Association Between Clinical, Para-Clinical and Environmental Indicators for Tooth Decay in Children with Pyelonephritis
Sirma Angelova*
Department of Pediatric Dentistry, Medical University-Varna, Bulgaria
Submission: March 05, 2019; Published: March 28, 2019
*Corresponding author: Sirma Angelova, Medical University-Varna, Faculty of Dental Medicine, Department of Pediatric Dentistry, Varna, Bulgaria
How to cite this article: Sirma A. Association Between Clinical, Para-Clinical and Environmental Indicators for Tooth Decay in Children with Pyelonephritis. Adv Dent & Oral Health. 2019; 10(4): 555795. DOI: 10.19080/ADOH.2019.10.555795
Abstract
Tooth decay is a multi-factorial disease. Its basic etiological factors are the enamel with its quality and quantity characteristics, cariogenic microorganisms of Streptococcus mutans and Streptococcus sobrinus, fermentable carbohydrates, time. Saliva plays the essential function of a co-factor for the dynamics of the carious process. Contemporary studies represent solid evidence of the anti-microbial effects of non-organic nitrates for some organs and systems, including the gastro-intestinal tract, oral cavity and skin. Bacterial infections of the urinary tract occur in different age, including the breast-feeding period and early childhood, and are often characterized with urine reflux dysfunction. The aim of this study is to investigate and evaluate correlations between some clinical, para-clinical and environmental indicators for tooth decay in children with pyelonephritis. Significant negative correlation between the number of carious lesions and pH and great negative correlation between the number of carious lesions and salivary nitrites have been established in the group of children with kidney disorder. These participants are characterized with extremely high positive correlation between the plaque index PLI and gingival index GI; significant negative correlation between the index of PLI and salivary pH level; significant negative correlation between PLI and salivary nitrites. There is a definite accent on the considerable interrelations between the number of cavity and non-cavity carious lesions, level of oral hygiene and gingival status, pH and nitrites in saliva and frequency of tooth-brushing in children suffering from the renal disorder of pyelonephritis.
Keywords: Clinical indicators; Environmental factors; Para-clinical parameters; Tooth decay; Pyelonephritis in children
Introduction
Bacterial infections of the urinary tract occur in different age, including the breast-feeding period and early childhood, and are often characterized with urine reflux dysfunction. This infectious process affecting the excretory system is ranked second in incidence preceded by respiratory tract infections in child’s age. Principally, the condition of fever and symptoms of anemia with undifferentiated origin is related to the disease of pyelonephritis [1]. A ratio of 4, 6 to 10% of all the hospitalized children are suffering from urinary tract infections [2]. The diagnosis of pyelonephritis in pre-school and school age is associated to symptoms of intoxication, fever attacks, paleness, pain discomfort in the lumbar-sacral region, miction-dysuric disorder, secondary enuresis [3]. In terms of established symptomatic pyelonephritis there is an urgent necessity of parenteral antibiotic therapy based on antibiogram. In cases of non-manifested symptoms can be applied combined medicines of Trimethoprim- Sulfamethoxasole (Biseptol, Trimezol). The duration of antibiotic treatment equals to 10 days, and in condition of severe infections- up to 14 days [3-5].
Tooth decay is a multi-factorial disease. Its basic etiological factors are the enamel with its quality and quantity characteristics, cariogenic microorganisms of Streptococcus mutans and Streptococcus sobrinus, fermentable carbohydrates, time. Saliva plays the essential function of a co-factor for the dynamics of the carious process [6]. If not treated, the disease of tooth decay is accompanied by specific pain symptoms, with considerably negative impact upon quality of life in the aspect of physical state, psychological and emotional comfort, behavioral patterns and social activities [7]. Tooth decay is determining as an infectious disease with behavioral samples-related nature. Among the main etiological factors for that disease is accentuated on the role of both microorganisms, Streptococcus mutans and Streptococcus sobrinus [8,9]. The process of dental plaque accumulation is associated to increase of the concentration of these acids-producing and acidophilic species with great cariogenic potential. In condition of intensive metabolism these microbes produce acids which cause decrease of the pH level and induce the destructive process of de-mineralization of enamel [10-12]. Regarding the traits of dental plaque as a medium of favorable conditions for caries, lots of investigations have been performed about the role of regular tooth-brushing procedures controlled by parents for efficient prevention of the disease. Other researches concern the necessity of utilization of additional oral hygiene products and means for ensuring proper mechanical reduction and chemical control of dental plaque [13-15].
In the last decade there has been a tendency of exponentially increased interest oriented to the time-saving and minimally invasive assays based on the properties of saliva as a diagnostic medium in clinical conditions [16-20]. In norm saliva is related to maintenance of the dynamic equilibrium of homeostasis, providing protection for hard teeth structures and soft tissues in oral cavity [21-24]. Salivary ingredients’ concentrations of calcium and phosphate ions, glycoproteins, trace of albumin, urea, ammonia, bicarbonates, immunoglobulins and enzymes influence on the dynamics of carious process [25-27]. Contemporary studies represent essential evidence of the anti-microbial effects of non-organic nitrates for some organs and systems, including the gastro-intestinal tract, oral cavity and skin [28,29].
The aim of this study is to investigate and evaluate correlations between some clinical, para-clinical and environmental indicators for tooth decay in children with pyelonephritis.
Material and Methods
Subject of the current study are 21 patients from 0 to 18 years of age, both males and females, with the diagnosis of the kidney disorder of pyelonephritis. These children were hospitalized at the Department of Pediatrics at the University Hospital “St. Marina” in Varna, Bulgaria. A control group of 10 children without common health disorders is also included into the investigation. This study received ethics approval by the Human Ethics Committee of the Medical University-Varna. Declaration of informative consent was assigned by a parent or legal guardian of each of the participants into the study.
A combination of different methods has been implemented in the context of the study. By the means of the inquiry we obtain information about the common health status of the individual, age, oral hygiene habits, diet regime with accent on intakes of sugar-containing foods and drinks. The clinical methods concern registration not only of cavity lesions of irreversible destruction of hard dental structures, but also record of no-cavity carious lesions of the type of caries incipience, indicated as D1a and D1b according to the classification system by ICDAS [10]. The level of plaque accumulation and status of gingival tissue have been evaluated by application of the indices of PLI Silness-Lȍe and GI Lȍe-Silness. Colorimetric method of determination of the level of salivary pH and nitrites was used. For assessment of interrelations between different indicators the statistical method of correlation based on the coefficient by Pearson was implemented (Figure 1 & 2).
Results
We have established definite levels of correlation between the recorded clinical indicators, salivary para-clinical parameters and environmental factors among the participants into the investigation with the diagnosis of pyelonephritis:
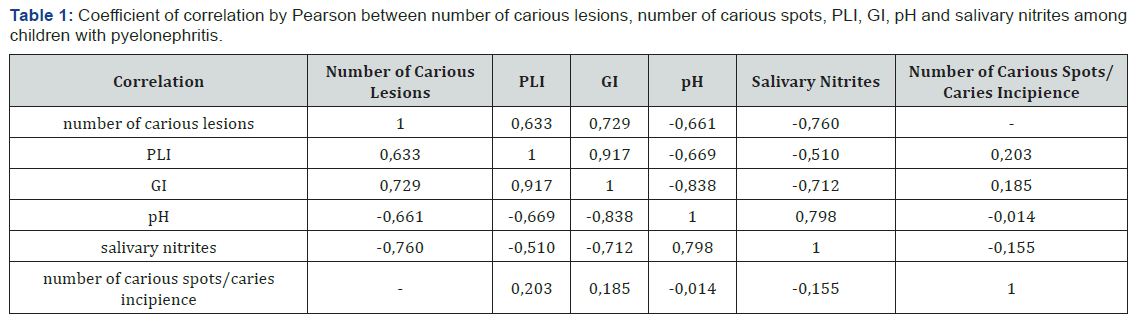
a. Significant positive correlation between the number of registered carious lesions and the plaque index of PLI; great positive correlation between the number of carious lesions and the gingival index of GI;
b. Significant negative correlation between the number of carious lesions and pH; great negative correlation between the number of carious lesions and salivary nitrites;
c. Extremely high positive correlation between the plaque index PLI and gingival index GI; significant negative correlation between the index of PLI and salivary pH level; significant negative correlation between PLI and salivary nitrites;
d. Slight positive correlation between the number of carious spots and PLI; great negative correlation between GI and salivary pH; great negative correlation between GI and salivary nitrites; great positive correlation between the level of pH and nitrites in saliva; slight negative correlation between the number of carious spots and salivary nitrites (Table 1).
Based on application of the statistical method of correlation we have registered these levels of correlation between the same indices in children not suffering from common health disorders. Namely:
a. Great positive correlation between the number of carious lesions and PLI; significant positive correlation between the number of carious lesions and GI;
b. Significant negative correlation between the number of carious lesions and pH; great negative correlation between the number of carious lesions and the content of salivary nitrites;
c. Extremely high positive correlation between PLI and GI; extremely high negative correlation between PLI and pH; extremely high negative correlation between PLI and salivary nitrites; great positive correlation between PLI and number of carious spots;
d. Great negative correlation between GI and pH; extremely high negative correlation between GI and salivary nitrites; significant positive correlation between GI and number of carious spots;
e. Extremely high positive correlation between salivary nitrites and level of pH into oral cavity;
f. Great negative correlation between the number of carious spots and pH; great negative correlation between the number of carious spots and salivary nitrites (Table 2).

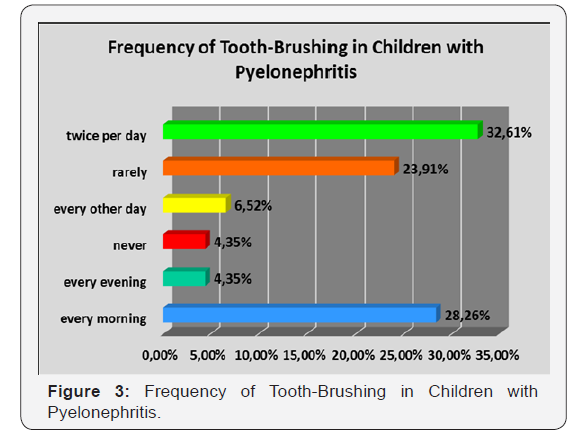
Aproximately 1/3, namely 32.61 %, of the participants suffering from pyelonephritis perform regular individual oral hygiene procedures twice per day, morning and evening before going to bed. Simultaneously, never till the moment or rarely exercise the habit of tooth-brushing a ratio of 28.26 % of all the examined children with kidney disorder (Figure 3).

In parallel, more than ¾, respectively 75.60%, of healthy children included into the study demonstrate steady and adequate oral-hygiene habits, with tooth-brushing each evening and morning. Rare procedures of tooth-brushing perform only 7.32 % of the included healthy controls (Figure 4).
Most of the children of both groups, more precisely 2/3 of these with the diagnosis of pyelonephritis and ½ of healthy controls, are characterized with weak /+/ concentration of nitrites in saliva. The highest concentration of nitrites, strong /+++/, concerns 3 of the patients with kidney disorder and 1 healthy child (Figure 5).

Discussion
Scientific literature accentuates on the significance of the oral-hygiene status, respectively the quality of performed professional and individual oral hygiene procedures, as a basic criterion of assessment of tooth decay risk [30-33]. Dynamics of the level of oral hygiene cares plays the role of a definite risk-determining factor with variable nature [34]. Accumulation of considerable amount of dental plaque on surfaces of teeth correlates to intensified colonization of cariogenic microorganisms [35,36] and serves as a clinical sign of high risk for caries [22]. Motivation for maintenance of perfect oral hygiene, including oral-hygiene products appropriate for definite age periods, is essential tool of oral diseases’ control not only on personal level, but also among pediatricians and general practitioners responsible for the common health of children [13-15].
Kidney disorders can be associated to nitrites in their function of biomarker with diagnostic value [37]. In concentrations not toxic for the human organism nitrites inhibit metabolic substrates of Str. mutans and other acids-producing microorganisms of A. naeslundii and L. casei [38]. In the context of our study among the examined children with diagnosed state of pyelonephritis there has been established great negative correlation between the number of carious lesions and nitrites in saliva. Significant negative correlation has been recorded between the plaque index PLI and the content of nitrites in saliva. Between the gingival index GI and concentration of salivary nitrites has been registered great negative correlation. Great positive correlation has been evaluated between the level of pH and nitrites in saliva. Simultaneously, slight negative correlation has been calculated between the number of carious spots and nitrites in saliva. The obtained results into our investigation ascertain the role of salivary nitrites as an essential protective factor against caries-related pathogenic micro-flora into oral cavity, including in children suffering from pyelonephritis and healthy ones [39]. Our results correspond to regularities determined by other authors. Namely, people characterized with high concentration of nitrites in saliva and oral micro-flora with great capacity for reduction of nitrates to nitrites, are less affected by caries in comparison to those with low level of salivary nitrates and suppressed activity of transformation of nitrates to nitrites [40]. It has been established that nitrites, produced by residual bacteria of the dorsal surface of the tongue, have cytotoxic and cytostatic effects against pathogenic microorganisms responsible for tooth decay [40-42] and periodontal diseases [43]. Salivary glands have the properties to react against processes of periodontal destruction by the means of mechanisms stimulating the protective potential of saliva [44-45]. Some authors accentuate on the diagnostic capacity and functionality of nitrites in saliva as a biomarker for renal disorders [37].
According to data represented by A. Ivanova, A. Krasteva-Panova and Z. Krastev the average value of pH in mixed saliva in adults is equal to 6,7, and in children it amounts to 7,2 [46]. The level of pH and buffer capacity in saliva influence the ions’ exchange during processes of de- and re-mineralization of enamel, resulting in oversaturation of the medium with calcium and phosphate ions in condition of pH=7 and in combination with fluorides. On the other hand, the concentration of hydrogen cations upon the dental surfaces have impact upon the process of de-mineralization [47,48].
The infectious diseases of the urinary tract system, including pyelonephritis, are inflammatory processes and their adequate therapy serves as an inevitable prerequisite for proper recovery of the organism [3-5]. Among medicines of first choice of treatment are antibiotics of the classification units of penicillin’s and cephalosporins in combination with antipyretics and non-steroid anti-inflammatory drugs. A study devoted to the influence of prolonged application of amoxicillin during the period of early childhood accentuates on its role for disturbed mineralization of enamel in permanent teeth. The deterioration of the process of enamel matrix formation and mineralization has been ascertained as a predisposing factor for increased caries activity of dentition among patients going through these therapy protocols [49]. Morphological alterations of enamel vary in wide range in clinical conditions- from findings of minimal loss of enamel manifested as isolated, single grooves, dots, furrows and pits, to generalized locations of de-mineralized rough, porous or cracked enamel surface. The histological analysis represents ameloblasts without adequate organization, with increased quantity of vacuoles into cytoplasm and slightly elongated nuclei with decreased condensation. The dosage-dependent effects of amoxicillin upon ameloblasts reflect negatively on their proper function, particularly during the stages of mineralization and maturation, causing quality- and/or quantity-related defects of hypo-mineralization [50]. Other authors identify infectious diseases in child’s age and exposure of children to various environmental toxins as etiological factors related to the occurrence of demarcation zones of disturbed opacity of enamel surface, often associated to manifestation of enamel hypoplasia. The diffusive regions of altered transparency and lack of smoothness correlate to prolonged usage of antibiotics of penicillin’s group (amoxicillin) in condition of high frequency of acceptance [51].
Parallel to the definite intensity of application of antibiotics and corticosteroids in state of infectious and autoimmune diseases, the dynamics of body temperature curve and frequent episodes of sub-febrile and febrile disorder can also make an impact on processes of formation of hard teeth tissues. The disturbance of balanced and coordinated build-up of specific architectonics of apatite crystals is related to the considerable vulnerability of enamel to a great variety of cariogenic factors in oral cavity [52].
Conclusion
In conclusion, there is a definite accent on the considerable interrelations between the number of cavitated and non-cavitated carious lesions, level of oral hygiene and gingival status, pH and nitrites in saliva and frequency of tooth-brushing in children suffering from the renal disorder of pyelonephritis.
References
- Palazzi DL, Campell JR (2019) Acute cystitis in children older than two years and adolescents.
- Devarajan P. Acute kidney injury in children: clinical features, aetiology, evaluation and diagnosis.
- Ivanov I (2005) Internal diseases. Explanative notes or practical approach to their diagnosis and treatment. Sofia-Moskva, Pensoft, pp. 221-296.
- Bliznakova D (2010) Pediatrics for dental medicine doctors. Varna, Bulgaria, pp. 227-272.
- Herold G (2011) Internal diseases. Ch II, Sofia, MI “Sharov”, pp. 182- 233.
- Batchelor PA, Sheiham A (2004) Grouping of teeth surfaces by susceptibility to caries: a study in 5-16-year-old children. BMC Oral Health 4(1): 2.
- Adulyanon S, Vourapukjaru J, Sheiham A (1996) Oral impacts affecting daily performance in a low dental disease Thai population. Community Dent Oral Epidemiol 24(6): 385-389.
- Alaluusua S, Malmivirta R (1994) Early plaque accumulation- A sign for caries risk in young children. Community Dent Oral Epidemiol 22(10): 273-276.
- Kidd EAM (2005) Essentials of dental caries. The disease and its management 3rd edn. Oxford University Press, UK.
- Peneva M (2008) Tooth decay in XXI century. Sofia, East-West, p. 292.
- Featherstone JD (2004) The continuum of dental caries-evidence for a dynamic disease process. J Dent Res 83: C39-C42.
- Featherstone JD (1999) Prevention and reversal of dental caries: role of low-level fluoride. Community Dent Oral Epidemiol 27(1): 31-40.
- Carl JJ (2005) Development and integration of oral health services for pre-school-age children. Pediatr 27(4): 323-330.
- Caspary G, Krol DM, Boulter S, Keels MA, Romano-Clarke G (2008) Perceptions of oral health training and attitudes toward performing oral health screening among graduating pediatric residents. Pediatrics 122(2): e465-e471.
- Douglass AB, Douglass JM, Krol DM (2009) Educating pediatricians and family physicians in children’s oral health. Acad Pediatr 9(6) 452-456.
- Liu J, Duan Y (2012) Saliva: A potential media for disease diagnostics and monitoring. Oral Oncol 48(7): 569-577.
- Malamud D (2006) Salivary diagnostics: the future is now. J Am Dent Assoc 137(3): 284-286.
- Nunes LA, Brenzikofer R, Macedo DV (2011) Reference intervals for saliva analytes collected by a standardized method in a physically active population. Clin Biochem 44(17-18): 1440-1444.
- Nunes LA, Mussavira S, Bindhu OS (2015) Clinical and diagnostic utility of saliva as a non-invasive diagnostic fluid: a systematic review. Biochem Med (Zagreb) 25(2): 177-192.
- Sun F, Reichenberger EJ (2014) Saliva as a source of genomic DNA for genetic studies: review of current methods and applications. Oral Health Dent Manag 13(2): 217-222.
- Axelsson P (2000) Diagnosis and risk prediction of dental caries. V2 Illinois, Quintessence Publishing Company, USA, p. 307.
- Edgar WM, Dawes C, O’Mullane DM (2004) Saliva and oral health. (3rd edn), BDA, London, UK, p. 146.
- Tabak LA (2001) A revolution in biomedical assessment: the development of salivary diagnostics. J Dent Educ 65(12): 1335-1339.
- Ten Cate AR ( 1998) Oral histology: development, structure and function. 5th edn, St Louis, Mosby, USA, p. 497.
- de Almeida Pdel V, Grégio AM, Machado MA, de Lima AA, Azevedo LR (2008) Saliva Composition and Functions: a Comprehensive Review. J Contemp Dent Pract 9(3): 72-80.
- Berkovitz BKB, Holland GR, Moxham BJ (2002) Oral anatomy, histology and embryology. (3rd edn), Mosby, New York, USA, p. 378.
- Humphrey SP, Williamson RT (2001) A review of saliva: normal composition, flow and function. J Prosthet Dent 85(2): 162-169.
- Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, et al. (1994) Stomach NO synthesis. Nature 368(6471): 502.
- Weller R, Price RJ, Ormerod AD, Benjamin N, Leifert C (2001) Antimicrobial effect of acidified nitrite on dematophyte fungi, candida and bacterial skin pathogens. J Appl Microbiol 90(4): 648-652.
- Blue C, Isringhausen K, Dils E (2011) Raising Oral Health Awareness Among Nephrology Nurses. J Dent Hyg 85(2): 151-157.
- Lewis CW, Boulter S, Keels MA, Krol DM, Mouradian WE, et al. (2009) Oral health and pediatricians: results of a national survey. Acad Pediatr 9(6): 457-461.
- Ramos-Gomez FJ, Crystal YO, Ng MW, Crall JJ, Featherstone JD (2010) Pediatric dental care: prevention and management protocols based on caries risk assessment. J Calif Dent Assoc 38(10): 746-761.
- Ramos-Gomez FJ, Crystal YO, Domejean S, Featherstone JD (2012) Minimal intervention dentistry: Part 3. Paediatric dental care- prevention and management protocols using caries risk assessment for infants and young children. Br Dent J 213(10): 501-508.
- Nowak AJ, Casamassimo PS (2002) The dental home. A primary care oral health concept. J Am Dent Assoc 133(1): 93-98.
- Litt MD, Reisine S, Tinanoff N (1995) Multidimensional causal model of dental caries development in low-income pre-school children. Public Health Reports 110(5): 607-617.
- Nicolau B, Marcenes W, Bartley M, Sheiham A (2003) A life course approach to assessing causes of dental caries experience: The relationship between biological, behavioural, socio-economic and psychological conditions and caries in adolescents. Caries Res 37(5): 319- 326.
- Karova ES (2013) Factor for maintenance of oral homeostasis. Sofia, CMB, p. 94.
- Radcliffe CE, Akram NC, Hurrell F, Drucker DB (2002) Effects of nitrite and nitrate on the growth and acidogenicity of Streptococcus mutans. J Dentistry 30(7-8): 325-331.
- Mohebbi SZ, Virtanen JI, Vahid-Golpayegani M, Vehkalahti MM (2008) Feeding habits as determinants of early childhood caries in a population where prolonged breastfeeding is the norm. Community Dent Oral Epidemiol 36(4): 363-369.
- Doel JJ, Hector MP, Amirtham CV, Al-Anzan LA, Benjamin N, et al. (2004) Protective effect of salivary nitrate and microbial reductase activity against caries. Eur J Oral Sci 112(5): 424-428.
- Radcliffe CE, Lamb R, Blinkhorn AS, Drucker DB (2003) Effect of sodium nitrite and ascorbic acid on the growth and acid production of Streptococcus mutans. J Dent 31(5): 367-370.
- Silva Mendez LS, Allaker RP, Hardie JM, Benjamin N (1999) Antimicrobial effect of acidified nitrite on cariogenic bacteria. Oral Microbiol Immunol 14(6): 391-392.
- Allaker RP, Silva Mendez LS, Hardie JM, Benjamin N (2001) Antimicrobial effect of acidified nitrite on periodontal bacteria. Oral Microbiol Immunol 16(4): 253-256.
- Bayindir YZ, Polat MF, Seven N (2005) Nitric oxide concentrations in saliva and dental plaque in relation to caries experience and oral hygiene. Caries Res 39(2): 130-133.
- Henskens YM, van den Keijbus PA, Veerman EC, Van der Weijden GA, Timmerman MF, et al. (1996) Protein composition of whole and parotid saliva in healthy and periodontitis subjects. Determination of cystatins, albumin, amylase and IgA. J Periodontal Res 31(1): 57-65.
- Krastev Z, Kiselova-Yaneva A, Kolarov R (2009) Oral Medicine. Sofia, Ivan Sapundjiev EOOD, 2009. p. 49-53; p. 197-232; p. 407-420.
- Cunha-Cruz J, Scott J, Rothen M, Mancl L, Lawhorn T et al. (2013) Salivary characteristics and dental caries: Evidence from general dental practices. J Am Dent Assoc 144(5): e31-e40.
- Ranganath LM, Shet RG, Rajesh AG (2012) Saliva: a powerful diagnostic tool for minimal intervention dentistry. J Contemp Dent Pract 13(2): 240-245.
- Mihalaş E, Matricala L, Chelmuş A, Gheţu N, Petcu A, et al. (2016) The Role of Chronic Exposure to Amoxicillin/Clavulanic Acid on the Developmental Enamel Defects in Mice. Toxicol Pathol 44(1): 61-70.
- Russell MW, Hajishengallis G, Childers NK, Michalek SM (1999) Secretory immunity in defence against cariogenic mutans streptococci. Caries Res 33(1): 4-15.
- Arrow P (2009) Risk factors in the occurrence of enamel defects of the first permanent molars among schoolchildren in Western Australia. Community Dent Oral Epidemiol 37(5): 405-415.
- Beentjes VE, Weerheijm KL, Groen HJ (2002) Factors involved in the aetiology of molar-incisor hypomineralisation (MIH). Eur J Paediatr Dent 3(1): 9-13.






























