Retrospective Analysis of Clinical and Laboratory Data in Systemic Lupus Erythematosus (SLE) Patients
Nermin Keni Begendi1* and Kamil Dilek2
1Afyonkarahisar Health Science University Hospital, Internal Medicine, Hematology Department, Afyonkarahisar, Turkey
2Uludag University Hospital, Internal Medicine, Rheumatology and Nephrology Department Bursa, Turkey
Submission: June 12, 2024; Published: June 28, 2024
*Corresponding author: Nermin Keni Begendi, Afyonkarahisar Health Science University Hospital, Internal Medicine, Hematology Department Afyonkarahisar, Turkey
How to cite this article: Nermin Keni B. Retrospective Analysis of Clinical and Laboratory Data in Systemic Lupus Erythematosus (SLE) Patients. Ortho & Rheum Open Access J. 2024; 23(4): 556116. DOI: 10.19080/OROAJ.2024.23.556116
Abstract
Aim: By retrospectively examining clinical and laboratory data in patients diagnosed with Systemic Lupus Erythematosus (SLE), we aimed to evaluate their relationship with each other and with organ involvements with high morbidity and mortality, especially Lupus Nephritis, and to characterize the clinical and laboratory data in patients with late onset of lupus (LSLE).
Patients and Method: The archived file information of 180 patients who applied to Uludağ University, Faculty of Medicine Rheumatology/Nephrology Department between 1980 and 2010 years and were diagnosed with SLE according to American College of Rheumatology (ACR) criteria and followed up were retrospectively examined; age, gender, disease age, follow-up period, disease activation periods, current treatments and laboratory data were analyzed. In the analysis of the files, information at the time of diagnosis was mostly used, and current information during follow-up was obtained from the files or from the patients themselves. Descriptive statistics were calculated for all data in our study. Standard error was given as a measure of variability. The normality assumption of continuous variables was determined by the Kolmogorov-Smirnov test. Mann-Whitney U test was used for comparisons between groups. Pearson chi-square and Fisher’s exact chi-square tests were used to compare categorical variables. Chi-square and binomial tests were used to compare the rates. Data analysis was done in the SPSS-13 package program. A value of p < 0.05 was accepted for statistical significance. Ethics committee approval was received for the study, dated January 11, 2011, numbered 2011-2/20.
Findings: In the comparison of laboratory and clinical findings between cases with and without Lupus Nephritis; It was observed that the mean disease age, follow-up period, sedimentation values and activation indicators were significantly higher in patients with Lupus Nephritis. Significant associations were also seen between clinical and laboratory data. It was observed that the frequency of Lupus Nephritis and the findings accompanying active disease such as fever and malar rash decreased in late onset of lupus patients.
Conclusion: Careful examination of clinical and laboratory data is of great importance in the diagnosis and follow-up of patients diagnosed with SLE, as well as in the monitoring of organ involvements with high mortality and in order to make an effective treatment plan. Knowing that the characteristics of late-onset of lupus patients are different is important in disease management.
Keywords: Systemic lupus erythematosus; Clinical data; Laboratory data; Lupus nephritis; Late-onset of lupus disease
Abbreviations: SLE: Systemic Lupus Erythematosus; ACR: American College of Rheumatology; Fas: Fibroblast associated; LN: Lupus Nephritis; WHO: World Health Organization; ISN/RPS: International Society of Nephrology/Renal Pathology Society; RF: Rheumatoid Factor
Introduction
Systemic lupus erythematosus (SLE); is a heterogeneous, multisystemic, chronic, autoimmune, inflammatory disease caused by tissue and organ damage caused by pathogenic autoantibodies and immune complexes. The affected organs are mainly skin, joints, kidney, central nervous system, cardiovascular system, serosal membranes, hematological and immune system. The disease progresses with exacerbations and remissions in affected individuals [1]. Although SLE can be seen at any age and in any gender, it is more common in women between the ages of 20-40, especially in women of reproductive age. While the female-male ratio is 8-13/1 in adults, it is 1.4-5.8/1 in children and 2-3/1 in older ages [1]. The incidence is reported to be between 15-50 per 100,000 people [1,2].
Although the etiology of the disease has not been clearly elucidated, genetic, hormonal and environmental factors that lead to inflammatory response play a role in the etiology. In studies, the role of various molecules involved in apoptotic pathways, especially fibroblast associated (Fas) and the cross-linked form of Fas (Fas ligand, FasL), in the pathogenesis of autoimmune diseases has gained importance. Studies have shown that defective expression of Fas ligand in mice causes systemic autoimmunity and lymphoproliferation [3]. The ensuing production of autoantibodies and immune complexes, autoreactive T cells and B cells, complement activation, and cytokine release result in widespread tissue damage, manifesting as the clinical picture of SLE [4-6].
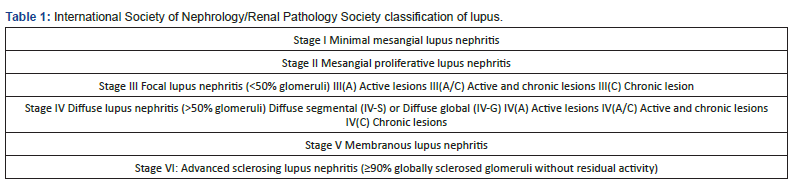
Renal involvement is a significant cause of morbidity and mortality and is associated with poor prognosis. It is usually seen in the first 5 years after diagnosis, and its frequency has been reported to be between 30-90% in studies. According to the American College of Rheumatology (ACR) criteria, the incidence of lupus nephritis (LN) 5 years after diagnosis is stated to be 60% [1,4]. Urinary abnormalities are present in approximately 50% of patients at the time of diagnosis and are observed in approximately more than half of the cases throughout the course of the disease. Renal involvement according to ACR criteria is defined as the presence of proteinuria of more than 0.5 g per day or 3(+) or more in a complete urine examination, and the presence of 5 or more cellular elements (erythrocytes, leukocytes) and casts (granular, tubular, mixed) in each area in the urine sediment [1,7,8]. While the most prominent finding in renal involvement is proteinuria, nocturia and newly developing hypertension are also warning signs for diagnosis. While proteinuria has been reported in almost all cases, microscopic hematuria is seen in approximately 80% of the cases throughout the course of the disease, and macroscopic hematuria is rare. If serum albumin is low in SLE, the first reason that comes to mind should be LN. Anti-dsDNA positivity with low complement and anti-Ro positivity with rheumatoid factor (RF) carry a high risk for LN [7-9]. LN was first classified by the World Health Organization (WHO) in 1974 [8]. It was revised by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) in 2003 [2,5,9] (Table 1).
While the definition of late-onset of lupus (LSLE) is used for patients diagnosed with SLE after the age of 50, this patient group constitutes approximately 4-20% of all patients. It is stated that the female-male ratio, which is stated as 8-13/1 in adults, is seen as 2/1 in older ages [10]. The characteristics of clinical findings in IYBL disease have been reported differently in different studies. Musculoskeletal system involvement, fever, weight loss, arthritis and serositis are most commonly reported [10].
Results
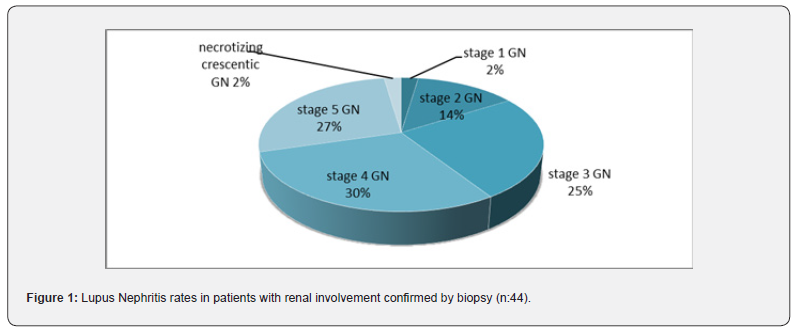
166 (92.2%) of the patients were female and 14 (7.8%) were male. The average age was 41.6 ±0.9 years. During the diagnosis and treatment process, kidney biopsy was performed in 48 cases for the purpose of confirming kidney involvement and histopathological staging; 42 of them (87.5%) were women and 6 (12.5%) were men. In 44 of 54 patients with renal involvement high rates of stage 3 (25%), stage 4 (29.5%) and stage 5 (27.3%) lupus nephritis were found in the biopsies of the patients (Figure 1). The average disease age is 90.4±5.4 months; It was 113±11.0 months in those with kidney involvement and 80.7±5.9 months in the others (p=0.014). The average activation period was 21.5±2.1 months; It was 22.8±3.8 months in patients with renal involvement and 20.9±2.6 months in other patients (p=0.024). Kidney involvement was detected in a total of 54 patients (30%) (Table 2: The most common clinical findings in our patients).
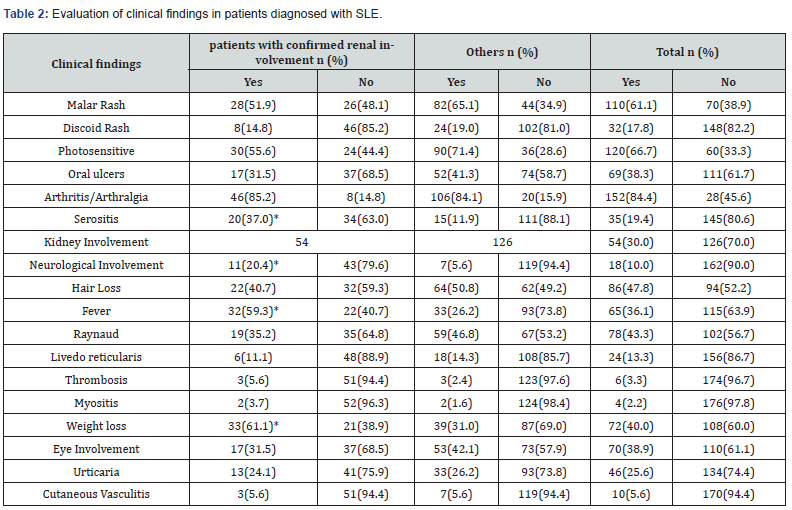
(*: p<0.05).
Laboratory findings of leukopenia, lymphopenia and thrombocytopenia, proteinuria, rich urine sediment and low complement were observed more frequently in patients with confirmed LN than in others; The difference was statistically significant (p<0.05). Similarly, creatinine levels were determined to be higher in cases with confirmed LN (p=0.005). In the evaluation made for autoantibodies in all patients; Among specific autoantibodies, ANA positivity was seen in 94.4% in total and anti-Ds DNA antibody positivity was seen in 28.9%, while the fact that anti-Histone antibody positivity was higher in the group with LN was found to be statistically significant (p<0.05). LSLE was observed in 23(12.8%) of our patients. Their average age was 60.7 years.
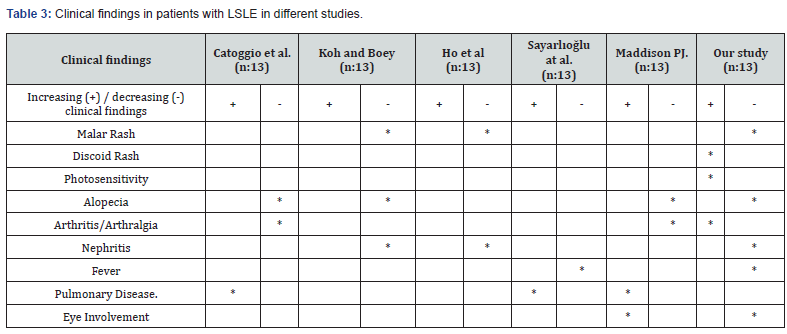
Of the patients diagnosed with LSLE, 14 (60.9%) had malar rash, 8 (34.8%) had oral aphthous ulcers, 20 (87%) had arthritis/ arthralgia (for all of these p> 0.05). Livedo, myositis and thrombosis were not observed. Lupus nephritis rate was calculated as 21.7% in this patient group (Table 3: Clinical features seen in other studies and our study).
Discussion
SLE, like other autoimmune diseases, is a disease that affects women more frequently, has a heterogeneous etiopathogenesis, and its etiology has not yet been clearly elucidated. The femalemale ratio, which is stated as 8-13/1 in adults, was found to be 11.8/1 in our study. In SLE, clinical and laboratory data guide the diagnosis and follow-up of the disease and the choice of treatment in the presence of organ involvement. Treatment should be individualized on a patient-by-patient basis after detailed evaluation of the data. The disease is mostly seen between the ages of 20-40. In our study, the average patient age was 41.6 years. The slightly higher mean age in women was associated with the use of the age of the cases in the year the study was conducted. The mean disease age, mean follow-up period and mean activation times were found to be higher in patients with confirmed lupus nephritis than in other patients. The difference between the groups was statistically significant.
Ethnicity and geography are of great importance in disease surveillance and renal prognosis. It is known that the disease, which has a poor prognosis in blacks and Asians, has a good prognosis in whites and Caucasians and the risk of renal involvement is low [10]. The majority of the population in Turkey’s geography and the Southern Marmara region, which we included in our study, is white and consists of a community of heterogeneous races. Lupus nephritis, which is reported to be between 30-60% according to ACR, was observed with a frequency of 30.0% in our patients, while this rate was 27.9% in the Euro-Lupus Project study, while the rate was 40.2% in the study series conducted in America and 74% in the studies conducted in Asia. The low rate found in our study may be associated with the significant impact of ethnic, racial and geographical characteristics on disease pathogenesis.
Frequency of serositis in our study was lower than stated in the literature, it was found to be higher in men, as in the Euro- Lupus Project study. Neurological involvement; as in our study, was seen less frequently in the other three studies than in the literature, as the reason; the difficulty in associating involvement with SLE in the presence of accompanying comorbid conditions may be shown. The higher frequency of Sicca syndrome/ocular involvement and Raynaud syndrome in our study than in the literature can be explained by the presence of accompanying rheumatoid arthritis, sjögren’s syndrome, dermatomyositis and other overlap syndromes in our cases. In studies, while malar rash, photosensitivity and arthritis/arthralgia are more common in women, serositis has been reported more in men [11].
In our study, consistent with the literature, serositis which usually accompanies active disease was detected with a frequency of 28.6% in men and 18.7% in women. Malar rash, photosensitivity and arthritis/arthralgia; in our patient population, it was observed more frequently in women, in line with the literature, and the difference caused by photosensitivity was observed to be statistically significant (p = 0.03). Renal involvement usually accompanies clinical and laboratory active disease findings (serositis, neurological involvement, high fever, leukopenia, lymphopenia, thrombocytopenia, high sedimentation, hypocomplementemia, proteinuria and rich urine sediment).
In our center, lupus nephritis was confirmed by biopsy, which is the gold standard method, in 44 of 54 patients with renal involvement. High rates of stage 3 (25%), stage 4 (29.5%) and stage 5 (27.3%) lupus nephritis were found in the biopsies of these cases; This can be explained by the fact that later biopsy stages of patients whose biopsies were not available at the time of diagnosis were used in data collection and that a transition from one histological pattern to another was observed over time. It has been reported in the literature that ESRD develops in around 5-20%. While the low rate of 3.4% in our cases can be explained by the small number of patients included in the study, it also shows the effectiveness and benefits of the treatments.
In examining LSLE patients; In the Euro-Lupus Project study (n:1000), cases with LSLE were observed with a frequency of 9%, and in our study (n:180); The frequency of LSLE seen in 23 patients (12.8%), was consistent with the literature. Renal involvement, which was found to be 30.0% in all our cases, decreased to 21.7% in these cases, consistent with the literature. When the clinical findings of the patients are examined; the frequency of malar rash, oral aphtha, raynaud’s phenomenon and renal involvement decreased in cases with LSLE; it was found that the frequency of discoid rash and arthritis/arthralgia increased in parallel with some studies. Serositis, neurological involvement and ocular involvement, which were seen to be highly prevalent in studies in the older age-onset patient population, were observed less frequently in our LSLE patient population. The positivity rates of specific autoantibodies used in the diagnosis of the disease and their relationship with clinical findings may show racial differences. In addition, anti-Histone antibody positivity with serositis, anti-nucleosome and anti-histone antibodies with high fever, anti Jo-1 antibody with thrombosis, anti-Ro-52 antibody with sjögren’s/eye involvement and urticaria, anti RNPSm and anti-RNP-Sm with raynaud phenomenon were observed compatible.
Conclusion
In order to determine the course of the disease in our country, to make an accurate treatment plan for organ involvement, and to contribute to disease surveillance, multi-center studies with large patient groups need to be conducted in our geography. Once the etiology of the disease is clarified, more target-specific treatments will come to the fore and treatment success will increase. Randomized studies with large patient populations are needed to understand the characteristics and course of LSLE disease and to make follow-up and treatment plans.
Limitations
This study was planned retrospectively and patients from one region of Turkey were included in the study and the number is partially sufficient to contribute to science.
References
- Goldman L (2000) Systemic Lupus Erythematosus In: Goldman L. (eds). Cecil Textbook of Medicine. (21st edition); New York: Elsevier Saunders pp. 1509-1517.
- Appel GB, Radhakrishnan J, D’ Agati V (2007) Secondary glomerular disease. In: Brenner BM, Levine SA, (eds). The Kidney. (8th edition). Philadelphia: WB saunders pp. 1067-1148.
- De Albuquerque D A, Ebling F M, Lienesch DW, Ram Raj S, Bevra Hahn H (2004) Analyses of serum levels of type1, type2 and type3 cytokines reveal multiple abnormalities in lupus-prone (NZB-NZW) F1 mice. APLAR J Rheum 7(1): 3-10.
- Karrar S, Cunninghame Graham DS (2018) Abnormal B cell development in systemic lupus erythematosus: what the genetics tell us. Arthritis Rheumatol 70(4): 496-507.
- Kiremitci S, Ensari A (2014) Classifying lupus nephritis: An ongoing story. Scientific World J 2014: 580620.
- Schieppati A, G. Remuzzi G (2008) Novel therapies of lupus nephritis. In: Barry M. Brenner (Eds). Current opinion in Nephrology and Hypertension, (21st Edition), Wolters Kluwer health/ Lippincott Williams & Wilkins 17: 156-165.
- Mok CC, Tang SSK (2004) Incidence and predictive factors of renal disease in Chinese patients with systemic lupus erythematosus. Am J Med 117(10): 791-795.
- Cameron JS (1999) Lupus nephritis. J Am Soc Nephrol 10(2): 413-411.
- Weening JJ, D’ Agati V, Schwartz MM, Surya VS, Charles EA (2004) The classification of glomerulonephritis in systemic lupus eryhematosus revisited. Kidney Int 65(2): 521-30.
- Cervera R, Espinosa G, D’ Cruz D (2009) Systemic Lupus Erythematosus: Pathogenesis, Clinical Manifestations and Diagnosis. Eular course Rheum Dis 1-27.
- Cervera R (2006) Europan Lupus Project. Autoimmunity Rev 5(3): 180-186.






























