Efficacy of Avosoya®300 (Avocado/Soybean Unsaponifiable) on Cartilage Biomarkers among Egyptian Patients with Early Symptomatic Knee Osteoarthritis. A multicenter Double Blind Randomized, Placebo Controlled Cross-over Study
Hesham Hamoud1*, Yasser A.Elmotaleb1, Ahmed Elyasaki2, Sherif Refaat3, Ali El deeb4, Ayman Darwish5, Rawhia Rabie5, Hassnaa Ragab6, Sabrien Hamza6, Mohamed Abdelkader7, Mohamed O Elshabrawy7, Mohamed I.Elattar7, Ayman Y Eltabbaa7, Abd Elhalem Abo Elsoud8 and Ashraf Eltabiey9
1Department of Rheumatology and Rehabilitation, Al-Azhar University, Egypt
2PMR, Ain Shams University and Program director of P&O, AHS, Galala University
3Department of Rheumatology and Rehabilitation, Mansoura University, Egypt
4Department of Rheumatology and Rehabilitation, Tanta University, Egypt
5Department Rheumatology and Rehabilitation, Al Menia University, Egypt
6Department Rheumatology and Rehabilitation, MOH Hospitals, Egypt
7Department of orthopedics, MOH Hospitals, Egypt
8Department of Neurosurgery, MOH Hospitals, Egypt
9Department of orthopedics, Al-Azhar University, Egypt
Submission:February 27, 2022; Published: March 17, 2023
*Corresponding author: Hesham Hamoud, Department of Rheumatology and Rehabilitation, Al-Azhar University, Egypt
How to cite this article: Hesham Hamoud, Yasser Abdulmutallab, Ahmed Elyasaki, Sherif Refaat, Ali El deeb, et al. Efficacy of Avosoya®300 (Avocado/ Soybean Unsaponifiable) on Cartilage Biomarkers among Egyptian Patients with Early Symptomatic Knee Osteoarthritis. A multicenter Double Blind Randomized, Placebo Controlled Cross-over Study. Ortho & Rheum Open Access J. 2023; 21(3): 556065. DOI: 10.19080/OROAJ.2023.21.556065
Abstract
Background: In osteoarthritis (OA), biochemical catabolic biomarkers receive increasing attention due to their superior sensitivity and objectivity. OA biochemical biomarkers have previously been studied in relation to structural changes. However, this study focused on early OA stages associated with knee pain. As a result of early diagnosis, patients with OA could be better managed, allowing for more personalized treatment options.
Objective: In this multicenter Double Blind Randomized, placebo Controlled Cross-over Study we aimed to estimate the symptomatic efficacy and carry-over effects of AVOSOYA®300 one capsule/day on patients with early symptomatic knee OA “ESKOA”.
Patients and Methods: This study included 275 patients with early symptomatic knee osteoarthritis (ESKOA) according to Migliore, et al. [1] criteria. The patients were randomized to receive either ASU (n=140) or the same capsule placebo / day ( n=135) for the first 6 months. clinical assessment for pain by Visual Analogue Scale (100mm scale), Frequency of occurrence of pain/week and the necessity to use NSAID (and/or) analgesics/week was done. Laboratory urinary CTX-II (uCTX-II) and serum COMP (sCOMP) at the baseline, at six months and at 1year.
Results: Our results showed that, the final included patients were 275 cases. Their ages ranged from 23 to 49 (41.17+5.73); They were 218 females and 57males. Among the ASU group, uCTXII, sCOMP, VAS score, pain frequency and NSDAIDs use /week were significantly improved after 12 months compared to that at baseline, 6 months and to that of placebo group.
Conclusion: This RCT study showed that, in early symptomatic knee OA patients, AVOSOYA300mg/day supplement improve both subjective and objective parameters. Subjectively, it decreases the pain frequency and severity; Also minimize the NSAIDs/pain killer needs. Objectively, this is confirmed through regression of catabolic cartilage biomarkers as uCTXII in the first 6 months of treatment and both uCTX-II and sCOMP afterwards.
Keywords: Early symptomatic knee osteoarthritis (ESKOA); urinary CTX-II (uCTX-II) and serum COMP (sCOMP); Avocado/Soybean Unsaponifiables (ASU)
Introduction
Osteoarthritis (OA) is a low-grade inflammatory disease of synovial joints and by far the most common form of arthritis [1]. Chronic OA will result in chronic pain, physical, psychic and social disability in older individuals [2]. Histologically, it is characterized by progressive deterioration and loss of hyaline cartilage [3] with concomitant structural and functional changes in the whole joint components, including the synovium, meniscus, periarticular soft tissues, and subchondral bone [4]. Cartilage homeostasis relies on the controlled catabolism of matrix proteins such as collagen and the replacement with newly synthesized proteins by chondrocytes [5]. Cross-linked C-telopeptides of type II collagen (CTX-II) and Cartilage oligomeric matrix protein (COMP) are two biochemical markers. They are degradation products of joint tissues, especially the cartilage extracellular matrix and can potentially predict the destruction of articular cartilage in OA. Many studies of early OA diagnosis have only measured one of these two biomarkers (CTXII and COMP) at a single time point during the pathological process of OA [6]. In a previous study published in 2020 by Hamoud et al., the concentration of urinary CTX-II (uCTXII) was significantly higher in the Early-Stage Knee Osteoarthritis (ESKOA) patients at the base line before using ASU and markedly decreased after 6 months of therapy [7]. Also, the uCTXII level is highly sensitive to the very early structural changes in the articular collagen.
Cartilage oligomeric matrix protein (COMP) is a tissue specific protein, binds to type II collagen fibers and can stabilize the collagen fiber network of articular cartilage. When the articular cartilage is destroyed, COMP is released into the synovial fluid and absorbed by the serum. Accordingly, the serum levels of CTX-II and COMP reflect the metabolism of type II collagen fibers [8]. Serum COMP (sCOMP) concentrations were found to be significantly higher in knee osteoarthritis with early signs of cartilage damage. The serum level of cartilage oligomeric matrix protein sCOMP levels were the highest significantly associated with the development of radiographic OA changes and painful radiographic knee OA [8]. In a previous study, there was a non-significant decrease in the median serum COMP level at 6 months treatment, because COMP decreased significantly only at a long period of OA changes with the disease progression [9].
Avocado/Soybean Unsaponifiables (ASU) are natural vegetable extracts made from avocado and soybean oils, consisting of the leftover fraction (approximately 1%) that cannot be made into soap after saponification. ASU is composed of one third Avocado and two thirds Soybean; the major components of ASU are phytosterols β-sitosterol, camp sterol and stigmasterol; which are rapidly incorporated into cells. ASU is a complex mixture of many compounds including fat-soluble vitamins, sterols, triterpene alcohols and possibly furan fatty acids. The identity of the active component remains unknown. The sterol contents of Avocado/soybean preparations are the primary contributors to biological activity in the articular chondrocytes [10]. Avocado/soybean unsaponi fiables possesses chondroprotective, anabolic and anticatabolic properties. It inhibits the breakdown of cartilage and promotes cartilage repair by inhibiting a number of molecules and pathways implicated in OA, also stimulates the synthesis of collagen and aggrecan by inhibiting inflammatory cytokines such as IL-1, IL-6, IL8, TNF and PGE2 through modulation of NF-kappa B [11].
Objectives
To estimate the symptomatic efficacy and carry-over effects of AVOSOYA®300 one capsule/day on patients with early symptomatic knee OA “ESKOA”.
The primary Endpoints were to monitor the changes in uCTX-II & sCOMP levels at the baseline, 6 and 12 months; and to monitor the associated changes in VAS at the baseline, 6 and 12 months.
The Secondary Endpoints were to compare the changes of the frequency of knee pain/week and NSAIDs/analgesic use for knee pain at baseline, 6month and at 12 months.
Patients and Methods
This multicenter double blind placebo control study was conducted among a total number of 878 patients with Early Symptomatic Knee Osteoarthritis (ESKOA). The patients were initially assessed for eligibility in this study. The study duration was 18 months, during the period from June 2021 till December 2022. All patients provided signed informed consent prior to the study start. The study nurse or the co-investigator made telephone calls to the patients to check on patient compliance and comfort. NSAIDs and paracetamol 500mg were allowed as rescue analgesia during the whole study period in case of severe pain and patients were asked to record the number of patient were asked to record the number of tablets used per day in a patient diary tablets used per day in a patient diary.
Inclusion criteria: Two mandatory symptoms according to Migliore et al. [1].
i. Any Knee pain without any recent trauma or injury and
ii. Very short joint stiffness, lasting 5-10 min, when starting movement;
iii. Lasting ˂6 months and
iv. Without risk factors.
Exclusion criteria
i. Active inflammatory arthritis or generalized pain;
ii. Patients aged ≥ 50 years;
iii. Patients aged ≥ 40 years if at least 1 risk factor present;
iv. Kallgren and Lawrence ˃1 [12] and
v. Any recent trauma or injury.
Starting (at Week0-4), by screening the patients, receiving informed consent, taking detailed medical history including demographic data; Then general physical examination (vital signs, weight, height and BMI) followed by meticulous local knee examination. Blood and urine sample were collected at baseline, 6months and 1 year. All patients were made to rest for half an hour in seated position. Blood samples were drawn in late midday to avoid diurnal variations. Five ml whole venous blood sample were drawn in syringe and collected in plain vial with aseptic technique. Also, all subjects’ urine samples were taken in early morning on the same day. Samples were sent to laboratory for estimation of Serum COMP and urinary CTX-II levels by ELISA technique. After a 1-week NSAID washout period, patients were randomized to receive either ASU (n=140) or the same capsule placebo / day (n=135) for the first 6 months, then subdivision and Cross-over was done with switching the medication after 6 month in a randomization pattern, where ASU group divided into G1(ASU- ASU n=80), G2 (ASU-Placebo n=60); Placebo group divided into G3 (Placebo-Placebo n=67), G4 (Placebo-ASU n=68). Figure 1 Medication-refill on regular follow-up every 1month till the end of the study duration (12month). Adverse events, if applicable, were reported immediately in a separate form. uCTX-II and sCOMP were measured at base-line, at 6 months, then after 12 months.
Statistical Analysis

Data recording, processing and statistical analysis after completion of the study. All the data were transferred to IBM card, using IBM-PC with statistical program “Statistical Package for Social Sciences” (SPSS Inc., Chicago, Illinois, USA), Version-20.0. Test for Normality was applied to the data. Descriptive statistics was performed for all variables of the study. For quantitative variables, the mean, range, standard deviation (+SD), Standard Error of the Mean (+SEM) was calculated. For categorical variables, absolute counts as well as percentages were generated. Comparison between two groups with parameteric data was done by Student “t” test, for non-parametric data by Wilcoxon rank sum test. Comparison of two related parametric variables was done by Paired “t” test; Wilcoxon rank sign test if non-parametric. Comparison between more than one group was done by ANOVA test (F-test); If non-parametric by Kruskal-Wallis (K), and if significant, continuation of analysis by post-HOC tests to clarify the actual difference between specific groups. Related repeated variables analyzed by Friedman test. Comparison of categorical data was done using the Chi-square test (X2-test). P-value is considered significant if less than 0.05. Data was graphically represented using HGW- program.
Results
275 patients were included in our final analysis. There were 218 females and 57 males, age ranged from 23 to 49 years old (41.17+5.73). They were randomly divided into two groups, one receiving ASU (n=140), and another receiving placebo (n=135). Table 1 show that both groups were matched in age, sex, disease duration (DD), OA grading and BMI.
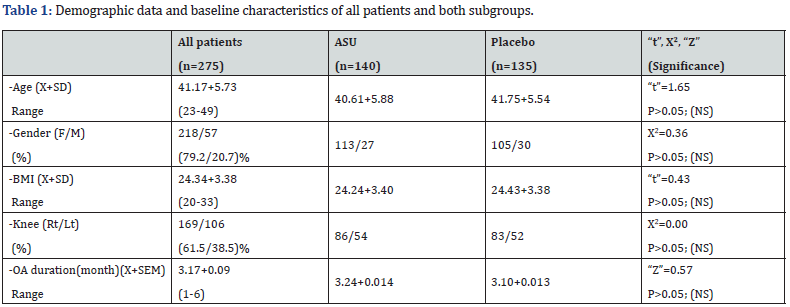
(X+SD)= Mean+/-Standard deviation, SEM= Standard error of the mean; NS = Not significant
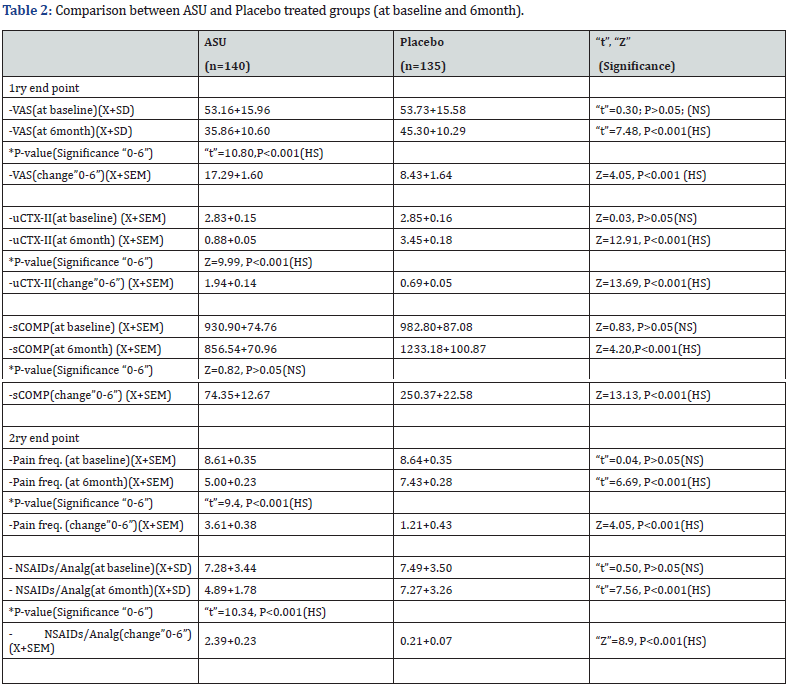
(X+SD)= Mean+/-Standard deviation, SEM= Standard error of the mean, NS = Not significant
Table 2 show data from baseline to 6month:
I- In the ASU group (n=140):
a. uCTX-II: Compared to the base line level (2.83+0.15ng/ ml) there was a highly significant decline in urinary CTX-II levels after 6 months (0.88+0.05ng/ml), where Z=9.99, P<0.001. Over the course of six months, the overall mean change was 1.94+0.14.
b. sCOMP: There was a non-significant decrease in serum COMP levels after 6 months of therapy (856.54+70.96pg/ml) compared to the baseline level (930.90+74.76pg/ml), Z=0.82, P>0.05.
c. VAS: VAS score at 6 months showed a highly significant improvement, declining to (35.86+10.60mm) compared to (53.16+15.96mm) at the baseline. Between baseline and 6 months, the overall mean change was 17.29+1.60.
d. Pain freq/week: Improved significantly from 8.61+0.35 to 5.00+0.23, with “t”=9.4, P<0.001. A mean change of 3.61+0.38 was seen between baseline and six months.
e. NSAIDs needed/week: There was a highly significant improvement from 7.28+3.44 to 4.89+1.78, with “t”=10.34, P<0.001. Compared to the baseline, the mean change in uCTXII from baseline to six months was 1.94+0.14. The uCTXII is therefore a more reliable laboratory marker than sCOMP because its improvement coincides with the improvement in VAS, pain frequency, and the use of NSDAIDs. Therefore, uCTXII decline is associated with early improvement of knee OA.
II- In the Placebo group (n=135):
a. At the base line: There was no significant difference between the placebo group and the ASU treated group regarding uCTX-II, sCOMP, Pain frequency/week, NSAIDs use/week and VAS.
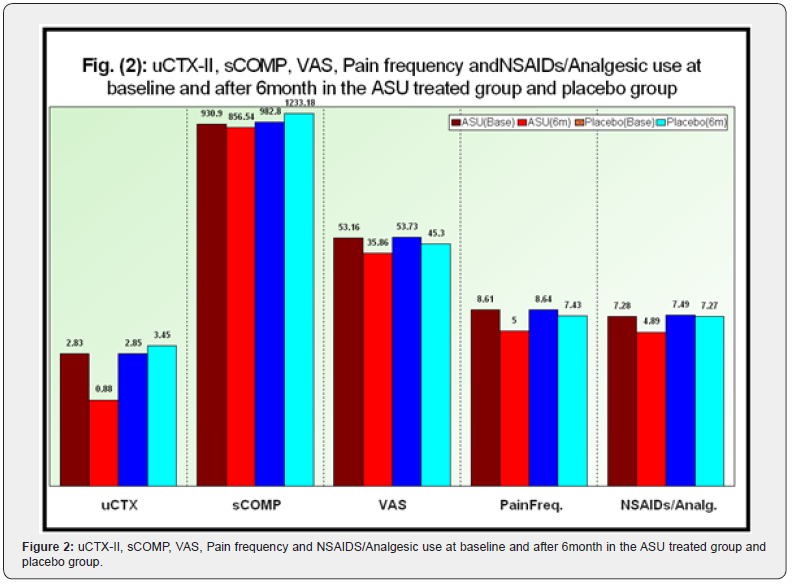
b. After 6month: The whole measured parameters except sCOMP (uCTX-II, , Pain frequency/week, NSAIDs use/week and VAS) were significantly less in placebo compared to ASU group. As compared with placebo group, ASU treated group showed significant mean change (baseline-6month change) in favor of ASU for VAS, and highly significant for the other parameters. Based on the above results, ASU usage for 6 months improved both subjective (VAS, Pain frequency, and the use of NSAIDs) as well as objective parameters (uCTXII) (Table 2 & Figure 2).
Table 3 show data from 6month till end of the study (1year):
I- The ASU group (n=140):
1- G1 :ASU and continue as ASU (n=80):
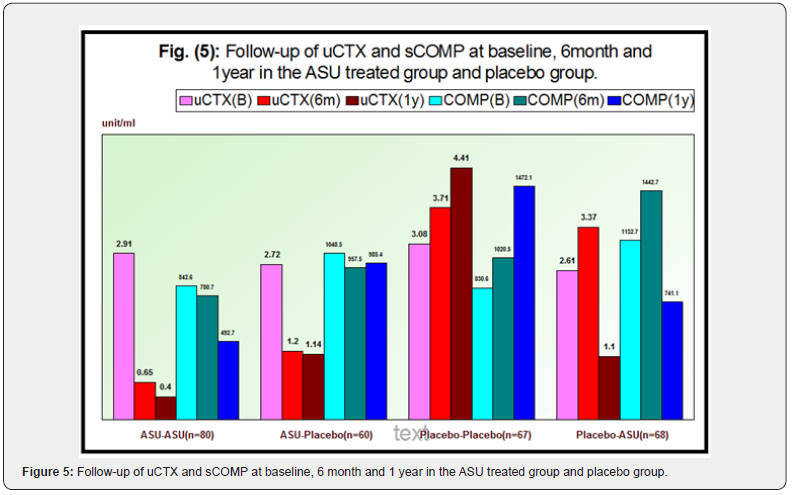
a. The uCTX-II was decreased from 2.91+0.22 (at baseline) to 0.65+0.05 (at 6 months) and 0.40+0.03 (at 1 year). There was a highly significant improvement from the baseline until the end of study, where, F=157.00, P<0.001. Between 6 months and 1 year, there was an overall mean change of 0.24+0.02, and between baseline and year 1, it was 2.50+0.21.
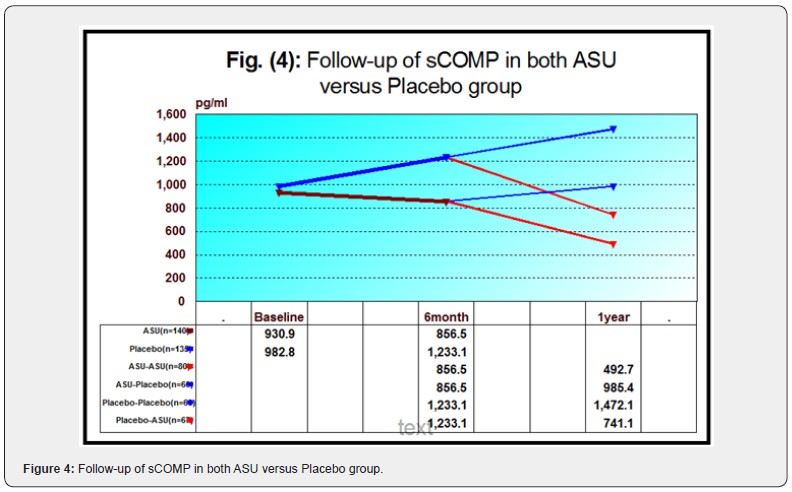
b. In terms of sCOMP, we observed a non-significant decrease from 842.64+92.43 (at baseline) to 780.77+88.14 (at 6months) where P˃0.05 however ,there was a highly significant decline after one year (492.72+49.48). The improvement was highly significant by the end of study where F=129.32, P<0.001. Between 6 months and 12 months, the mean change was 288.05+45.03, where it was 349.91+48.55 from baseline to end of the study.
c. VAS was decreased from 52.44+16.05 at baseline to 34.78+10.13 at 6 months, and 22.21+8.11 at 12 months. The change was highly significant with F=95.62, P<0.001. From 6 months to 1 year, the mean change was 12.56+1.48 and from baseline to end of the study, it was 30.22+2.00.
d. Pain frequency/week: was reduced from 8.30+3.97 (at baseline) to 5.00+2.77 (at 6 months) and 3.40+1.60 (at 12 months). The improvement was highly significant with F=81.80, P<0.001. From 6 months to end of the study, the mean change was 1.60+0.23 and from baseline to 1year, it was 4.90 +0.44.
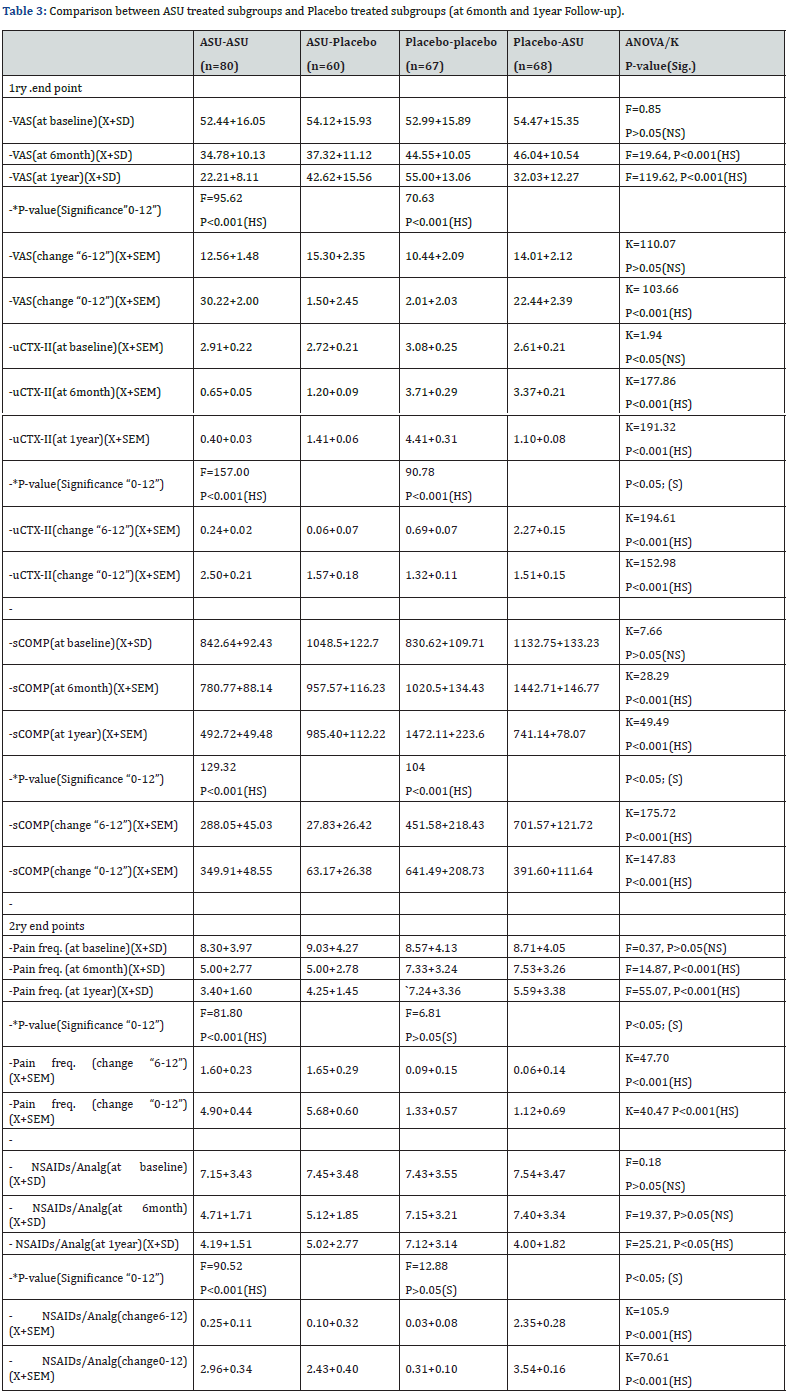
e. The amount of NSAIDs needed per week was significantly reduced from 7.15+3.43 (at baseline) to 4.71+1.71 (at 6 months) and 4.19+1.51(at 12 months). With F=90.52, P<0.001. The overall mean change was 0.26+0.11 from 6month to 12 months and 2.95+0.34 from baseline to end of the study.
2-G2: ASU and switched to Placebo (n=60)
a. uCTX-II: was decreased from 2.72+0.21 (at baseline) to 1.20+0.09 (at 6month) and maintained 1.41+0.06 (at 12 months). The overall mean change was 0.06+0.07 from 6month to 1year and 1.57+0.18 from baseline to 1year, where P<0.001. There was a non-significant difference between uCTX-II levels after 6 months until end of the study indicating the residual effects of ASU after switching to placebo.
b. In terms of sCOMP: we observed a non-significantly decrease from 1048.5+122.7(at baseline) to 957.57+116.23 (at 6month) and 985.40+112.22 (at 12 months) (P˃0.05). The overall mean change was 27.83+26.42 from 6month to 1year and 63.17+26.38 from baseline to 1year.
c. VAS: was decreased from 54.12+15.93 (at baseline) to 37.32+11.12 (at 6month), then slightly elevated to 42.62+15.56 (at 1year) but still significantly lower than the baseline level where P<0.001 indicating the carry over effects of ASU after switching to placebo. Between 6 months and end of the study. The overall mean change was 15.30+2.35 from 6month to 12 months and 1.50+2.45 from baseline to end of the study.
d. Pain frequency /week: was reduced from 9.03+4.27 (at baseline) to 5.00+2.78 (at 6month) and then slightly decreased to 4.25+1.45 (at 1year) but still significantly lower than the baseline level where P<0.001 indicating the carry over effects of ASU after switching to placebo. The overall mean change was 1.65+0.29 from 6month to 1year and 5.68+0.60 from baseline to 1year.
e. The amount of NSAIDs needed per week: was significantly decreased from 7.45+3.48 (at baseline) to 5.12+1.85 (at 6month) and sustained 5.02+2.77 (at 1year), where P<0.001 confirming the carry over effects of ASU after switching to placebo. The overall mean change was 0.10+0.32 from 6month to 12 months and 2.43+0.40from baseline to end of the study.
II- The Placebo group (n=135)
1- G3: placebo and continue as placebo (n=67):
a. uCTX-II: showed a non-significant increase from 3.08+0.25 (at baseline) to 3.71+0.29 (at 6 months) then a highly significant increased after 1year (4.41+0.31) with F=90.78, P<0.001. The overall mean change was 0.69+0.07 from 6 months to 1year and 1.32+0.11 from baseline to 1year.
b. sCOMP: Increased from 830.62+109.71(at baseline) to 1020.5+134.43 (at 6 months) and 1472.11+223.6 (at 1year) with F=104.00, P<0.001. The overall mean change was 451.58+218.43 from 6month to 1year and 641.49+208.73 from baseline to 1year.
c. VAS: there was Initial drop from 52.99+15.89 (at baseline) to 44.55+10.05 (at 6 months), with more increase to 55.00+13.06 (at 1year). This fluctuation was statistically highly significant with F=70.63, P<0.001. The overall mean change was 10.44+2.09 from 6 month to 1year and 2.01+2.03 from baseline to 1year.
d. Pain freq/week: showed a non-significant reduction from 8.57+4.13 (at baseline) to 7.33+3.24 (at 6month) and 7.24+3.36 (at 1year) P˃0.05. The overall mean change was 0.09+0.15 from 6month to 1year and 1.33+0.57 from baseline to 1year.
e. The amount of NSAIDs needed per week: showed a non-significant reduction from 7.43+3.55 (at baseline) to 7.15+3.21 (at 6 months) and 7.12+3.14 (at 1year), P˃0.05. The overall mean change was 0.03+0.08 from 6month to 1year and 0.31+0.10 from baseline to 1year.
2 G4: placebo and switched to ASU (n=68)
a. uCTX-II: was increased from 2.61+0.21 (at baseline) to 3.37+0.21 (at 6 months), followed by a highly significant reduction to 1.10+0.08 (at 1year) P<0.001. The overall mean change was 2.27+0.15 from 6month to 1year and 1.51+0.15 from baseline to 1year.
b. sCOMP: was increased from 1132.75+133.23 (at baseline) to 1442.71+146.77 (at 6 months), followed by a highly significant reduction to 741.14+78.07 (at 1year) P<0.001. The overall mean change was 701.57+121.72 from 6 months to 1year and 391.66+111.64 from baseline to 1year.
c. VAS: showed a non-significant drop from 54.47+15.35 (at baseline) to 46.04+10.54 (at 6 months), followed by a highly significant reduction to 32.03+12.27 (at 1year). The overall mean change was 14.01+2.12 from 6month to 1year and 22.44+2.39 from baseline to 1year.
d. Pain freq/week: showed a non-significant drop from 8.71+4.05 (at baseline) to 7.53+3.26 (at 6month), followed by more drop to 5.59+3.38 (at 1year) P<0.001. The overall mean change was 0.06+0.14 from 6month to 1year and 1.12+0.69 from baseline to 1year.
e. The amount of NSAIDs needed per week: showed a non-significant drop from 7.54+3.47 (at baseline) to 7.40+3.34 (at 6 months), followed by a highly significant reduction to 4.00+1.82 (at 1year). The overall mean change was 2.35+0.28 from 6month to 1year and 3.54+0.16 from baseline to 1year.
ANOVA test was used to compare all the four groups regarding each individual variable. The four groups were matched regarding the baseline data. In follow-up data, all analysis done showed significant and highly significant differences between the four groups. Post-hoc analysis showed that the difference was more pronounced between ASU-ASU group versus Placebo-Placebo group. Tables and figures are provided below (Table 3 Figures 3-6).

Discussion
Patients commonly use alternative therapeutic options with naturally derived compounds, such as ASU, alone or in combination with current pharmaceutical treatments to reduce pain and functional disability in OA [13]. Radiological irreversible changes and pain caused by joint tissue destruction are usually used to diagnose knee OA. Hence, it is essential to develop assays that can detect biochemical markers released from cartilage indicating its turnover as early as possible.31 Detecting early joint damage through biomarkers is possible in such diseases. A biomarker can be a valuable diagnostic and prognostic tool because it can reflect disease-relevant biological activity in the joint and predicts the course of the disease. Additionally, they can be used as an alternative tool to determine how well disease modifying OA drugs work [14]. So, we conducted this multicentric double blind, randomized, placebo-controlled, cross-over study to evaluate the disease modifying effects of ASU on early knee OA (ESKOA) and to assess the carry-over effects of AVOSOYA®300 when treatment was stopped.
Our study revealed a highly significant decline in the urinary CTX-II levels (P<0.001) and high significant improvement in VAS scores from 53.16+15.96 at baseline to 35.86+10.60 (P<0.05) Compared to the placebo arm in early symptomatic knee osteoarthritis (ESKOA) patients receiving 300 mg/d Avocado/ soybean Unsaponifiable (ASU) treatment after six months. Also pain freq/ week and NSAIDs/analgesics needed/week were significantly reduced from (8.61+0.35 to 5.00+0.23) & from (7.28+3.44 to 6.40+2.54) respectively after 6 months compared to the baseline frequencies (P<0.001). this is in consistent with some observational and clinical studies that shown a significant improvement in functional ability and joint pain accompanied by a reduction in the proportion of patients using analgesics and NSAIDs from 58.8% at the baseline visit to 24.9% at the last visit 3 (P<0.001) [15-17]. A very similar NSAID-sparing effect was reported previously in 3 & 6-month randomized, placebo-controlled trials [18-20]. However, sCOMP level after 6 months showed a non-significant reduction in the (ASU) group compared with placebo group.
In consistent with our previously published study, the urinary uCTX-II level is highly sensitive to the very early structural changes in the articular collagen, however the serum level of sCOMP increased significantly only after a long period osteoarthritic change and with the disease progression [7]. Daghestani et al. [21] demonstrated that, uCTX-II and sCOMP were significantly associated with the incidence (defined as KL<2 at baseline and KL2 at follow-up) and progression (defined as KL1 increase between baseline and follow-up) of OA [21].
There have been a limited number of studies during the past few years that attempted to validate existing OA biomarkers in the context of a clinical DMOAD trial. Kluzek et al. [22] studied uCTX-I, uCTX-II and serum osteocalcin in the context of two large phase III knee OA trials of oral salmon calcitonin and demonstrated that sCOMP levels are predictive of subsequent structural changes and incidence of painful KOA, independently of age and BMI. To study the carry over effect of ASU after 6 months, we subdivided randomly each of the studied groups into two subgroups; where 80 out of 140 ASU group (G1) and 67 out of 135 placebo group (G3) continued to receive ASU 300mg/d, however 60 out of 140 ASU group (G2) were switched to receive placebo medication and 68 out 135 placebo group (G4) were switched to receive ASU medication.
In our study, the primary efficacy endpoints (VAS, uCTXII, sCOMP) of G1 (ASU - ASU) showed a highly significantly improvement after 12 months compared to the baseline levels (52.44+16.05 to 22.21+8.11; mean change 57.6%), (2.91+0.22 to 0.40+0.03; mean change 86.2%), and (842.64+92.43 to 492.72+49.48 mean change 41.5%) respectively where P<0.001. However, the mean change of sCOMP after 6 months 8.4% ( 842.64+92.43 at baseline to 780.77+88.14 after 6month) was insignificant where P>0.05 as agreed with our previous study [7]. Regarding the secondary efficacy endpoints, about 59% of patients reported “no pain” by the end of the study as agreed with Gluszko P et al. [23]. Also, analgesics and various NSAIDs were used less frequently by 45% of patients (real life study) at the end of the study. About 40% of patients with symptomatic OA did not use NSAIDs because of safety concerns, contraindications or ability to tolerate pain without medication [17]. The defined daily dosage of NSAIDs among G1 was also significantly reduced. It should be highlighted, that a valuable NSAID-sparing effect is providing convincing evidence of symptomatic efficacy of ASU treatment [24].
Simental-Mendia M et al. conducted a systematic review and meta-analysis in randomized placebo-controlled trials to evaluate the symptom-modifying effects and safety of ASU on knee or hip OA and demonstrated that ASU therapy has a positive impact exclusively in symptomatic knee OA but without significant structural cartilage changes [25]. Regarding G2 (ASU - Placebo) The highly significantly improvement in (VAS and uCTXII ) that was achieved after 6 months showed a slight increase at 12months but still significantly lower than the baseline levels (54.12+15.93 to 32.21+8.11; mean change reduced from 57.6% to 39.3%), (2.72+1.66 to 1.14+0.53; mean change reduced from 86.2% to 58%), respectively where P<0.001. So, once ASU was switched to placebo, pain started to provoke again in placebo group while the symptomatic benefits observed at the end of treatment in the ASU group was persevered for a further 6 months, indicating its carry-over or residual effects. As in our previous study, the mean change in sCOMP at 6 months was insignificant (P>0.05 ) [7].
In contrast to our result, the Chingford cohort study, which has 20 years of data on development of radiographic knee OA progression in a cohort of middle-aged women with Kellgren and Lawrence (KL) scores of zero at baseline, sCOMP levels in the highest quartile were significantly associated with the development of radiographic knee OA and painful radiographic knee OA and this may be explained by the very long follow up period [22]. Kraus VB et al. [26] stated that the uCTX-II and sCOMP were the most constantly associated biomarkers with the occurrence, frequency and progression of knee OA [26]. To the best of our knowledge, this was the first study to evaluate the efficacy of ASU on improving the clinical manifestations of ESKOA (VAS, pain frequency and NSAIDs usage) as indicated by the objective parameters at the molecular level (uCTXII and sCOMP) [27-32].
Limitations
i. The main limitation was the short duration of the study, as we encounter difficulties of following-up of our patients.
ii. Correlation with imaging as Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC).
iii. Small, recruited number are included in this study.
Conclusion
This RCT study concluded that, in early symptomatic knee OA patients, AVOSOYA300mg/day supplement improve both subjective and objective parameters. Subjectively, it decreases the pain frequency and severity; Also minimize the NSAIDs/pain killers needed/week. Objectively, this is confirmed through regression of cartilage catabolic biomarkers as uCTX-II in the first 6month of treatment (as uCTXII is highly sensitive to the very early structural changes in the articular cartilage) and both uCTX-II and sCOMP that decreases afterwards with long term control of the disease progression.
Recommendation
We recommend further long-term head-to-head comparative study with another chondroprotective drugs and in larger patient population to augment our findings.
References
- Alberto Migliore, Carlo Alberto Scire , Loreto Carmona , Gabriel Herrero-Beaumont , Emanuele Bizzi, et al. (2017) The challenge of the definition of early symptomatic knee osteoarthritis: a proposal of criteria and red flags from an international initiative promoted by the Italian Society for Rheumatology. Rheumatol Int 37(8): 1227.
- Neogi T (2013) The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 21(9): 1145-1153.
- Luyten FP, Denti M, Filardo G, Kon E, Engebretsen L (2012) Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc Off J ESSKA 20(3): 401–406.
- Martin JA, Buckwalter JA (2002) Aging, articular cartilage chondrocyte senescence and osteoarthritis. Biogerontology 3(5): 257e64.
- Buckwalter JA, Mankin HJ (1998) Articular cartilage: degeneration and osteoarthritis, repair, regeneration, and transplantation. Instr Course Lect 47: 487-504.
- Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF (1988) Obesity and knee osteoarthritis. The Framingham study. Ann.Intern Med 109(1): 18-24.
- Hesham Hamoud, Gamal Hosny, Mohamed A Hanafy, Mahmoud I Risha (2020) Efficacy of Avosoya®300 (Avocado/Soybean Unsaponifiables) on Cartilage Catabolic Biomarkers among Egyptian Patients with Early Symptomatic Knee Osteoarthritssis. Ortho & Rheum Open Access J 16(1): 555927.
- Baker-LePain JC, Lane NE (2010) Relationship between joint shape and the development of osteoarthritis. Curr Opin Rheumatol 22(5): 538-543.
- Schiphof D, Oei EHG, Hofman A, Waarsing JH, Weinans H, et al. (2014) Sensitivity and associations with pain and body weight of an MRI definition of knee osteoarthritis compared with radiographic Kellgren and Lawrence criteria: a population-based study in middle-aged females. Osteoarthr Cartil OARS Osteoarthr Res Soc 22(3): 440–446.
- Qazi AA, Folkesson J, Pettersen PC, Karsdal MA, Christiansen C, et al. (2007) Separation of healthy and early osteoarthritis by automatic quantification of cartilage homogeneity. Osteoarthr Cartil OARS Osteoarthr Res Soc 15(10): 1199–1206.
- Ling SM, Patel DD, Garnero P, Zhan M, Vaduganathan M, et al. (2009) Serum protein signatures detect early radiographic osteoarthritis. Osteoarthr Cartil OARS Osteoarthr Res Soc 17(1): 43-48.
- Sellam J, Berenbaum F (2013) Is osteoarthritis a metabolic disease? Joint Bone Spine 80(6): 568-573.
- Orlowsky EW, Kraus VB (2015) The role of innate immunity in osteoarthritis: when our first line of defense goes on the offensive. J Rheumatol 42(3): 363-371.
- Courties A, Gualillo O, Berenbaum F, Sellam J (2015) Metabolic stress induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage 23(11): 1955-1965.
- Blotman F, Maheu E, Wulwik A, Caspard H, Lopez A (1997) Efficacy and safety of avocado/soybean unsaponifiables in the treatment of symptomatic osteoarthritis of the knee and hip. A prospective,multicenter, three-month, randomized, double-blind, placebo-controlled trial. Rev Rhum Engl Ed 64(12): 825-834.
- Maheu E, Mazieres B, Valat JP, G Loyau, X Le Loet, et al. (1998) Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: a prospective, randomized, double-blind, placebo controlled, multicenter clinical trial with a six-month treatment period and a two-month follow-up. Arthritis Rheum 41(1): 81-91.
- Appelboom T, Scheurmans J, Verbruggen G, Y Henrotin, J Y Reginster, et al. (2001) Symptoms modifying effect of avocado/soybean unsaponifiables (ASU) in knee osteoarthritis. Scand J Rheumatol 30(4): 242-247.
- Driban, JB, RR Bannuru, CB Eaton, TD Spector, DJ Hart T, et al. (2020) The Incidence and Characteristics of Accelerated Knee Osteoarthritis among Women: the Chingford Cohort. BMC Musculoskeletal Disorders 21(2).
- Liu-Bryan R, Terkeltaub R (2015) Emerging regulators of the inflammatory process in osteoarthritis. Nat Rev Rheumatol 11(1): 35-44.
- Mobasheri A, Matta C, Z_ak_any R, Musumeci G (2015) Chondrosenescence: definition, hallmarks and potential role in the pathogenesis of osteoarthritis. Maturitas 80(3): 237-244.
- Daghestani HN, Jordan JM, Renner JB, Doherty M, Wilson AG, et al. (2017) Serum Npropeptide of collagen IIA (PIIANP) as a marker of radiographic osteoarthritis burden. PLoS One 12(12): e0190251.
- Kluzek S, Bay-Jensen A-C, Judge A, Karsdal MA, Shorthose M, et al. (2015) Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers 20(8): 557-564.
- Głuszko P, Stasiek M (2016) Symptom-modifying effects of oral avocado/soybean unsaponifiables in routine treatment of knee osteoarthritis in Poland. An open, prospective observational study of patients adherent to a 6-month treatment. Reumatologia 54(5): 217-226.
- Blotman F, Maheu E, Wulwik A, H Caspard, A Lopez (1997) Efficacy and safety of avocado-soybean unsaponifiables in the treatment of symptomatic osteoarthritis of the knee and hip. A prospective, multicenter,three-month, randomized, duble blind, placebo-controlled trial. Rev Rheum 64(12): 825-834.
- Greene MA, Loeser RF (2015) Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23(11): 1966-1971.
- Kraus VB, Collins JE, Hargrove D, Losina E, Nevitt M, et al. (2017) Predictive validity of biochemical biomarkers in knee osteoarthritis: data from the FNIH OA Biomarkers Consortium. Ann Rheum Dis 76(1): 186-195.
- Aspden RM, Scheven BA, Hutchison JD (2001) Osteoarthritis as a systemic disorder including stromal cell differentiation and lipid metabolism. Lancet 357(9262): 1118-1120.
- Bliddal H, Leeds AR, Christensen R (2014) Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons e a scoping review. Obes Rev 15(7): 578-586.
- Baert IAC, Mahmoudian A, Nieuwenhuys A, Jonkers I, Staes F, et al. (2013) Proprioceptive accuracy in women with early and established knee osteoarthritis and its relation to functional ability, postural control, and muscle strength. Clin Rheumatol 32(9): 1365–1374.
- Aigner T, Zhu Y, Chansky HH, Matsen FA 3rd, Maloney WJ, et al. (1999) Reexpression of type IIA procollagen by adult articular chondrocytes in osteoarthritic cartilage. Arthritis Rheum 42(7): 1443-1450.
- Maheu E, Mazieres B, Valat JP, G Loyau, X Le Loet, et al. (1998) Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial with a six-month treatment period and a two-month follow up demonstrating a persistent effect. Arthritis Rheum 41(1): 81-91.
- Sellam J, Berenbaum F (2010) The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat Rev Rheumatol 6(11): 625-635.






























