Curcumin Effect on Nitropropane Alterations on the IL-1β, Tnfα & IL 6 in Submandibular Gland of Mice
Badawy M Abd El Hamied1* and Atef I Ahmed2
1Department of Oral Biology, Assiut University, Egypt
2Department of Oral Biology, Al-Azhar University, Egypt
Submission: April 08, 2019; Published: May 16, 2019
*Corresponding author: Badawy M Abd El Hamied, Lecturer, Department of Oral Biology, Faculty of Dentistry, Assiut University, Egypt
How to cite this article: Badawy M Abd El Hamied, Atef I Ahmed. Curcumin Effect on Nitropropane Alterations on the IL-1β, Tnfα & IL 6 in Submandibular Gland of Mice. Adv Dent & Oral Health. 2019; 10(5): 555800. DOI: 10.19080/ADOH.2019.10.555800
Abstract
Objectives: 2-Nitropropane (2-NP) is a rat liver carcinogen. Curcumin has shown some therapeutic activities. This study aimed to investigate if curcumin has a synergistic effect on the 2-NP alterations on the IL-1β, IL 6 & TNFα in submandibular gland of Mice.
Methods and materials: The gene expression levels of TNF-α, IL-1β & IL-6 were tested in submandibular gland of mice by RT-qPCR. 30 adult BALB/c male mice were divided randomly into 3 equal groups. Group I, a control group (n=10), injected with vehicle alone (canola oil, 5mL/kg). The latter two groups (n=20) by intraperitoneal injection of 2-NP (100mg/kg body weight dissolved in canola oil) 2 time/week for 4 weeks. At the same time of 2-NP injection, mice of group III were orally drinking water supplemented with curcumin 50(Mg/Ml)/4weeks. Submandibular gland tissues were histologically studied.
Results: the gene expression analysis showed a statistically significant upregulation of TNF-α, IL-1β, and IL-6 in group II. While, group III showed significantly downregulated expression. The apparent histopathological changes manifested in group II was less in group III.
Conclusions: Curcumin may modulate the alterations of 2-NP by down-regulating proinflammatory cytokines IL-1β, IL6 and TNF α in BALB/c mice.
Keywords: Curcumin; 2-Nitropropane; IL-1; IL-6; TNFα; Submandibular salivary glands
Introduction
The submandibular glands are major salivary glands located beneath the floor of the mouth. Interleukins are a group of cytokines that expressed by leukocytes [1]. IL1 β is participating in the regulation of immune responses, inflammatory reactions, and hematopoiesis [2]. IL-6 plays a role in the final differentiation of B cells into immunoglobulin-secreting cells [3]. Several genetic modifications or mutations associated with dysregulated IL-1 activity and autoinflammatory disorders were identified [4]. Expression of IL-1 is up regulated in different tumor phenotypes and is implicated as an important factor in tumor progression via expression of metastatic, angiogenic genes and growth factors. Therefore, down regulation of expression of IL-1 may be able to inhibit cancer progression [5]. The miR146a and miR147b were associated with increased expression of genes related to the immune/inflammatory response. Overexpression of miR147b reduced the expression of the pro-inflammatory mediators IL-6 after IL-1β stimulation [6].
Nitropropane (2-NP) is a colorless, oily liquid with a mild odor. It is used as a solvent, principally in blends, and has many industrial applications [7]. Previously, severe liver damage, as well as some kidney damage, has been observed in workers fatally poisoned from acute inhalation exposure to high concentrations of 2-nitropropane [8]. The results indicated that 2-NP inflicted DNA damage in the bone marrow cells and thus could be leukemogenic [9]. Studies were carried out in the rat indicated that 2-NP induced chromosome aberrations as well as DNA repair in vivo [10].
Curcumin and its two related compounds are the main secondary metabolites of Curcuma longa [11]. Curcuma longa has been used in herbal medicines for the treatment of a variety of ailments such as rheumatism, diabetic ulcers, anorexia, cough and sinusitis. It has also been shown to have significant wound healing properties [12].
It has been reported to have anti-inflammatory, antioxidant, anti-rheumatoid, and anti-atherosclerosis role. It can also reduce lipid, eliminate free radicals and inhibit the growth of the tumor. Many reports had suggested that curcumin has shown great potential in the treatment of tumors by inducing apoptosis. Use of curcumin mouthwash has shown antiplaque and antigingivitic properties. It could be effectively used as an adjunct to scale and root planning [13]. Also, it showed that ultra-soluble curcumin could prove useful as a therapeutic intervention in Sjögren’s syndrome [14]. Also, it inhibited IκB kinase β kinase activity in the saliva of head and neck squamous cell carcinoma cancer, and this inhibition correlated with reduced expression of several cytokines [15].
The aim of this study was to investigate if curcumin has a synergistic effect on the nitropropane alterations and the role of IL-1β, IL 6 and TNFα.
Material and Methods
Experimental design and doses
A total of 30 adult BALB/c male mice weighting 25-30g/each were used in this experiment. Mice were purchased from the Institute of Theodor Bilharz (Cairo, Egypt).
Ethical considerations
All experimental animals maintained and monitored in a specific pathogen-free environment. All experimental animal protocols were performed according to regulations set by the Institutional Animal Care and Use Committee and were approved by ethical committee of faculty of veterinary medicine, Assiut University (approved by faculty council number 4, at 25/4/2018). All animal procedures were also performed according to the Declaration of Helsinki and the guidelines for the care and use of experimental animals established by the National Institutes of Health (NIH). All animals could acclimatize in plastic cages (five animals per cage) inside a well-ventilated room for one week prior to the experiment. The animals were maintained under standard laboratory conditions (temperature of 23 °C, relative humidity of 60-70%, and a 12h light/dark cycle) and were fed a diet of standard commercial pellets and water containing libitum. We made every effort to minimize animal stress.
Grouping and experimental planning
After 1 week of acclimatization, mice were randomly categorized into three main groups (10 mice each).
a. Group I (control mice).
b. Group II (2- Nitropropane inject group mice).
c. Group III (2- Nitropropane inject group treated with curcumin).
Oral toxicity was induced in mice in the latter two groups (n = 20) by intraperitoneal (i.p.) injection of 2- Nitropropane (100mg/ kg body weight dissolved in canola oil) 2 time/week for 4 weeks; mice in the control group were injected with vehicle alone (canola oil, 5mL/kg). At the same time of 2- NP injection, mice were orally drinking water supplemented with curcumin 50 (Mg/Ml)/ 4 weeks.
Sample collection
All animals were sacrificed at day 31 post- 2- NP injection. The submandibular salivary gland was removed and cut into small pieces in sterile saline. The pieces were suspended in Trizol for RNA extraction and gene expression analysis.
Histopathological analysis
The salivary gland pieces fixed in 10% formalin were microtechnically processed in order to obtain paraffinized tissue sections. The tissue sections were stained with hematoxylin and eosin (H&E) for morphological examination and viewed under light microscope.
Gene expression analyses
The effect of curcumin administration of both on the expression of a panel of selected genes involved in the inflammation and toxicity of oral mucosa were investigated using real-time quantitative PCR.
Primers used in RT-PCR
The primers used for quantitative real-time PCR analysis were designed using the Primer Express 1.5 software (Applied Biosystems). The mouse primers were designed as following:
a. TNF-α Forward: 5′- ATGAGCACAGAAAGCATGA-3′, Reverse 3′-AGTAGACAGAAGAGCGTGGT- 5′.
b. IL-6 Forward: 5′- ACCGAGCTCTGTTGACAAG-3′, Reverse 3′- TCCTCGCCACACTTCTCTTT- 5′.
c. IL-1β Forward: 5′- GCACTACAGGCTCCGAGATGAAC-3′, Reverse 3′ TTGTCGTTGCTTGGTTCTCCTTGT- 5′ and normalized using GAPDH Forward: 5′- GTTGTCTCCTGCGACTTCA-3′, Reverse 3′- GGTGGTCCAGGGTTTCTTA - 5′.
tissues
After performing the indicated treatments, RNA was extracted and then the corresponding cDNA was prepared, and then real time PCR was applied for gene expression analysis as previously described [16]. In brief, all cDNA samples were processed in a 96- well plate using the following cycling conditions: 10 minutes at 95℃, and 40 cycles at 95℃ for 15 seconds ended by one min. at 60℃. These data were analyzed according to Livak et al. [17].
Statistical analysis
We used one-way analysis of variance ANOVA for determination of the statistical significance of differences between mean values. A probability of ≤ 0.05 defined this significance.
Results
Histopathological results
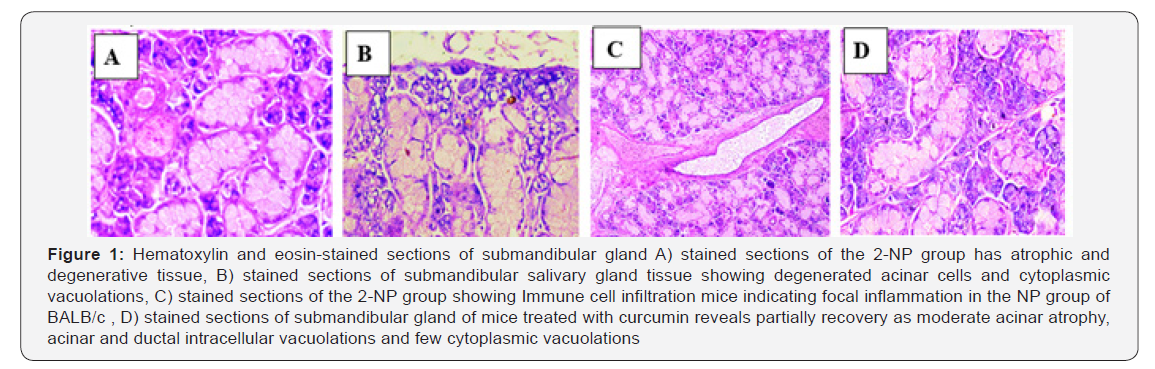
Microscopically, the submandibular glands of BALB/c male mice of the control group appeared with a well-known histologic feature with no observable lesions. Mice in 2- Nitropropane treated group appeared with marked atrophy and degeneration of the acinar cells and ducts (Figure 1A). Glands in this group showed also cytoplasmic vacuolations (Figure 1B). Ductal inflammatory cell foci in the submandibular glands were apparent in this group (Figure 1C). In animals treated with curcumin there was apparent improvement from the 2-NP deleterious effect. The acinar architecture of the gland appeared near normal (Figure 1D). However, this reversal of 2-NP effect did not acquire the full normal case of the gland.
Gene expression analyses results
Real time- PCR and mouse primers specific for IL-1β, IL-6 and TGFα were used to detect the presence of mRNAs. IL-1, IL-6 and TGFα could be detected in mouse submandibular gland in all groups. The data shown in Table 1 indicate a dramatic difference between different study groups. The means of IL-1β, IL-6 and TGFα levels demonstrated a significant increase in mice with 2-NP when compared to controls (p<0.001). In contrast, use of curcumin revealed that the relative mRNA levels of the IL-1β, IL-6 and TGFα decreased significantly compared with the 2-NP treated mice (Figures 2-4).
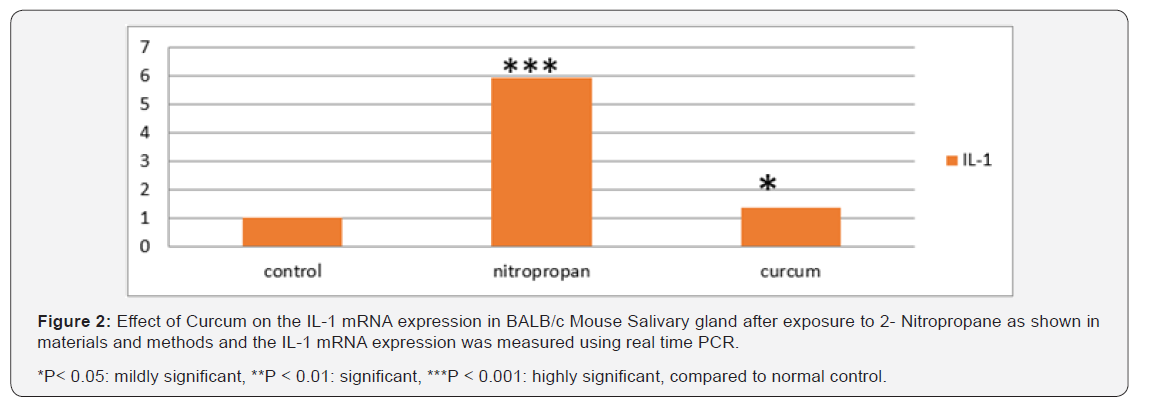
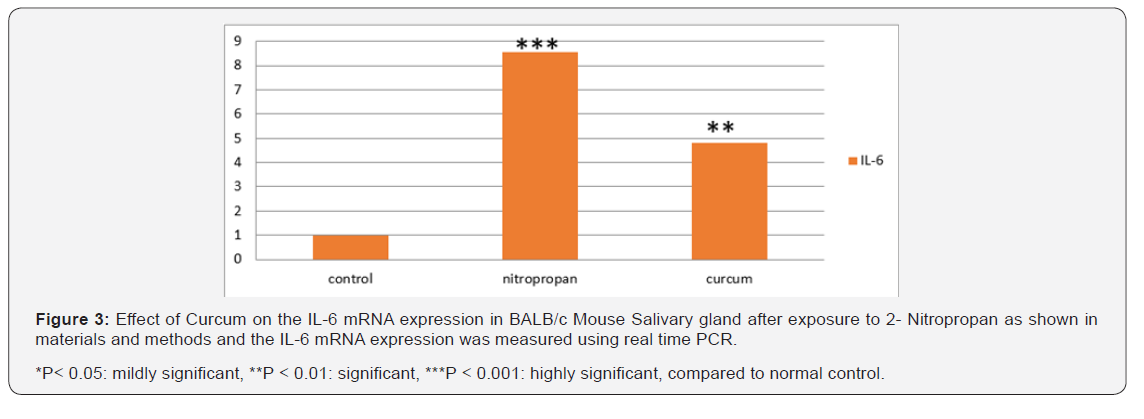

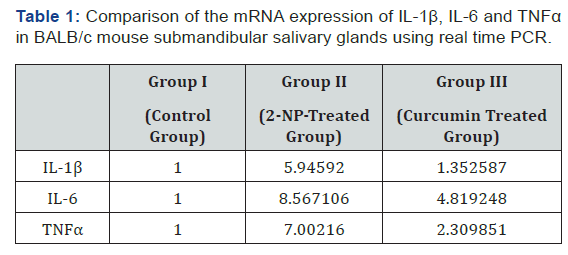
Figure 2 shows that expression of mRNA for IL-1β genes steadily increased with the administration of 2-NP (P < 0.001). Significantly lower levels were observed with curcumin treatment P< 0.05. As shown in Figure 4, the expression of IL-6 in tissue samples was significantly lower after curcumin. The differences in mean IL-6 expression appeared statistically significant, with a P<0.01. However, tissue expressions of IL-6 in 2-NP treated group had risen. As shown in Figure 4 expressions of TNFα were significantly lower (P< 0.05) after curcumin administration.
Discussion
Nowadays there is great evidence of the possible health benefits of curcumin, but little is known about the effects on the salivary glands. In the present work it was investigated that the expression levels of IL-1β, IL-6 and TNF-α in the submandibular glands of BALB/c mice were significantly higher in the 2-NP treated animals compared to the control group. The histopathologic analysis stated that the acinar cells were atrophic and degenerated. In addition, these cells showed cytoplasmic vacuolations and ductal inflammatory cell foci indicating focal inflammation. These changes were comparable to those demonstrated in other studies [18,19]. It was suggested that the liver damage induced by 2-NP is related to oxidative damage, lipid peroxidation [20]. Previous results indicated that 2-NP inflicted DNA damage in the bone marrow cells [9] and induced chromosome aberrations as well as DNA repair [10]. In contrast, the findings of this study suggested that the expression levels of IL-1 IL-6 and TNF-α in the submandibular glands from mice treated with curcumin were significantly decreased. Histologically, there was partial improvement from the 2-NP deleterious effect.
These results may be correlated with several studies which evidenced possible health benefits of curcumin. A few studies had shown that curcumin can be prevention and a chemotherapeutic agent for colon, skin, oral and intestinal cancers. Curcumin was also well known for its ant inflammatory and antioxidant, anticoagulant and anti-infective effects [12,21,22]. The anticancer effect of curcumin is attributed to activation of apoptotic pathways in cancer cells, as well as inhibition of inflammation and angiogenesis in the tumor microenvironment and suppression of tumor metastasis. Several reports had demonstrated that curcumin inhibits angiogenesis in a wide variety of tumor cells through the modulation of various cell signaling pathways which involve transcription factors, protein kinases, growth factors and enzymes [23]. Oral administration of curcumin beneficially modulates many diseases including diabetes, fatty liver disease, atherosclerosis, arthritis, cancer and neurological disorders [24]. Curcumin given 24 hours before irradiation reduced the structural damage to the salivary glands [25].
In respect to the role of cytokines, expression of IL-1 is up regulated in different tumor phenotypes and is implicated as an important factor in tumor progression via expression of metastatic, angiogenic genes and growth factors. Therefore, down regulation of expression of IL-1 may be able to inhibit cancer progression [5].
Similarly, Interleukin 6 (IL6) and TNF α are involved in a wide variety of biological functions. Tumor necrosis factor receptor-1 (TNFR1) is involved in apoptosis through extrinsic pathway initiation. The level of soluble TNFR1 is reported increased in primary Sjögren’s syndrome patients [26].
Furthermore, it was found that stimulation with IL-1β and TNF-α increased submucosal gland secretion in a concentrationdependent manner. The cytokine effect was dependent on cAMP. Most importantly, IL-1β- and TNF-α-stimulated secretion was blocked by the cystic fibrosis transmembrane conductance regulator (CFTR) blocker. Also, during bacterial infections and resulting release of proinflammatory cytokines, the glands are stimulated to secrete fluid, and this response is mediated by cAMP activated CFTR, a process that would fail in patients with CF [27]. It was demonstrated that IL6 pretreatment prevented both senescence and salivary gland hypofunction via a mechanism involving enhanced DNA damage repair. It was indicated that cellular senescence is a fundamental mechanism driving radiationinduced damage in the salivary gland and suggested that IL6 pretreatment may represent a promising therapeutic strategy to preserve salivary gland function in head and neck cancer patients undergoing radiotherapy [28].
Additionally, higher expression of IL-6 was found in salivary gland cancer. There was a high association of cytomegalovirus CMV antigen presence with the presence of IL-6, and with the IL-6 expression intensity. Positive expression of CMV antigens in a high percentage of SGC cells suggested that it might play an important role in carcinogenesis by increasing IL-6 production and leading to inhibition of apoptosis and tumor development [29].
Likewise, protein expression levels of IL-17 and IL-6 were detected parotids and submandibular glands by ELISA. Compared with the normal group, mRNA transcriptional levels and protein expression levels of IL-17 and IL-6 were significantly up-regulated after administration of deionized water [30].
In This study there was a strong association between administration of curcumin and the expression levels of IL-1β, IL-6 and TNF-α which can be interpreted by many authors. Elevated expression and protein level of IL-1α and IL-1β were found in lymphoma bearing mice, which were significantly down regulated by curcumin treatment. Similarly, curcumin treatment down regulated activation of IL-1α and IL-1β via AP-1 and NF-IL-6 respectively (5). It has been shown to interfere with multiple cell signaling pathways, including cell cycle, apoptosis, proliferation, survival, invasion, angiogenesis, metastasis and inflammation [31].
Finally, the findings of this study conclude that curcumin may attenuate the alterations of 2-NP by down-regulating proinflammatory cytokines IL-1β, IL6 and TNF α in BALB/c mice.
References
- Brocker C, Thompson D, Matsumoto A, Nebert DW, Vasiliou V (2010) Evolutionary divergence and functions of the human interleukin (IL) gene family. Hum Genomics 5(1): 30-55.
- Sims JE, March CJ, Cosman D, Widmer MB, MacDonald HR, et al. (1988) cDNA expression cloning of the IL-1 receptor, a member of the immunoglobulin superfamily. Science 241(4865): 585-589.
- Lütticken C, Krüttgen A, Möller C, Heinrich PC, Rose-John S (1991) Evidence for the importance of a positive charge and an alpha-helical structure of the C-terminus for biological activity of human IL-6. FEBS Lett 282(2): 265-267.
- Palomo J, Dietrich D, Martin P, Palmer G, Gabay C (2015) The interleukin (IL)-1 cytokine family--Balance between agonists and antagonists in inflammatory diseases. Cytokine 76(1): 25-37.
- Das L, Vinayak M (2014) Curcumin attenuates carcinogenesis by down regulating proinflammatory cytokine interleukin-1(IL-1α and IL- 1β) via modulation of AP-1 and NF-IL6 in lymphoma bearing mice. Int Immunopharmacol 20(1): 141-147.
- Van Scheppingen J, Mills JD, Zimmer TS, Broekaart DWM, Iori V, et al. (2018) miR147b: A novel key regulator of interleukin 1 beta-mediated inflammation in human astrocytes Glia 66(5): 1082-1097.
- Andrae U, Homfeld H, Vogl L, Lichtmannegger J, Summer KH (1988) 2-Nitropropane induces DNA repair synthesis in rat hepatocytes in vitro and in vivo. Carcinogenesis 9(5): 811-815.
- U S Environmental Protection Agency (1999) Integrated Risk Information System (IRIS) on 2-Nitropropane. National Center for Environmental Assessment, Office of Research and Development, Washington, DC, USA.
- Deng XS, Tuo J, Poulsen HE, Loft S (1997) 2-Nitropropane-induced DNA damage in rat bone marrow. Mutat Res 391(14): 165-169.
- George E, Burlinson B, Gatehouse D (1989) Genotoxicity of 1- and 2-nitropropane in the rat. Carcinogenesis 10(12): 2329-2334.
- Lestari M, Indrayanto G (2014) Curcumin. Profiles Drug Subst Excip Relat Methodol 39: 113-204.
- Akbik D, Ghadiri M, Chrzanowski W, Rohanizadeh R (2014) Curcumin as a wound healing agent. Life Sci 116(1): 1-7.
- Chatterjee A, Debnath K, Rao NKH (2017) A comparative evaluation of the efficacy of curcumin and chlorhexidine mouthrinses on clinical inflammatory parameters of gingivitis: A double-blinded randomized controlled clinical study. J Indian Soc Periodontol 21(2): 132-137.
- Kurien BT, Harris VM, Quadri SM, Coutinho-de Souza P, Cavett J, et al. (2015) Significantly reduced lymphadenopathy, salivary gland infiltrates and proteinuria in MRL-lpr/lpr mice treated with ultrasoluble curcumin/turmeric: increased survival with curcumin treatment. Lupus Sci Med 2(1): e000114.
- Kim SG, Veena MS, Basak SK, Han E, Tajima T, et al. (2011) Curcumin treatment suppresses IKKβ kinase activity of salivary cells of patients with head and neck cancer: a pilot study. Clin Cancer Res 17(18): 5953- 5961.
- Ismail IA, Kang HS, Lee HJ, Chang H, Yun J, et al. (2013) 2-Hydroxycinnamaldehyde inhibits the epithelial-mesenchymal transition in breast cancer cells. Breast Cancer Res Treat 137(3): 697- 708.
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4): 402-408.
- Soames JV, Southam JCJV (1999) Oral pathology (3rd edn), Oxford: Oxford Univ Press, UK, pp. 247-265.
- Bauchinger M, Kulka U, Schmid E (1987) Analysis of cytogenetic effect in human lymphocytes induced by metabolically activated 2-nitropropane. Mutation Research 190(3): 217-219.
- Borges LP, Nogueira CW, Panatieri RB, Rocha JB, Zeni G (2006) Acute liver damage induced by 2-nitropropane in rats: effect of diphenyl diselenide on antioxidant defenses. Chem Biol Interact 160(2): 99-107.
- Zheng BZ, Liu TD, Chen G, Zhang JX, Kang X (2018) The effect of curcumin on cell adhesion of human esophageal cancer cell. Eur Rev Med Pharmacol Sci 22(2): 551-560.
- Marchiani A, Rozzo C, Fadda A, Delogu G, Ruzza P (2014) Curcumin and curcumin-like molecules: from spice to drugs. Curr Med Chem 21(2): 204-222.
- Shakeri A, Ward N, Panahi Y, Sahebkar A (2018) Anti-angiogenic activity of curcumin in cancer therapy: a narrative review. Curr Vasc Pharmacol 17(3): 262-269.
- Ghosh SS, He H, Wang J, Gehr TW, Ghosh S (2018) Curcumin-mediated regulation of intestinal barrier function: The mechanism underlying its beneficial effects. Tissue Barriers. 6(1): e1425085.
- Lopez-Jornet P, Gómez-García F, García Carrillo N, Valle-Rodríguez E, Xerafin A, et al. (2016) Radioprotective effects of lycopene and curcumin during local irradiation of parotid glands in Sprague Dawley rats. Br J Oral Maxillofac Surg 54(3): 275-279.
- Fletes-Rayas AL, Palafox-Sánchez CA, Muñoz-Valle JF, Orozco-Barocio G, Navarro-Hernández RE, et al. (2016) TNFR1-383 A˃C polymorphism association with clinical manifestations in primary Sjögren’s syndrome patients. Genet Mol Res 15(2).
- Baniak N, Luan X, Grunow A, Machen TE, Ianowski JP (2012) The cytokines interleukin-1β and tumor necrosis factor-α stimulate CFTRmediated fluid secretion by swine airway submucosal glands. Am J Physiol Lung Cell Mol Physiol 303(4): 327-333.
- Marmary Y, Adar R, Gaska S, Wygoda A, Maly A, et al. (2016) Radiation- Induced Loss of Salivary Gland Function Is Driven by Cellular Senescence and Prevented by IL6 Modulation. Cancer Res 76(5): 1170- 1180.
- Radunovic M, Tomanovic N, Novakovic I, Boricic I, Milenkovic S, et al. (2016) Cytomegalovirus induces Interleukin-6 mediated inflammatory response in salivary gland cancer. J BUON 21(6): 1530-1536.
- Lu Y, Chen Y, Wang YN, Liu H, Zhang JS, et al. (2015) Effect of Banxia Qinlian Decoction on Th17/IL-17 Immune Inflammatory Way of Sjögren’s Syndrome NOD Model Mice: Zhongguo Zhong Xi Yi Jie He Za Zhi 35(5): 612-617.
- Anand P, Sundaram C, Jhurani S, Kunnumakkara AB, Aggarwal BB (2008) Aggarwal Curcumin and cancer: An ‘‘old-age” disease with an ‘‘age-old” solution. Cancer Lett 267(1): 133-164.






























