Alkaptonuria: A Case Report and Review of the Musculoskeletal Manifestations
Dr. Andrea Montalbano*, Tessa Phillips, Dr. Osama Elattar and Dr. Hossein Elgafy
Department of Orthopedic Surgery, University of Toledo, USA
Submission: February 12, 2024; Published: February 23, 2024
*Corresponding author: Dr. Andrea Montalbano, Department of Orthopedic Surgery, University of Toledo, 3000 Arlington Ave. Toledo OH 43614, USA
How to cite this article: Dr. Andrea Montalbano*, Tessa Phillips, Dr. Osama Elattar and Dr. Hossein Elgafy. Alkaptonuria: A Case Report and Review of the Musculoskeletal Manifestations. Ortho & Rheum Open Access J. 2024; 22(): 556100. DOI: 10.19080/OROAJ.2024.22.556100
Abstract
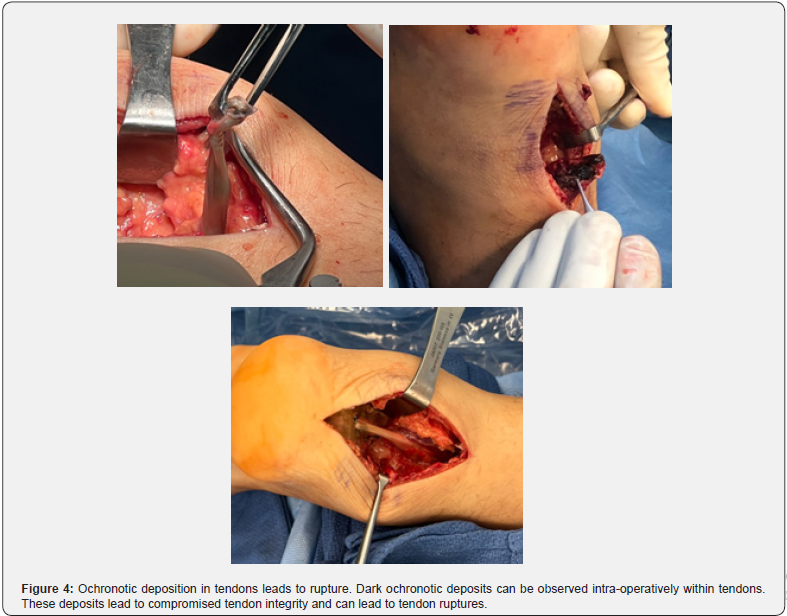
Alkaptonuria is a rare metabolic disorder that involves the inability to break down homogentisic acid, which then accumulates in connective tissues. This accumulation of ochronotic deposits in the intra-articular cartilage leads to brittle cartilage, which is susceptible to fragmentation and ultimately presents as early onset arthropathy. In tendons, the deposits lead to compromised tendon integrity and can lead to tendon ruptures. The earliest signs of alkaptonuria involve observation of dark pigment in the urine when the urine is oxidized or alkalized. However, frequently the disease progresses into its later stages when the diagnosis is made. In the case presented, we follow a 40-year-old male who initially presented to our institution with degenerative spinal changes going back several years and a history of Achilles tendon rupture. He continued to be observed further for his progressive spinal degeneration and experienced contralateral distal Achilles tendon rupture, of which dark discoloration of the tendon was found during the surgical repair. His history of degenerative spinal changes, bilateral Achilles tendon ruptures, and urinalysis led to the diagnosis of alkaptonuria.
Introduction
Alkaptonuria is a rare autosomal recessive metabolic disorder that involves a deficiency in the enzyme homogentisic acid oxidase (HGD) [1]. This deficiency leads to an accumulation of homogentisic acid (HGA). Early signs of alkaptonuria involve excess HGA being excreted in the urine, which can be observed as a dark pigment in the urine upon oxidation or alkalinization. Later signs involve degradative changes to connective tissue, which can be observed intraoperatively as deposits of dark pigment in tendons and joints [2]. This phenomenon is defined as ochronosis. The most significant complications of alkaptonuria ochronosis are ochronotic arthropathy, cardiovascular ochronosis, genitourinary tract obstruction, and ocular and cutaneous ochronosis [1]. Musculoskeletal involvement is a very serious complication of this disorder [2]. The mechanism by which HGA deposits in connective tissue is relatively unknown, but it is hypothesized the deposits cause cartilage to become brittle which decreases elasticity, leading to fragmentation. These degradative changes lead to the common ochronotic arthropathy that is observed with alkaptonuria [3]. The most common joints to be affected are weight bearing joints, with the spine and knee being the most prevalent. The spine is most often the first to show signs of ochronotic arthropathy, which includes loss of lumbar lordosis, disc prolapse, and osteophyte formation [4]. It is also common to observe degradative changes to tendons, which can be recognized intraoperatively with dark deposits in the tissues. Involvement of the Achilles tendon and patellar tendon are the most common [5]. Here we present a case of a patient who was presented at the age of 40 with multiple musculoskeletal manifestations of alkaptonuria, including severe ochronotic arthropathy of the spine and bilateral Achilles tendon involvement.
Body
The pathophysiology of alkaptonuria starts with the inability to break down HGA due to mutations in the homogentisate 1,2-dioxygenase (HGD) gene, resulting in a defective enzyme. This defect results in elevated levels of HGA. More recent studies have further investigated the mechanism by which HGA accumulation is producing the pathologic changes seen in patients with alkaptonuria. HGA has been hypothesized to be the driving force behind local inflammation and oxidation in the affected tissue [6]. HGA induced protein oxidation, which subsequently leads to lipid peroxidation (LPO), has been shown to play a key role in cartilage degradation [7]. LPO produces HCN which alters cell viability and the metabolic activity of chondrocytes, leading to cell death of chondrocytes [7]. These degradative changes at the cellular level leads to complex changes seen on a larger scale, such as the ochronotic arthropathy seen in AKU.
Ochronotic arthropathy characteristically presents early onset spinal and large joint changes. It is defined by the ochronotic pigment deposits in the cartilage of the joints. These deposits lead to the degradative changes seen in patients with alkaptonuria. Taylor et al. [8] describes the pathophysiology behind the degradative joint changes at the cellular level. Ochronotic pigment deposition increases the stiffness of the cartilage, making the matrix more susceptible to mechanical damage. Therefore, joints that endure significant mechanical stress are first to show symptoms. Further histologic evaluation of the chondrocytic changes in AKU samples showed abnormalities in cellularity, with zones of flattened ellipsoidal chondrocytes localized to regions of damage, increased lipid peroxidation corresponding to areas of increased oxidative stress and increased apoptotic bodies [7]. The clinical signs and symptoms of a patient experiencing these degradative changes will first present with signs of arthropathy at an abnormally early age typical for osteoarthritis. Frequently, patients will present with these changes in the 4th decade of life. AKU patients presenting with spinal changes have commonly reported stiffness. Imrich et al. [9] showed the most common radiographic findings to be intervertebral disc space narrowing, calcifications, vacuum phenomena, and osteophytes. They also observed a higher severity score when the spinal changes were in the lumbosacral and thoracic region.
The ochronotic pigment observed in the pathogenesis of joint degradation can also involve connective tissues, including tendon, heart, eyes, and ears. Due to the high collagen content in the tendons, there is a propensity to observe increased deposition of the ochronotic pigment. The accumulation of homogentisic acid inhibits collagen cross linking, ultimately compromising the integrity of tendon, leading to tendinopathy and tendon ruptures, with the patellar and Achilles tendons being the most common [4].
Case Presentation
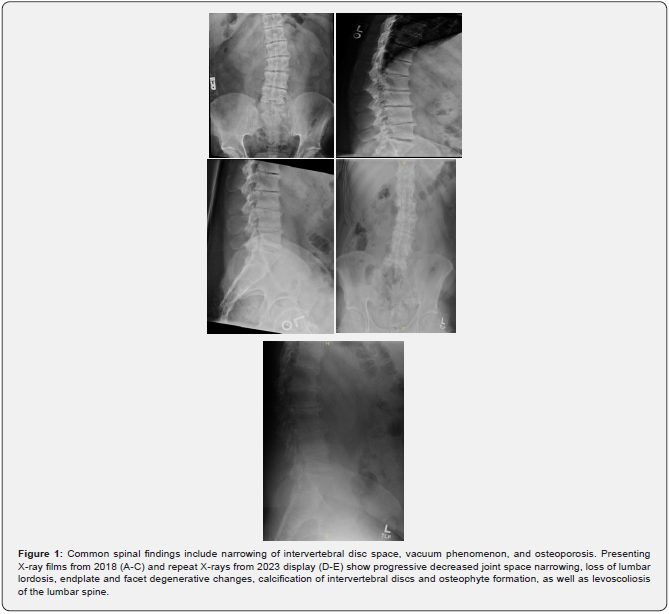
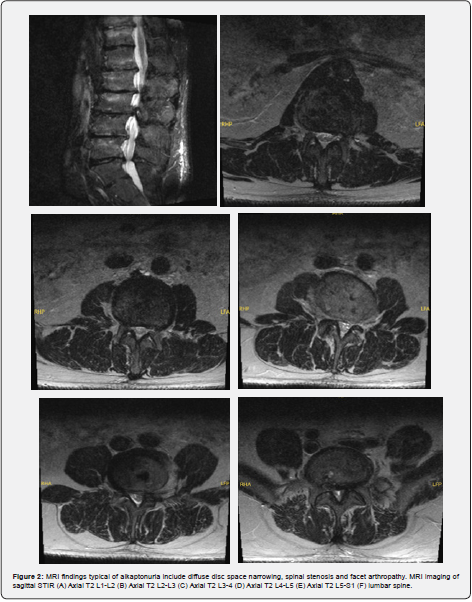
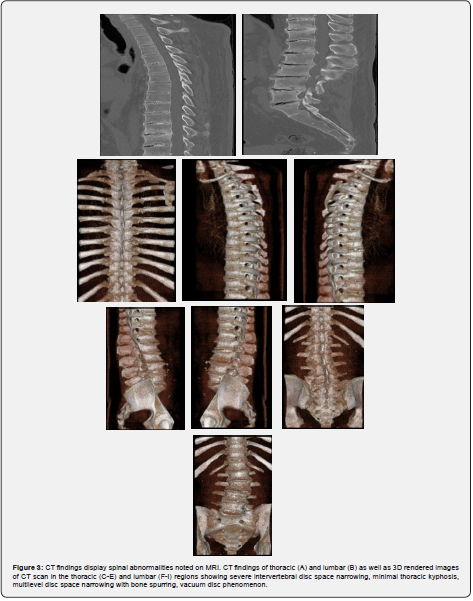
Patient is a 40-year-old Caucasian male with a history of bilateral Achilles tendon ruptures, degenerative spine changes, and discoloration of urine. In 2017, the patient was presented with a partial, acute, spontaneous right Achilles tendon rupture. The posterior aspect of the tendon remained intact, but the anterior portion was disrupted. The tendon was repaired with #2 Fiber Wire sutures in a whipstitch fashion with 4.75mm SwiveLock anchors. The repair was supplemented with a gastrocnemius resection lengthening via the Vulpius procedure, as there was a significant gap following repair. Tendinous discoloration was not noted at this time. The postoperative course was unremarkable. In 2018, the patient presented with complaints of lower back pain and displayed signs of degenerative disc disease and levoconvex lumbar spinal curvature (Figure 1). CT and MRI revealed severe intervertebral disc space narrowing, minimal thoracic kyphosis, multilevel disc space narrowing with bone spurring, and vacuum disc phenomenon. CT of the lumbar spine confirmed spinal abnormalities previously seen on MRI (Figures 2 & 3). Conservative management for low back pain was initiated at this time. In December of 2021, the patient presented with acute, spontaneous distal contralateral left Achilles rupture. At this time, a blue-black discoloration was noted within the Achilles tendon (Figure 4). As the Achilles tendon was not amenable to repair, it was decided to supplement it with a flexor hallucis longus (FHL) tendon transfer. The FHL was whipstitched with Fiber loop and fixation was augmented with an Endo button through the calcaneus and Biotenodesis screw fixation. Tendon samples were sent to the lab for tendon culture and anaerobic culture, which was negative. The post-operative course was uncomplicated. In 2023, the patient presented with an interval increase in low back pain (Figure 1). At this time, the patient was referred to pain management for a continuation of conservative management. The patient was tested for homogentisic acid deficiency, which was markedly elevated (836 mmol/mol creatinine; ref values <11). This value was diagnostic for alkaptonuria.
Conclusion
Alkaptonuria is a rare metabolic disease that results in a multitude of degenerative changes in collagen rich areas of the body. Early onset of ochronotic arthropathy consistently presents in the spine, leading to long term debilitating changes. Tendon involvement presents as tendon ruptures, commonly in the Achilles, due to the accumulation of ochronotic pigment, resulting in compromised tendons that are more susceptible to rupture. These common signs of alkaptonuria were seen in the patient discussed in this case, showcasing the importance of being able to distinguish a rare underlying condition, such as alkaptonuria, during multiple musculoskeletal pathologies.
References
- Gaines JJ Jr (1989) The pathology of alkaptonuric ochronosis. Hum Pathol 20(1): 40-46. Hum Pathol 20(5): 500.
- Mannoni A, Selvi E, Lorenzini S, Giorgi M, Airo P, et al. (2004) Alkaptonuria, ochronosis, and ochronotic arthropathy. Semin Arthritis Rheum 33(4): 239-248.
- Ranganath LR, Khedr M, Vinjamuri S, Gallagher JA (2021) Characterizing the alkaptonuria joint and spine phenotype and assessing the effect of homogentisic acid lowering therapy in a large cohort of 87 patients. J Inherit Metab Dis 44(3): 666-676.
- Wu K, Bauer E, Myung G, Fang MA (2018) Musculoskeletal manifestations of alkaptonuria: A case report and literature review. Eur J Rheumatol 6(2): 98-101.
- Perry MB, Suwannarat P, Furst GP, Gahl WA, Gerber LH (2006) Musculoskeletal findings and disability in alkaptonuria. J Rheumatol 33(11): 2280-2285.
- Braconi D, Millucci L, Bernardini G, Santucci A (2015) Oxidative stress and mechanisms of ochronosis in alkaptonuria. Free Radic Biol Med 88(Pt A): 70-80.
- Millucci L, Giorgetti G, Viti C, Ghezzi L, Gambassi S, et al. (2015) Chondroptosis in alkaptonuric cartilage. J Cell Physiol 230(5): 1148-1157.
- Taylor AM, Boyde A, Wilson PJ, Jarvis JC, Davidson JS, et al. (2011) The role of calcified cartilage and subchondral bone in the initiation and progression of ochronotic arthropathy in alkaptonuria. Arthritis Rheum 63(12): 3887-3896.
- Imrich R, Sedlakova J, Ulehlova M, Gornall M, Jackson R, et al. (2022) Radiological evolution of spinal disease in alkaptonuria and the effect of nitisinone. RMD Open 8(2): e002422.






























