Abstract
Inclusion Body Myositis (IBM), Polymyositis (PM), and Dermatomyositis (DM) are distinct autoimmune-mediated inflammatory myopathies that primarily affect skeletal muscles but differ in their clinical presentation, pathophysiology, and management. IBM is most commonly seen in older individuals and is characterized by asymmetric, progressive muscle weakness, particularly in the distal arm and proximal leg muscles. It is also resistant to immunosuppressive treatments. In contrast, PM and DM exhibit symmetric proximal muscle weakness, with DM additionally presenting distinctive dermatological manifestations such as heliotrope rashes and Gottron’s papules. Both PM and DM are associated with extra muscular complications, including interstitial lung disease and, in DM, increased cancer risk, particularly in older adults. Diagnostic evaluation relies on clinical assessment, elevated muscle enzymes, electromyography, and muscle biopsy, with distinct histological features guiding diagnosis. While IBM may present with rimmed vacuoles and protein aggregates, PM and DM show endomysial lymphocytic infiltration and vasculopathy. Treatment strategies for PM and DM involve glucocorticoids combined with disease-modifying antirheumatic drugs (DMARDs), with additional therapies like intravenous immunoglobulin (IVIG) and rituximab in refractory cases. IBM management focuses on supportive care, physical therapy, and addressing comorbidities. Despite progress in understanding these disorders, therapeutic options remain limited, especially for IBM. Early diagnosis, individualized treatment, and ongoing research into biomarkers and targeted therapies are essential for improving patient outcomes and quality of life in these challenging conditions.
Keywords:Inflammatory Myopathies; Inclusion Body Myositis; Polymyositis; Dermatomyositis
Abbreviations:IBM: Inclusion Body Myositis; PM: Polymyositis; DM: Dermatomyositis; SLE: Systemic Lupus Erythematosus; UES: Upper Esophageal Sphincter; EMG: Electromyography; PFTs: Pulmonary Function Tests; HRCT: High-Resolution Computed Tomography; CK: Creatine Kinase; IVIG: Intravenous Immunoglobulin; DMARD: Disease-Modifying Antirheumatic Drug; RIM: Rituximab in Myositis (trial); Anti-tRNA: Anti-aminoacyl Transfer RNA; IV: Intravenous; FDA: Food and Drug Administration; FVC: Forced Vital Capacity; FEV1: Forced Expiratory Volume in 1 Second; TLC: Total Lung Capacity; FRC : Functional Residual Capacity; RV: Residual Volume; CHF: Congestive Heart Failure; ILD: Interstitial Lung Disease; HRCT: High-Resolution Computed Tomography; MTX: Methotrexate; AZA: Azathioprine; MMF: Mycophenolate Mofetil; TPMT: Thiopurine Methyltransferase; PPI: Proton Pump Inhibitor; UV: Ultraviolet; PMN: Polymorphonuclear; ICU: Intensive Care Unit
Introduction
Inflammatory myopathies are a group of rare diseases that involve chronic (long-standing) muscle inflammation, weakness, and in some cases, pain. Myopathy is a general term used to describe a number of conditions affecting the muscles [1-3]. Inflammatory myopathies are an important topic due to their impact on both the quality of life and functional ability of affected individuals. This condition can lead to chronic muscle weakness, pain, and, in some cases, significant disability. Early diagnosis and effective treatment are crucial, as timely intervention can help manage symptoms, prevent progression, and improve long-term outcomes [1-3].
Furthermore, understanding the underlying mechanisms of these conditions can contribute to developing more targeted therapies and better care strategies. Given their complexity and the potential for serious complications, inflammatory myopathies represent a critical area of research and clinical attention in neurology and rheumatology [1-3]. Early treatment helps to manage inflammation, slow disease progression, and reduce the risk of complications such as difficulty swallowing, breathing problems, or joint deformities. Moreover, prompt diagnosis allows for identifying the specific type of inflammatory myopathy, enabling personalized treatment plans that target the underlying causes. Addressing the condition early can help preserve muscle function, improve the quality of life, and reduce the disease’s longterm impac.
Epidemiology and Demographic Considerations
Inclusion body myositis (IBM)
Inclusion body myositis (IBM) is a rare, progressive inflammatory disease primarily affecting individuals over 50 years old, making it the most common acquired myopathy in older adults [4]. Its global prevalence varies by region, ranging from 1 to 71 cases per million people [5]. Regions with advanced healthcare systems, such as North America, Western Europe, and Australia, report higher prevalence rates due to better diagnostic capabilities. The condition is more prevalent in males, with a male-to-female ratio of approximately 3:1 [4]. IBM primarily affects Caucasians but has been documented in various ethnic groups, exhibiting differences in clinical presentation and disease progression. It commonly presents as asymmetric muscle weakness, particularly in the quadriceps and distal muscles like finger flexors, leading to challenges with mobility and fine motor tasks [6]. While familial cases are rare, they suggest a genetic component, including HLAassociated susceptibility. Environmental factors and age-related immune dysregulation are also believed to play a role in its development.
Unlike other inflammatory myopathies, IBM is resistant to immunosuppressive therapy and progresses slowly yet relentlessly, often resulting in significant disability [4]. Due to underdiagnosis or misdiagnosis, its true prevalence and incidence may be underestimated, highlighting the need for greater awareness and enhanced diagnostic approaches. Understanding the demographics of IBM is essential for accurate differential diagnosis and early recognition, given its distinct epidemiological and clinical characteristics. IIBM’s higher prevalence among Caucasians and its association with specific genetic markers, such as HLA class II haplotypes, can inform diagnostic and genetic counseling strategies for affected populations. Recognizing these demographic trends can help reduce misdiagnoses, which often occur due to symptom overlaps with other myopathies or neurodegenerative diseases like amyotrophic lateral sclerosis (ALS). Early identification based on demographic risk factors enables timely muscle biopsy and biomarker testing, facilitating an accurate diagnosis. Considering IBM’s resistance to immunosuppressive therapies, an accurate diagnosis is vital for patient counseling, managing disease progression, and planning supportive care.
Polymyositis (PM)
Polymyositis is an idiopathic inflammatory myopathy primarily affecting adults between the ages of 30 and 60 [7]. Its global prevalence ranges from 5 to 22 cases per 100,000 individuals, with an annual incidence of 1 to 5 cases per 100,000 [8]. The condition is more common in females, with a female-tomale ratio of approximately 2:1, and is rarely observed in childrenunlike dermatomyositis, which has a juvenile variant [7]. While the incidence of polymyositis is relatively consistent worldwide, prevalence rates vary depending on population studies. For instance, a 2003 study in Quebec reported a prevalence of 21.5 cases per 100,000, whereas a 2010 study in Japan estimated 13.2 cases per 100,000 [9]. U.S. hospital-based studies indicate that the average age of affected individuals is around 58 years, with hospitalizations for polymyositis being associated with more significant morbidity and higher healthcare costs compared to other conditions [4].
Understanding demographics enhances early suspicion and differential diagnosis in diseases like polymyositis by identifying patterns related to age, gender, and ethnicity. Recognizing these demographic trends helps differentiate polymyositis from other myopathies, such as inclusion body myositis, which typically occurs in males over 50, or juvenile dermatomyositis, seen in children. Ethnic variations, such as a slightly higher prevalence among African or African American populations, can also guide diagnostic priorities [4]. Additionally, awareness of demographiclinked risks, like interstitial lung disease or malignancy in older adults, facilitates targeted evaluations. By leveraging this information, clinicians can achieve earlier, more accurate diagnoses, ultimately improving patient outcomes.
Dermatomyositis (DM)
Dermatomyositis (DM) is a rare autoimmune inflammatory myopathy characterized by muscle weakness and skin rashes. It commonly affects individuals in two age groups: children (juvenile dermatomyositis) and adults, typically between 40 and 60 years [10]. The global incidence of dermatomyositis is between 1 and 10 cases per million people, with an estimated prevalence of 5 to 20 per 100,000 [11]. The disease is more prevalent in females than males, with a female-to-male ratio of approximately 2:1 [11]. While dermatomyositis occurs in all ethnic groups, higher prevalence rates have been reported among African American populations, particularly in adults [4]. Juvenile dermatomyositis is more frequently seen in children, especially girls, and tends to present with different clinical features, such as more rapid progression and greater systemic involvement [10]. Understanding the demographics of dermatomyositis is essential for early diagnosis and differential diagnosis. Age of onset, gender, and ethnicity offer key insights for clinicians. For example, an older adult with a characteristic skin rash (such as heliotrope rash or Gottron’s papules) highly suggests adult-onset dermatomyositis. At the same time, similar symptoms in a child may indicate juvenile dermatomyositis [11]. The higher prevalence of the disease in females and African American populations should prompt clinicians to consider dermatomyositis sooner when these groups present with unexplained muscle weakness or skin changes [10]. Early recognition, guided by demographic factors, improves diagnostic accuracy and facilitates timely treatment, which is crucial to prevent long-term complications such as fibrosis or malignancy.
Pathophysiology
Inclusion Body Myositis (IBM)
Inclusion body myositis is known to be an idiopathic inflammatory myopathy with an unclear underlying pathophysiology. Manifestations are noticed for the characteristic muscle involvement, preferentially affecting the finger flexor and knee extensor with an endomysial infiltration of CD8+ T cells and sarcoplasmic aggregation [12]. Recent research published by Pinto et al. has shown that the severity of endomysial inflammation correlates with the severity of dysphagia in patients with inclusion body myositis. However, it is of high value to mention that this study highlighted the heterogeneity of the disease by pointing out that respiratory dysfunction and dysphagia may progress independently of limb weakness [13].
Two main theories support the underlying mechanisms of inclusion of body myositis. One proposed theory currently being investigated in clinical trials pertains to the infiltrating CD8+ T cells expressing cell-surface markers such as KLGR1, CD57, and PD-1 while lacking CD28 expression. These markers are consistent with the hypothesis that these T cells are highly differentiated cytotoxic cells exhibiting a senescent phenotype. The alternative hypothesis suggests that the pathogenesis involves progressive myofiber degeneration that occurs independently of inflammation. This theory posits that many proteins form ubiquitinated sarcoplasmic aggregates, which accumulate within rimmed vacuoles in myofibers. One significant protein involved is TDP-43, an RNA-binding protein that is lost from the nucleus and forms cytoplasmic aggregates. TDP-43 is known to prevent the incorporation of cryptic exons into mature RNA, and muscle biopsies from patients with inclusion body myositis have demonstrated the expression of these cryptic exons. Moreover, TDP-43 aggregates can form self-templating seeds, suggesting a potential prion-like disease spread. With new and current clinical research, advancements in diagnostic imaging technology, and improvements in biomolecular diagnostic testing, our understanding of these underlying pathophysiological mechanisms continues to grow and propose new theories [12,14].
Polymyositis (PM)
Polymyositis is a humoral immune response factor, with approximately 80% of patients having positive antinuclear and specific antibodies. Antibodies specific to myositis are aminoacyl transfer RNA (tRNA) synthetase and antinuclear helicase [Mi- 2]. The tRNA synthetase antibody has been associated with the antisynthetase syndrome; some of the pathologies associated with this syndrome are myositis, interstitial lung disease, non-erosive arthritis, Raynaud phenomenon, skin rash of the hand, and the characteristic mechanic’s hand. Various antisynthetase antibodies are identified, all associated with a common condition known as interstitial lung disease. This disease may manifest before the onset of myositis or may present independently of it. B cells are integral to the pathophysiology of polymyositis, as they facilitate a humoral immune response by infiltrating muscle tissue [15]. Two cell patterns dominate the cell-mediated immune response in polymyositis; CD4+ T lymphocytes against blood vessels dominate the first distribution, while the second pattern has the presence of CD8+ T lymphocytes and CD4+ T lymphocytes against muscle fibers. The first pattern will have CD4+ predominantly present in perivascular and perimysial areas of tissue, accompanied by macrophages, B lymphocytes, plasma cells, and dendritic cells. The second pattern, led by activated CD8+ T lymphocytes and CD4+ T lymphocytes and accompanied by macrophages and dendritic cells, presents with an endomysial distribution. However, these patterns are not exclusive to each other, and both of them can present in polymyositis. In polymyositis, capillaries are decreased per muscle area compared to a healthy individual; these capillaries have different morphology, are thickened, and could present with hyperplasia and necrosis. Hence, these changes could promote ischemia, ending in muscle fiber damage [15].
Dermatomyositis (DM)
Dermatomyositis is believed to present as a vasculopathy with endothelial damage, showing ischemic changes in muscle biopsies, with histopathological manifestations like perifascicular atrophy and microinfarction. HLA A1-N8-DR3-DQ2 and HLA-DRB1 are known to be genetic risks of dermatomyositis. Dermatomyositis presents with increased circulating endothelial cells and an increased number of endothelial markers, Von Willebrand Factor, and P-selectin. Interferon type I plays a vital role in pathology and the prominent infiltration of CD4+ cells. Type I interferon activates T and B cells, leading to the induction of autoantibodies; this will induce MHC class I expression in the cells, resulting in endoplasmic reticulum stress and finally causing myocyte death [12,16]. The vascular involvement in dermatomyositis is the cause of clinical skin changes, and the capillary nail fold is caused by complement deposition. Moreover, since micro vessels are affected both in dermatomyositis and polymyositis, another clinical symptom secondary to hypoxia is the presence of muscle fatigue; the hypothesis to this clinical presentation has been tested since there is an attenuation of muscle fatigue after performing physical activity [15,16].
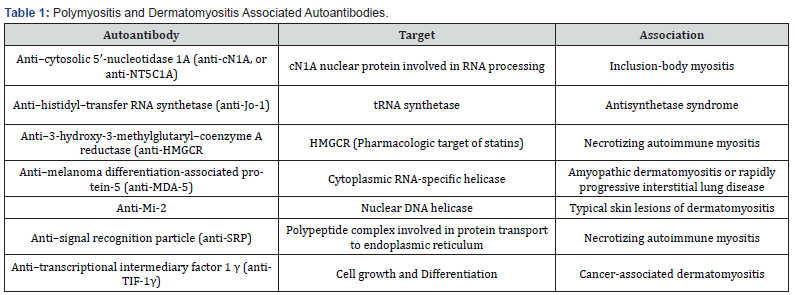
Autoantibodies associated with polymyositis and dermatomyositis are illustrated in Table 1. This complex group of diseases has a variety of symptoms, also called inflammatory muscle diseases, that are not exclusive to the muscle, and some of the symptoms may overlap. Patients face a variety of symptoms affecting their daily activities, mainly activities in which the proximal muscles are required. In inclusion body myositis, an early affection to the distal muscles affects the patients performing tasks, like buttoning or holding up objects; these clinical manifestations can also be seen in polymyositis and dermatomyositis but in an advanced stage. The presence of extramuscular manifestations is present in all inflammatory muscle diseases, rash, fever, interstitial lung diseases, and some cardiac complications like arrhythmias or ventricular dysfunction; thus, it is important to diagnose early and precisely to ensure the best prognosis and tailored treatment [12,17].
Clinical Manifestations
Although IBM, PM, and DM share similar pathophysiological features, the unique clinical manifestations of each disease share a key role in differentiating between diagnoses. Inclusion body myositis most commonly manifests in Caucasian males older than 30 years of age [18]. Classically, IBM presents with gradual asymmetric weakness of the distal arm and proximal leg, primarily affecting the quadriceps muscle, which can lead to falls and difficulty standing. 98% of patients with IBM reported an incidence of falls, and 60% of patients reported falling frequently in a survey conducted in 2014 [19]. In the upper extremities, weakness of the finger flexors would often lead to loss of dexterity, most commonly affecting the distal phalangeal flexors of the nondominant hand and sparing the thenar, hypothenar, and finger extensors [18]. Other rare initial manifestations of IBM include dysphagia, foot drop, and primary respiratory failure. Dysphagia most commonly is diagnosed late in IBM patients, thus leading to more severe presentations. Within the myopathy diseases, IBM reports the most frequent and severe manifestations of dysphagia, as well as a higher risk of aspiration pneumonia [20]. Multiple theories have been considered as to why this occurs in IBM, including reduced upper esophageal sphincter (UES) tone, UES spasms, fibrosis of cricopharyngeal muscle, suprahyoid muscle weakness, or impaired relaxation of UES [20]. Patients may report needing to swallow repeatedly, feeling stasis, regurgitation, and washing down solids with liquids. However, often, patients may not even recognize these extramuscular manifestations; thus, screening by the physician is vital. Cox et al. suggest that two of the most sensitive questions physicians must ask for diagnosis are “Does food get stuck in your throat?” and “Do you have to swallow repeatedly to get rid of food?” [21]. Furthermore, IBM is also associated with autoimmune disorders and should be considered in patients with coinciding systemic lupus erythematosus (SLE), Sjogren’s syndrome, thrombocytopenia, and sarcoidosis; some studies have also seen an association between IBM and HLADRB1- 03:01/01:01 [18].
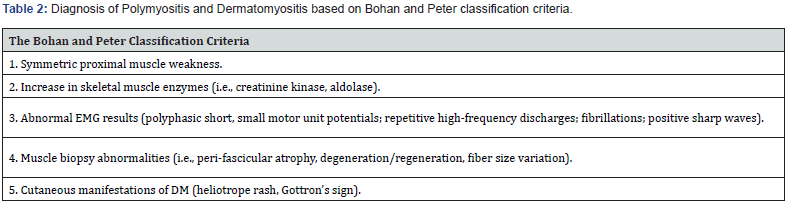
Polymyositis (PM) and Dermatomyositis (DM) also fall under the inflammatory myopathy classification and share many similarities. PM and DM are diagnosed through the classification described by Bohan and Peter (Table 2). Firstly, symmetric proximal muscle weakness, unlike the asymmetric muscle weakness seen in IBM. Next, laboratory values such as elevated creatinine kinase and aldolase, EMG evidence, histopathological evidence, and dermatological manifestations are seen only in DM [22]. PM is rare in childhood and most commonly seen in female patients after the second decade [22]. Classical musculoskeletal findings in both PM and DM are progressive symmetric proximal muscle weakness and truncal weakness. Patients may report trouble using proximal muscles, such as climbing steps, rising from a chair, and combing their hair, while there is a sparing of distal muscle actions, such as writing and buttoning a shirt [23]. Neck flexors are often the first group of muscle affected, followed by proximal lower extremities and shoulder abductors. If these symptoms are also seen early in disease presentation, there should be high suspicion of an additional neuromuscular disease. These myopathies are also associated with muscle tenderness and myalgia early in the disease course, with a more substantial prevalence in DM [22]. Unlike IBM, many extramuscular manifestations are seen in both PM and DM. Arthralgia and arthritis are often seen in both myopathies, with involvement, particularly in the wrists and small joints of the hand and knee; these symptoms will resolve with the therapy of the underlying inflammatory myopathy [24]. The key difference between PM and DM is the dermatological manifestations central to diagnosing DM. Heliotrope rash (Figure 1) in DM is characterized by purple erythematous discoloration along the distribution of the periorbital skin [25]. The rash can either follow the course of the disease or wax and wane parallel to disease activity.
Gottron’s papules are the erythematous scaly papules found over the bony prominences of the metacarpophalangeal joints and distal and proximal interphalangeal joints [25]. These symmetric lesions can also coincide with hyperpigmentation, hypopigmentation, and telangiectasia. This cutaneous manifestation in DM has also been termed “mechanic’s hands,” characterized by bilateral scaling, hyperkeratosis, and horizontal fissuring of the palms and hands [22]. They are often misinterpreted as contact dermatitis, psoriasis, or lichen planus. Thus, biopsy plays a key role in diagnosis. Dermatological findings often worsen with exposure to sunlight and may present with edema in areas with heliotrope rash and Gottron’s sign. Furthermore, periungual telangiectasia affects the nail folds in DM patients and is characterized by hypertrophy of the cuticle and small hemorrhagic infarcts within the cuticle [25]. Many patients also report scalp involvement ranging from moderate nonscarring alopecia to a pruritic psoriasis-like manifestation of the scalp [25]. Cutaneous calcinosis is a rare dermatological manifestation seen in the juvenile subset of DM. These subcutaneous calcium deposits often occur in areas of compression, such as the elbows, knees, and buttocks [26]. Cardiac involvement, although rare in PM and DM, is a leading cause of death in patients with inflammatory myopathy [27]. There are multiple reported cardiac manifestations seen in PM and DM, including arrhythmias, myocarditis, congestive heart failure (CHF), cardiac arrest, pericarditis, and secondary fibrosis [22]. Conduction abnormalities fall as the most common cardiac manifestation in these myopathies. They are often asymptomatic with subtle ECG changes such as bundle branch blocks, ST-T changes, PR prolongation, and Q-wave abnormalities [22]. Heart failure is also more commonly associated with DM and is attributed to hypertension secondary to long-term steroid use. Prevalence of heart failure in these patients has been theorized also to be caused by myocarditis, which causes left ventricular dysfunction and restrictive cardiomyopathy presentation [22]. Cardiac manifestation in PM and DM tend to resolve with immunosuppressive treatment, but may also be treated with betablockers, nitrates, and calcium antagonist diuretics [27].
Pulmonary manifestations of PM and DM occur secondary to respiratory muscle weakness and are a significant source of morbidity and mortality in this patient population. Progressive respiratory muscle weakness seen in the later course of these diseases leads to four main complications: hypoventilation, aspiration pneumonia, and Interstitial Lung Disease (ILD) [22]. Hypoventilation secondary to muscle weakness will present with a restrictive lung disease pathology on pulmonary function tests (PFTs): reduced lung volumes, maximum expiratory and inspiratory pressures, increased residual volumes, and standard FEV1: FVC ratio [28]. On chest x-rays, patients will present with reduced lung volume and basal atelectasis [22]. Secondly, aspiration pneumonia is a frequent and severe complication of DM and PM, often coinciding with extensive muscle and skin involvement. These patients are more likely to complain of dysphagia due to disease involvement of the striated muscles of the pharynx and upper esophagus [29]. Lastly, Interstitial Lung Disease (ILD) is a generalized restrictive lung disease characterized by fibrosis and infiltration of monocytes, neutrophils, and lymphocytes. The most frequently reported symptoms in myositis-associated ILD are cough and dyspnea; however, some patients may remain asymptomatic [22]. PFTs play a key role in diagnosing these patients, generating decreased total lung capacity (TLC), functional residual capacity (FRC), residual volume (RV), FEV1 and FVC, as well as an elevated FEV1: FVC ratio, and reduced diffusing capacity of the lung for carbon monoxide [22]. The gold standard of diagnosis is via highresolution CT (HRCT) and co-presence of antiaminoacyl:transfer RNA (tRNA) synthetase antibodies commonly occur in myositisassociated ILD [22]. Rupture of subpleural or pericardial blebs in ILD can rarely cause spontaneous pneumomediastinum and is more common in patients with DM [30].
Diagnostic Evaluation
Laboratory Testing
Creatine kinase (CK) levels are a critical initial test in evaluating inflammatory myopathies. Elevated CK levels are typical in PM and DM, reflecting ongoing muscle inflammation and damage. However, CK elevation is often modest or absent in IBM, complicating the diagnostic process. The identification of specific autoantibodies also aids in diagnosis. Anti-Jo-1 antibodies are associated with PM and DM and often indicate extramuscular manifestations such as interstitial lung disease. In contrast, anticN1A antibodies have been identified in patients with IBM and serve as a potential biomarker, although their sensitivity and specificity are limited [31-33].
Imaging
Magnetic resonance imaging (MRI) is valuable in evaluating muscle involvement and guiding biopsy. In PM and DM, MRI often reveals muscle edema, reflecting active inflammation. In contrast, patients with IBM may demonstrate chronic muscle atrophy and fatty replacement rather than active edema, correlating with the disease’s insidious onset and chronicity. Electromyography (EMG) is another critical imaging modality. PM and DM typically show irritative myopathy patterns with fibrillation potentials and myopathic motor unit action potentials. In IBM, the EMG pattern often includes mixed findings, such as chronic denervation and myopathic features, complicating differentiation from neurogenic diseases [32-35].
Muscle Biopsy
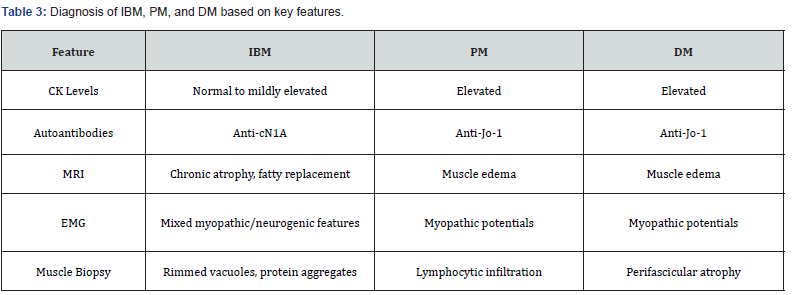
Histopathologic examination of muscle tissue remains the gold standard for diagnosing inflammatory myopathies. IBM is characterized by rimmed vacuoles and the accumulation of protein aggregates such as β-amyloid and TDP-43, highlighting its degenerative aspect. In PM, the biopsy reveals endomysial lymphocytic infiltration, predominantly CD8+ T cells targeting muscle fibers. Dermatomyositis, in contrast, demonstrates peri fascicular atrophy, capillary abnormalities, and complement deposition, consistent with its vasculopathy and immunemediated pathogenesis. These distinct histologic features are critical for accurate differentiation and guide management decisions [33-35]. While CK levels and autoantibody profiles provide valuable initial clues, imaging and muscle biopsy findings offer definitive diagnostic information. The complexity of these disorders necessitates a systematic approach, emphasizing the integration of clinical, laboratory, imaging, and histopathologic data. Early and accurate differentiation is vital to optimize management strategies and improve patient outcomes. A summary of the key diagnostic parameters for evaluating these disorders is presented in Table 3.
Treatment and Management Approaches
Inclusion Body Myositis (IBM)
The primary goal of therapy in inclusion body mуositis (IBМ) is to optimize muscle strength and function. Given the disease’s slowly progressive and variable course, it can be quite challenging to determine if treatment leads to an objective improvement in or stabilization of muscle strength. It is well known that immunosuppressive medications will lower muscle enzyme levels in ΙΒΜ patients despite the continued progression of ԝеаknеss and also that creatine kinase (CK) levels decrease with muscle atrophy. Therefore, CK levels cannot be used to monitor response to therapy in this disease. Based on the existing data, a trial of immunosuppressive medications is only considered in IΒΜ patients with an atypical presentation or another autoimmune disease [36-38].
Almost all patients with any degree of limitation in activities of daily living will benefit from physical and occupational therapy evaluation. A speech therapist should evaluate patients with ԁуѕрhagiа. Exercise is likely beneficial in all patients. In contrast to other inflammatory mуοраthiеѕ such as dermatomyositis and polymyositis, inclusion body myοsitis (ІΒМ) is relatively resistant to standard immunomodulatory therapies. Typically, a trial of immunosuppressive treatment is suggested when the diagnosis of ІΒМ is uncertain or in cases where there appears to be overlap with polymyositis as evidenced by early and prominent proximal ԝеаknеss (i.e., neck flexors, arm abductors, hip flexors) [36-40]. The optimal treatment for ІBM is unknown, and most interventions have demonstrated limited benefit. Nonpharmacologic interventions such as physical, occupational, and speech therapy can be helpful. An exercise program to maintain strength as long as possible, addressing concerns about falling and the use of orthoses and assistive devices such as canes and walkers, addressing difficulties with the activities of daily living such as finger flexor ԝеаknesѕ, helping them to learn techniques to minimize the risk of aspiration in patients with ԁуsphаgia, providing nutritional support or dietary counseling in patients with ԁyѕрhagia or obesity, respectively [36-37].
Polymyositis (PM) and Dermatomyositis (DM)
The goals of treatment are to improve muscle strength while preventing both relapse and treatment-associated adverse events. Some patients may select treatments primarily to address extramuscular disease manifestations (e.g., interstitial lung disease or cutaneous disease). Glսϲοϲοrtiϲoidѕ is the cornerstone of initial therapy for DМ and рοlуmyоѕitis (ΡM). There is a consensus that glucocorticoid therapy improves strength and preserves muscle function. In a National Institutes of Health series, for example, 39 percent of 113 glucocorticoid-treated patients had complete normalization of serum enzymes, and 25 percent regained full muscle strength. Mild muscle disease (i-e, near normal muscle strength), treated simultaneously with glսϲοϲοrtiϲоids and either methotrexate, azathioprine, or mycophenolate is suggested. For mild to moderate disease (i-e, with moderate muscle ԝеаknеѕs, the simultaneous treatment with systemic glսϲοϲοrtiсоidѕ and a disease-modifying antirheumatic drug (DMARD) such as azathioprine, methotrexate, or mycophenolate are suggested. For patients with moderate muscle disease (i-e, significant muscle ԝеаkոeѕѕ on examination), higher doses of glսϲοϲοrtiсоiԁs in addition to a DMARD are suggested [36-40].
Avoiding mеthοtrехаtе is suggested in patients with kidney function impairment, liver disease, or interstitial lung disease. Otherwise, choosing between mеthοtrеxаtе and аzаthiоprine is primarily dictated by the side effects associated with each agent and patient preference. Меthοtrехatе is more convenient than аzаthiοрriոе, requiring only once-a-week administration either orally or parenterally. Patients who are deficient in thiopurine methyltransferase may not be able to tolerate аzаthiоprine [38- 40]. Alternatives to these agents include mycophenolate mofetil (1 to 1.5 g twice daily) or tacrolimus (0.1 to 0.2 mg/kg daily, in two divided doses). The use of mycophenolate mofetil has become increasingly common, particularly for patients with DM who also have a skin disease. Tacrolimus doses should be adjusted to maintain a trough concentration between 5 to 20 ng/mL, although the optimal target concentration has not been established [37,39-40]. For patients with severe disease (e.g., ԁуѕрhagia, diaphragmatic ԝеakոеѕѕ, or ԝеakոеsѕ preventing self-care), the suggested treatment is with intravenous immսոοglοbսlin (IVIG; 2 g/kg, divided over two to five days, administered monthly) and IV methylprednisolone (1 g daily for three days), followed by prednisone 1 mg/kg daily (to a maximum dose of 80 mg daily) [36-40].
The Rituximab in Муоsitis (RIM) trial is the largest randomized, double-blind, placebo-controlled trial conducted in the inflammatory mуοpаthiеs. RIM enrolled 75 patients with РΜ, 72 patients with DМ, and 48 patients with juvenile DМ who were randomized to receive treatment with rituximab, either at baseline or after a delay of eight weeks. By the end of the study, 83 percent of patients demonstrated clinical improvement, although there was no statistically significant difference between the two groups. A post-hoc analysis demonstrated that the fastest response to rituximab was seen among patients with antisynthetase and anti- Mi-2 autoantibodies. Data supporting the use of cyclophosphamide for DМ or РМ are predominantly limited to small series, and it is generally not used for muscle or skin disease in the absence of concomitant interstitial lung disease [36-40]. In addition to drug therapy, there are a variety of other essential considerations in the treatment of patients with inflammatory mуоpathy.
These include the initiation of an ехеrcise program under the supervision of a physical therapist, steps to prevent аѕрiratiοո in patients with esophageal dysfunction, counseling about the need for patients with ԁеrmаtοmуοѕitis (DΜ) to avoid ultraviolet light, and prophylaxis against οѕtеοроrоѕiѕ and opportunistic infections. The lower esophageal sphincter may be ineffective in patients with mucositis and overlap with scleroderma, resulting in reflux esophagitis. Treatment with proton pump inhibitors decreases both the risk of stricture formation and reflux symptoms, but some patients have persistent symptoms. Cutaneous manifestations of DM are often photosensitive, and patients should be counseled to avoid direct sun exposure. Skin manifestations can persist despite adequate treatment of mуοѕitiѕ. Pruritus is often a significant complaint and may be treated with topical agents such as menthol, camphor, antihistamines, pramoxine, and lidocaine. Systemic agents include hydroxyzine, doxepin, and amitriptyline [36-40]. All patients treated with high-dose glսϲοϲοrtiϲoiԁѕ are at risk for glucocorticoid-induced οѕtеοроrоѕiѕ. Thus, all patients embarking upon treatment courses for inflammatory mуоpаthу are potential candidates for antiresorptive therapy designed to prevent bone loss. Because of the high-dose glucocorticoid therapy and other immunosuppressive medications used to treat inflammatory mуоpathy, patients with DΜ and рοlуmyоѕitis (PM) are at an increased risk for opportunistic infection, including Pneumocystis Jirovecii, fungal, mycobacterial, and viral infections. Patients are advised to receive appropriate immunizations before the institution of immunosuppressive therapies. It is suggested that attempts to conceive should be delayed until disease remission has been achieved for at least three months [36-40].
Prognosis and Follow-Up
The late diagnosis of IBM, combined with the lack of response to medications for other inflammatory myopathies (IIMs) and associated comorbidities, complicates management and may reduce life expectancy for patients [41,42,44]. Understanding the disease’s underlying mechanisms is essential for developing effective therapies. New biomarkers and imaging technologies promise to improve diagnostic accuracy and evaluate treatment outcomes in clinical trials [42,43]. Future diagnostic and follow-up tools should prioritize high sensitivity to track disease progression and therapeutic response more effectively. Polymyositis (PM), as traditionally defined, is an over-diagnosed entity today [45]. As specific antibodies are absent for PM to guide the diagnosis, many cases can be misdiagnosed due to suboptimal muscle biopsy samples [46]. PM patients who would have met the previous definition are now diagnosed with other disease processes, such as antisynthetase syndrome (ASyS), immunemediated necrotizing myopathy (IMNM), inclusion body myositis (IBM), or overlap myositis, reflecting improved understanding of distinct biopsy features and disease characteristics. [44,47,48].
When PM is correctly diagnosed, prognosis depends on early detection and treatment, with complications such as interstitial lung disease or cardiac involvement significantly influencing outcomes. The rest of the entities mentioned are not in the scope of this review. Dermatomyositis (DM) prognosis varies widely depending on disease severity and response to treatment [49,52]. Comorbidities such as Interstitial lung disease (ILD) often lead to a worse prognosis. Additionally, cancer is more prevalent in individuals with DM, especially those diagnosed at older ages [50,51]. Many individuals with dermatomyositis can achieve long-term survival with appropriate management. While muscle weakness can persist, skin manifestations of DM, such as heliotrope rashes and Gottron’s papules, often improve with treatment [52]. With the advent of newer therapeutic strategies, life expectancy for DM patients has improved [49].
Conclusion
Inclusion Body Myositis (IBM), Polymyositis (PM), and Dermatomyositis (DM) are distinct inflammatory myopathies with unique clinical, diagnostic, and therapeutic features. IBM is characterized by progressive, asymmetric muscle weakness and limited response to immunosuppressive treatments, while PM and DM share symmetric muscle weakness and extramuscular manifestations, such as dermatological signs in DM. Accurate diagnosis requires a combination of clinical evaluation, laboratory tests, imaging, and muscle biopsy. Treatment for PM and DM typically involves glucocorticoids and disease-modifying drugs, while IBM management focuses on supportive care, physical therapy, and monitoring comorbidities. Despite advances in understanding these diseases, challenges in treatment persist, and further research into biomarkers and targeted therapies is essential. Early diagnosis and a multidisciplinary approach to care are critical for improving patient outcomes and quality of life. As research progresses, personalized therapies may offer hope for better management and long-term prognosis in these complex conditions.
References
- National Institute of Neurological Disorders and Stroke. Inflammatory myopathies. National Institute of Neurological Disorders and Stroke website.
- Benveniste O (2024) Inflammatory myopathies in 2024: Better classify them to better treat them. Revue Neurologique 180(9): 963-970.
- Khoo T, Lilleker JB, Thong BYH, Leclair V, Lamb JA, Chinoy H (2023) Epidemiology of idiopathic inflammatory myopathies. Nat Rev Rheumatol 19(11): 695-712.
- Panginikkod S, Musa R (2020) Inclusion body myositis.
- Dimachkie MM, Barohn RJ (2014) Inclusion body myositis. Neurol Clin 32(3): 629-646.
- Lindgren U, Pullerits R, Lindberg C, Oldfors A (2022) Epidemiology, survival, and clinical characteristics of inclusion body myositis. Ann Neurol 92(2): 201-212.
- Sarwar A, Dydyk AM, Jatwani S (2020) Polymyositis.
- Medsger TA Jr, Dawson WN Jr, Masi AT (1970) The epidemiology of polymyositis. Am J Med 48(6): 715-723.
- Ohta A, Nagai M, Nishina M, Tomimitsu H, Kohsaka H (2014) Prevalence and incidence of polymyositis and dermatomyositis in Japan. Mod Rheumatol 24(3): 477-480.
- Kronzer VL, Kimbrough BA, Crowson CS, Davis JM 3rd, Holmqvist M, Ernste FC (2023) Incidence, prevalence, and mortality of dermatomyositis: A population-based cohort study. Arthritis Care Res (Hoboken) 75(2): 348-355.
- Bernatsky S, Joseph L, Pineau CA, P Bélisle, J F Boivin, et al. (2009) Estimating the prevalence of polymyositis and dermatomyositis from administrative data: age, sex, and regional differences. Ann Rheum Dis 68(7): 1192-1196.
- Anderson NC, Lloyd TE (2023) Inclusion body myositis: an update. Curr Opin Rheumatol 37(1): 80-85.
- Pinto MV, Laughlin RS, Klein CJ, Mandrekar J, Naddaf E (2023) Inclusion body myositis: correlation of clinical outcomes with histopathology, electromyography and laboratory findings. Rheumatology 61(6): 2504-2511.
- Lynch EM, Pittman S, Daw J, Ikenaga C, Chen S, et al. (2023) Seeding-competent TDP-43 persists in human patient and mouse muscle. Acta Neuropathol 145(3): 365-380.
- Nagaraju K, Lundberg IE (2022) Polymyositis and dermatomyositis: pathophysiology. Rheum Dis Clin North Am 37(2): 159-1571.
- Dalakas MC (2020) Inflammatory muscle diseases. N Engl J Med 372(18): 1734-1747.
- Kobayashi I (2023) Advances in juvenile dermatomyositis: pathophysiology, diagnosis, treatment, and interstitial lung diseases-a narrative review. Front Pediatr 11: 819853.
- Dimachkie MM, Barohn R J (2014) Inclusion body myositis. Neurologic clinics 32(3): 629-646.
- Hiscock A, Dewar L, Parton M, Machado P, Hanna M, Ramdharry, G (2014) Frequency and circumstances of falls in people with inclusion body myositis: a questionnaire survey to explore falls management and physiotherapy provision. Physiotherapy 100(1): 61-65.
- Mohannak N, Pattison G, Hird K, Needham M (2019) Dysphagia in patients with sporadic inclusion body myositis: management challenges. International Journal of General Medicine 12: 465-474.
- Cox FM, Verschuuren JJ, Verbist BM, Niks EH, Wintzen AR, et al. (2009) Detecting dysphagia in inclusion body myositis. Journal of neurology 256(12): 2009-2013.
- Khan S, Christopher-Stine L (2011) Polymyositis, dermatomyositis, and autoimmune necrotizing myopathy: clinical features. Rheumatic Disease Clinics 37(2): 143-158.
- Mammen AL (2010) Dermatomyositis and polymyositis: clinical presentation, autoantibodies, and pathogenesis. Annals of the New York Academy of Sciences 1184(1): 134-153.
- Schumacher HR, Schimmer H, Gordon GV, Bookspan MA, Brogadir, et al. (1979) Articular manifestations of polymyositis and dermatomyositis. The American Journal of Medicine 67(2): 287-292.
- Callen JP (2010) Cutaneous manifestations of dermatomyositis and their management. Current rheumatology reports 12(3): 192-197.
- Hoeltzel MF, Oberle EJ, Robinson AB, Agarwal A, Rider LG (2014) The presentation, assessment, pathogenesis, and treatment of calcinosis in juvenile dermatomyositis. Current rheumatology reports 16(12): 467.
- Lu Z, Guo‐chun W, Li M, Ning Z (2012) Cardiac involvement in adult polymyositis or dermatomyositis: a systematic review. Clinical Cardiology 35(11): 685-691.
- Dickey BF, Myers AR (1984) pulmonary disease in polymyositis/dermatomyositis. In Seminars in arthritis and rheumatism 14(1): 60-76.
- Fathi M, Lundberg, IE, Tornling G (2007) Pulmonary complications of polymyositis and dermatomyositis. In Seminars in respiratory and critical care medicine 28(4): 451-458.
- Le Goff B, Chérin P, Cantagrel A, Gayraud M, Hachulla E, et al. (2009) Pneumomediastinum in interstitial lung disease associated with dermatomyositis and polymyositis. Arthritis Care & Research 61(1): 108-118.
- Dalakas MC (2019) "Inflammatory Muscle Diseases." New England Journal of Medicine 372(18): 1734-1747.
- Allenbach Y, et al. (2020) "Pathogenesis of inflammatory myopathies." Current Opinion in Rheumatology 32(6): 518-525.
- Lloyd TE, et al. (2022) "Myositis-specific autoantibodies and their clinical implications." Rheumatology International 42(5): 781-791.
- Greenberg SA (2019) "Inclusion Body Myositis: Clinical Features and Pathogenesis." Nature Reviews Rheumatology 15(5): 257-272.
- Mammen AL (2020) "Dermatomyositis and Polymyositis: Clinical and Pathological Features." Annals of Internal Medicine 172(5): 301-309.
- TW Bunch, JW Worthington, JJ Combs, DM Ilstrup, AG Engel (1980) Azathioprine with prednisone for polymyositis. A controlled, clinical trial. Ann Intern Med 92(3): 365-369.
- MC Dalakas, I Illa, J M Dambrosia, S A Soueidan, D P Stein, et al. (1993) A controlled trial of high-dose intravenous immune globulin infusions as treatment for dermatomyositis. N Engl J Med 329(27): 1993-2000.
- CN Pisoni, MJ Cuadrado, MA Khamashta, GRV Hughes, DPD' Cruz (2007) Mycophenolate mofetil treatment in resistant myositis. Cruz Rheumatology 46(3): 516-518.
- Rohit Aggarwal, Christina Charles-Schoeman, Joachim Schessl, Mazen M Dimachkie, Irene Beckmann, et al. (2021) Prospective, double-blind, randomized, placebo-controlled phase III study evaluating efficacy and safety of octagam 10% in patients with dermatomyositis ("ProDERM Study"). Medicine 100(1): e23677.
- Nicoline B M Voet, Elly L van der Kooi, Ingrid I Riphagen, Eline Lindeman, Baziel G M van Engelen, et al. (2013) Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev
- 9:(7): CD003907.
- Naddaf E, Shelly S, Mandrekar J, Chamberlain AM, Hoffman EM, et al. (2022) Survival and associated comorbidities in inclusion body myositis. Rheumatology (Oxford) 61(5): 2016-2024.
- Mano T, Iguchi N, Iwasa N, Yamada N, Sugie K (2024) Compound muscle action potential of whole-forearm flexors: A clinical biomarker for inclusion body myositis. Clin Neurophysiol Pract 9: 162-167.
- Perez-Rosendahl M, Mozaffar T (2022) Inclusion body myositis: evolving concepts. Curr Opin Neurol 35(5): 604-610.
- Lundberg IE, Fujimoto M, Vencovsky J, Aggarwal R, Holmqvist M, Christopher-Stine L, Mammen AL, Miller FW (2021) Idiopathic inflammatory myopathies. Nat Rev Dis Primers 7(1): 86.
- Van der Meulen MF, Bronner IM, Hoogendijk JE, Burger H, van Venrooij WJ, et al. (2003) Polymyositis: an overdiagnosed entity. Neurology 61(3): 316-321.
- Xu S, Hu X, Wang J, Xu Q, Han Z, Zhou H, Gao M (2023) Polymyositis and dermatomyositis biomarkers. Clin Chim Acta 547: 117443.
- Halilu F, Christopher-Stine L (2022) Myositis-specific Antibodies: Overview and Clinical Utilization. Rheumatol Immunol Res 3(1): 1-10.
- "Clinical Manifestations of Dermatomyositis and Polymyositis in Adults (2024).
- Guo J, Wang W, Huang A, Mei C (2024) Pharmacological Strategies in Dermatomyositis: Current Treatments and Future Directions. Med Sci Monit 30: e944564.
- Marzęcka M, Niemczyk A, Rudnicka L (2022) Autoantibody Markers of Increased Risk of Malignancy in Patients with Dermatomyositis. Clin Rev Allergy Immunol 63(2): 289-296.
- Teboul A, Allenbach Y, Tubach F, Belin L, Cassius C, et al. (2024) Prognostic factors for patients with cancer-associated dermatomyositis: a retrospective, multicenter cohort study of 73 patients. Rheumatology (Oxford) 26: keae629.
- Robinson ES, Feng R, Okawa J, Werth VP (2015) Improvement in the cutaneous disease activity of patients with dermatomyositis is associated with a better quality of life. Br J Dermatol 172(1): 169-174.






























