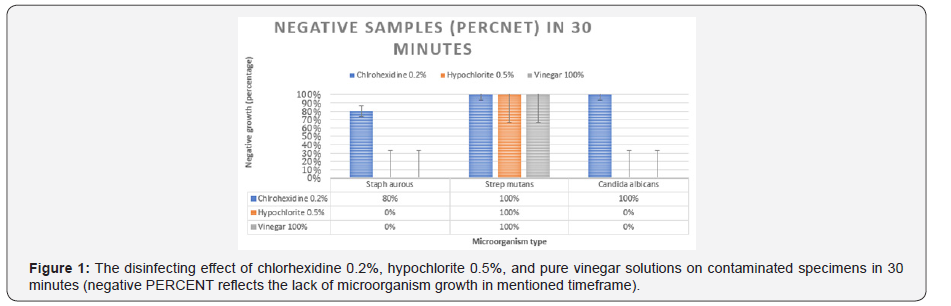
Figure 1 summarizes the results after 30 minutes: Chlorhexidine 0.2% was significantly more effective than hypochlorite 0.5% and pure vinegar in eliminating Candida albicans (P=0.008). Chlorhexidine 0.2% was more effective on Staph aureus (80% disinfection) compared to two other solutions (0% disinfection) (P=0.048). There was no significant difference in the effect of three disinfectants on Strep mutans (100% disinfection) (P= 1.00 for comparison between Chlorohexidine and Vinegar, P= 1.00 for comparison between Chlorohexidine and Hypochlorite).
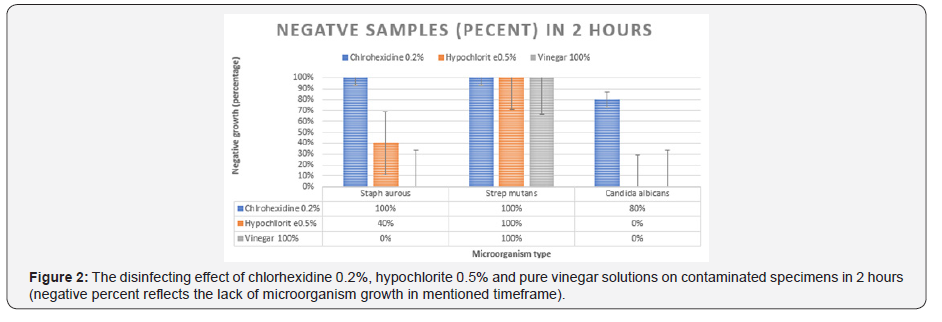
Figure 2 shows the materials effects after two hours: Chlorhexidine 0.2% eliminates 80% of Candida albicans colonies, that was significantly more than pure vinegar and hypochlorite 0.5% (P=0.44). The effect of chlorhexidine 0.2% on Staph aureus (100% disinfection) was significantly more than hypochlorite (40% disinfection) (P=0.008). However, all three solutions were completely effective on Strep mutans after 2 hours (100% disinfection).
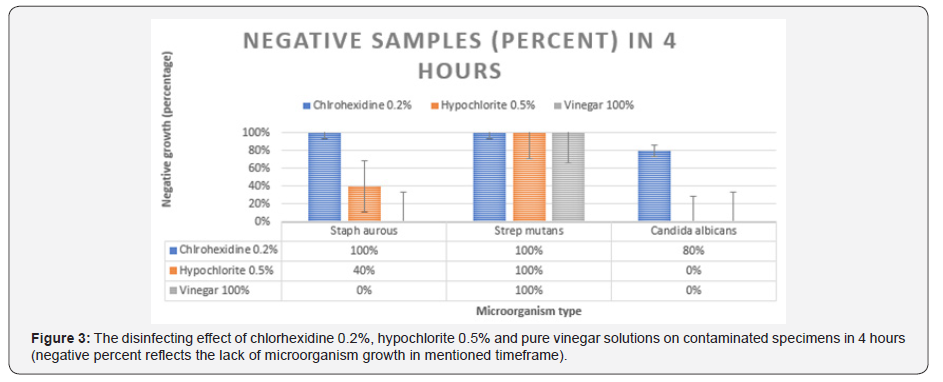
Figure 3 summarized the effects after 4 hours: Chlorhexidine 0.2% was more effective on Candida albicans and Staph aureus microorganisms (P= 0.048 for comparison between Chlorohexidine and Vinegar, P= 0.048 for comparison between Chlorohexidine and Hypochlorite). All three solutions showed acceptable effect on Strep mutans, and there was no statistically significant difference (Negative percent in all solutions= 100%) (P= 1.00).
Discussion
The overall resistance of the body to infectious diseases reduces with the age, and the importance of prevention increases consequently [13]. On the other hand, local infections can play an important role in systemic complications [2-5], and removable prostheses could be an active source of such infectious contaminations. The clinicians prefer to recommend safe, accessible, easily applicable, and less expensive disinfectants in low concentrations as preventive approach for decontamination of removable prostheses to increase patient cooperation and reduce the reverse effects. Strep mutans, Staph aureus, and Candida albicans are among the most prevalent microorganisms that could result in health problems in old age [6,14]. The present study aimed to evaluate the effectiveness of low concentration of prevalent and routine disinfectant solutions on disinfecting acrylic part of prostheses from these common microorganisms at different timeframes (30 minutes, 2 hours, and 4 hours).
Based on the results, pure vinegar, hypochlorite 0.5%, and chlorhexidine 0.2% were completely effective on Strep mutans in all timeframes. Therefore, 30-minute immersion in one of these disinfectants is enough to eliminate Strep mutans disinfection. When it came to Staph aureus bacteria, pure vinegar did not show any significant effect; Chlorhexidine 0.2% had ascending effect and eliminates 80% of Staph aureus colonies in 30 minutes, that increased to 100% at 2 and 4 hours. Hypochlorite 0.5% did not show an acceptable effect on these bacteria at 30 minutes; however, it eliminates 40% of colonies in 2 and 4 hours. Therefore, in order to eliminate this resistant bacterium from acrylic prostheses, Chlorhexidine 0.2% can be recommended for 30 minutes or more; while, Hypochlorite 0.5% should be applied for at least 2hours.
Candida albicans impregnated specimens, were only surrendered to chlorhexidine 0.2%, and this solution showed complete effectiveness at 30 minutes. As this microorganism causes the most common complication in denture wearers (denture stomatitis), the application of chlorhexidine 0.2% for 30 minutes or more could be effective and reliable [3,4].
In one study, Lavania examined the effectiveness of sodium hypochlorite 1%, chlorhexidine 2%, vinegar 100%, and sodium perborate 3.8% on disinfecting acrylic specimens infected with Staph aureus, Escherichia coli, and Candida albicans in a timeframe of 10 minutes. The source of microorganisms in the study was clinical specimens of patients [8]; In the present study, fresh colonies from international standard strains were used, and, the study was performed with lower materials concentrations as the higher solutions’ concentration could result in more damage to the prosthesis components. The present investigation results showed that vinegar cannot be considered as an acceptable prosthesis disinfectant. However, Chlorohexidine 0.2%, and Hypochlorite 0.5% could provide enough effect on eliminating microorganisms in 2 hours, and Chlorohexidine 0.2% is acceptable and reliable disinfectant for common denture infectious contaminations.
Several agents have been suggested for prosthesis disinfection like castor oil [15], glutaraldehyde [16], vinegar [8], sodium hypochlorite [11], and chlorohexidine [14]. Each has its own advantages and problems. Knowing about the material efficiency, commercially availability, and the least effective concentration/ time along with the cognition of material characteristics is necessary to prescribe a patient specific material. More investigations focused on the least effective concentration/time for sodium hypochlorite and chlorohexidine considering the material effects on mechanical and physical properties of denture is suggested.
Conclusion
Considering the limitations of the present study, the following conclusions can be made: 1. Chlorhexidine 0.2% was significantly more effective than hypochlorite 0.5% and pure vinegar in eliminating Candida albicans (P=0.008 in 30 mins, and P=0.44 in 2 hours). 2. In 30 minutes, Chlorhexidine 0.2% was significantly more effective on Staph aureus compared to two other solutions (P=0.048). 3. Chlorhexidine 0.2% was more effective on Candida albicans and Staph aureus microorganisms at 4-hours timeframe (P<0.05). 4. There was no significant difference in the effect of three disinfectants on Strep mutans (P>0.05). 5. Chlorhexidine 0.2% was the most effective option between disinfecting solutions tested. 6. Chlorhexidine 0.2% caused complete elimination of Strep mutans and Candida albicans after 30 minutes, and Staph aureus after 2 hours. 7. Hypochlorite 0.5% was 100% effective on Strep mutans after 30 minutes and eliminated 40% of Staph aureus colonies after 2 hours. 8. Pure vinegar showed the least effectiveness as disinfectant material and was effective only on Strep mutans (100%).
References
- Leite VM, Pinheiro JB, Pisani MX, Watanabe E, de Souza RF, et al. (2014) In vitro antimicrobial activity of an experimental dentifrice based on Resinus communis. Braz Dent J 25(3): 191-196.
- Hibino K, Samaranayake LP, Hägg U, Wong RW, Lee W (2009) The role of salivary factors in persistent oral carriage of Candida in humans. Arch Oral Biol 54(7): 678-683.
- Atashrazm P, Sadri D (2011) Prevalence of oral mucosal lesions in a group of Iranian dependent elderly complete denture wears. J Contemp Dent Pract 14(2): 174-178.
- Salerno C, Pascale M, Contald M, Esposito V, Busciolano M, et al. (2011) Candida-associated denture stomatitis. Med Oral Patol Oral Cir Bucal 16(2): 139-143.
- Kossioni AE (2011) The prevalence of denture stomatitis and its predisposing conditions in an older Greek population. Gerodontology 28(2): 85-90.
- Sharaffedin F, Sadeghi AR, Kohanteb G (2005) Comparison of the effect of Deconex (solarsept), Micro10 and Cidex in disinfecting dental instruments. J Dent Shiraz univ Med Sci 6(1,2): 38-46.
- Jnanadev KR, Satish Babu CL, Shilpa Shetty S, Surendra Kumar GP, Sheetal HS (2011) Disinfecting the acrylic resin plate using electrolyzed acid water and 2% glutaraldehyde: a comparative microbiological study. J Indian Prosthodont Soc 11(1): 36-44.
- Lavanya, Kumar C (2015) Study to Assess the Disinfection of Dental Prostheses to Clear Locally Prevalent Microbial Strains. Journal of Evidence Based Medicine and Healthcare 2(46): 8304-8307.
- Yildirim-Bicer AZ, Peker I, Akca G, Celik I (2014) In vitro antifungal evaluation of seven different disinfectants on acrylic resins. BioMed research international 519098: 9.
- Hashizume LN, Hosharuk MF, Morira MJS (2015) Effect of affordable disinfectant solutions on Candida albicans adhered to acrylic resin for dental prosthesis. RGO Rev Gaúch Odontol 3(63): 309-314.
- Badaro MM, Salles MM, Leite VM, Arruda CN, Oliveira VD, et al. (2017) Clinical trial for evaluation of Ricinus communis and sodium hypochlorite as denture cleanser. J Appl Oral Sci 25(3): 324-334.
- Valentini-Mioso F, Maske TT, Cenci MS, Boscato N, Pereira-Cenci T (2018) Chemical hygiene protocols for complete dentures: A crossover randomized clinical trial. J Prosthet Dent 121(1): 83-89.
- Procópio AL, da Silva RA, Maciel JG, Sugio CY, Soares S, et al. (2018) Antimicrobial and cytotoxic effects of denture base acrylic resin impregnated with cleaning agents after long-term immersion. Toxicology in Vitro 52: 8-13.
- Tuncdemir AR, Uğur AR, Özdemir B, Kahraman KT (2018) Antimicrobial Activity of Different Solutions on Denture Base Materials. J Pediatr Infect Dis 13(4): 308-312.
- Silva CHL, Salles MM, Oliveria VC, Souza RF, Paranhos HFO (2015) Antimicrobial action of sodium hypochlorite and castor oil solutions for denture cleaning - in vitro Braz Oral Res 29(1): 1-6.
- Ebadian B, Pursina F, Saghaee S (2007) In Vitro Evaluation of the Atimicrobial Effect of Two Disinfectant Chemical Solutions on Conventional Heat- Cured AcrylicDenture Base Resin. Mashhad Journal of dental research 31(3): 217-222.
- Lima EM, Moura JS, Del Bel Cury AA, Garcia RC, Cury JA (2006) Effect of enzymatic and NaOCl treatments on acrylic roughness and on biofilm accumulation. J Oral Rehabil 33(5): 356-362.
- Abu BA, Md Rasol R, Nordin Y, Md Noor N, bin Mohd Ali MK (2015) Turbidity Method to Measure the Growth of Anaerobic Bacteria Related to Microbiologically Influenced Corrosion. Trans Tech Publications 227: 298-301.
- Nascimento PL, Ribeiro RB, Gadê-Neto CR, Dias AH (2015) Incorporation of disinfectants for obtaining dental stone: microbiological and dimensional evaluation. Rev odontol UNESP 44(1): 24-30.
- Salvia AC, dos Santos Matilde F, Rosa FC, Kimpara ET, Jorge AO, et al. (2013) Disinfection protocols to prevent cross-contamination between dental offices and prosthetic laboratories. Journal of infection and public health. 6(5): 377-382.






























