Evaluation of the Impact Vitamin D Supplementation on Bone Mineral Density in Breast Cancer Patients using or not taking Aromatase Inhibitors: 5-year follow-up
Marcelo Antonini1*, Gisela Rosa Franco Salerno2, Natalia Daniela Kovalinski Vieira1, Odair Ferraro1, André Mattar3, Reginaldo Guedes Coelho Lopes1 and Juliana Monte Real1
1Breast Pathology Sector of the Hospital do Servidor Público Estadual Francisco Morato de Oliveira (HSPE-FMO), Brazil
2Department of Physiotherapy at the Center for Biological and Health Sciences at Universidade Presbiteriana Mackenzie (UPM), Brazil
3Pérola Byington Hospital, Brazil
Submission:February 19, 2022; Published: March 03, 2023
*Corresponding author: Marcelo Antonini, Breast Pathology Sector of the Hospital do Servidor Público Estadual Francisco Morato de Oliveira (HSPE-FMO), Rua Cayowaa, 1575, ap 72, São Paulo, Brazil
How to cite this article: Marcelo Antonini, Gisela Rosa Franco Salerno, Natalia Daniela Kovalinski Vieira, Odair Ferraro, André Mattar, et al. Evaluation of the Impact Vitamin D Supplementation on Bone Mineral Density in Breast Cancer Patients using or not taking Aromatase Inhibitors: 5-year follow-up. Ortho & Rheum Open Access J. 2023; 21(3): 556063. DOI: 10.19080/OROAJ.2023.21.556063
Abstract
Objective: Verify the impact of aromatase inhibitors use in postmenopausal women undergoing treatment for breast cancer and verify whether vitamin D supplementation modified the progression to osteopenia and osteoporosis.
Methodology: Comparative prospective cohort study with 240 women divided in 4 groups; G1 with insufficient vitamin D, G2 with sufficient vitamin D in treatment with aromatase inhibitors; G3 with insufficient vitamin D and G4 with sufficient vitamin D in another type of treatment as control group. Mineral density of the lumbar spine, femoral neck, and total femur was analyzed before, after 6 months and 5 years of treatment. Blood tests to measure serum calcium, ionic calcium, Vitamin D, and parathyroid hormone were performed before and after 5 years. Data were initially analyzed by the KS, Kruskal-Wallis and Mann-Whitney tests, considering p≤0.05.
Results: After 6 months, progression to osteoporosis had an index of G1 (12%), G2 (4%) and G3 (5%). After 5 years, the progression to osteopenia had an index of G1 (55%), G2 (42%), G3 (73%) and G4 (50%), with G3 being significant compared to G1, G2 and G4 (0.036, <0.001 and 0.009). Bone mass loss was significant in all groups, the greatest loss was found in the spine, where G1 showed an 8-fold increase in loss and G3 showed a 43-fold increase.
Conclusion: Postmenopausal Bone loss may be related to the use of aromatase inhibitors compared to other types of treatment. Vitamin D supplementation, regardless of breast cancer treatment, seems to be a protective factor in the progression to osteoporosis.
Keywords: Breast Neoplasms; Aromatase Inhibitors; Bone Density; Vitamin D Deficiency
Introduction
Breast cancer is the neoplasm with the highest incidence among women in the world, without considering non-melanoma skin tumors. It is the leading cause of death from malignant neoplasms in females in both developing and developed countries. However, recent treatment regimens have shown advances in reducing recurrence and mortality [1]. Approximately 67% of women with breast cancer are hormone receptor (HR) positive. Because of this, adjuvant endocrinotherapy is frequently used and can be performed with a selective estrogen receptor modulator (SERM) or an aromatase inhibitor (AI). The ATAC study (Arimidex, Tamoxifen, Alone or in Combination) [2] and the MA.27 study showed that AI treatment in postmenopausal women has superior efficacy and greater tolerability than tamoxifen [3].
Estrogen plays a central role in maintaining bone mineral density (BMD) in women, and in postmenopausal women, low estradiol levels are associated with increased bone turnover, reduced bone mineral density and increased risk of fracture.
Although estrogen receptors are predominantly in osteoblasts, the main role of this hormone is in bone resorption. The binding of estrogens or their analogues to osteoblast receptors inhibits the release of osteoclast-stimulating factors and increases the activity of their inhibiting factors, which is essential for bone maintenance [4,5].
The mean reduction in BMD in postmenopausal white women was 2.5% per year at the lumbar spine and 1.8% per year at the femoral neck, with no appreciable change before this period. Black women have lower percentage losses: 2.2% per year in the spine, 1.4% in the femoral neck: Japanese and Chinese women had greater losses in the femoral neck: 2.1% and 2.2% per year, respectively [5,6]. As tamoxifen has partial estrogen agonist activity, there is bone protection, especially in trabecular bones such as the lumbar spine, unlike what occurs with the use of AI [6].
Studies show that declines in BMD are related to the menstrual transition, that is, rapid bone mass loss is identified within 3 years of menopause. However, density begins to decline about 1 year before this period and continues to decline in early postmenopausal women, with a slight reduction in the rate of loss around 2 years later. For this reason, researchers refer to this period as transmenopause (perimenopause and early menopause), and it is better defined based on the date of completion of the menstrual period due to the great variability among women in the duration of these periods and in menstrual bleeding patterns [4].
BMD varies between individuals due to a complex mechanism that depends on several factors, such as body weight, frequency of physical activity, amount of calcium in the diet, smoking, amount of circulating vitamin D, alcohol consumption, and longterm use of corticosteroids. The classic action of vitamin D is the regulation of calcium and phosphorus metabolism through the control of intestinal absorption and renal reabsorption processes, maintaining them in plasma concentrations sufficient to ensure adequate mineralization and bone growth in children and adolescents and the overall bone health at all stages of life [7-9].
Obesity has been discussed as an important protective factor for bone mineral density, especially in post-menopause. These women have a higher level of circulating estrogen precisely because it is synthesized on a larger scale in peripheral tissues, mainly in abdominal fat, due to the action of aromatase. Studies show that patients with low weight (less than 58 kg) have a higher risk of osteoporosis and fractures [10]. Due to the prevalence of breast cancer in postmenopausal women and the relevance of AI in adjuvant treatment, it is essential to discuss their possible effects on bone mass loss and determine which patients are at greater risk of developing osteoporosis. Interpreting these findings, a more effective preventive treatment can be performed in accordance with world guidelines. Therefore, the objective of this study was to verify whether vitamin D supplementation in postmenopausal women who use aromatase inhibitors for breast cancer treatment modifies the progression to osteopenia and osteoporosis.
Methods
This is a prospective, comparative, cohort study carried out in the mastology sector of the Hospital do Servidor Público Estadual Francisco Morato de Oliveira (HSPE-FMO), having been previously approved by the ethics and research committee via Plataforma Brasil (CAAE 47317215.6. 0000.5463). After the informed consent of the participants, 275 women with hormone receptor positive (RH+) and hormone receptor negative (RH- ) breast cancer were selected, excluding women who had osteoporosis prior to the intervention, loss of follow-up in the proposed Treatment and incomplete data.
The women were divided into 4 groups of 60 participants, being; G1 (RH+) with insufficient vitamin D, G2 (RH+) with sufficient vitamin D in treatment with aromatase inhibitors (AI), which of choice was anastrozole; G3 (RH-) with insufficient vitamin D and G4 (RH-) with sufficient vitamin D as control groups. During the study period, all study participants performed bone densitometry annually at the HSPE imaging service on the same dual-ray emission absorptiometry (DXA) device, Lunar Prodigy Advance GE Healthcare, and we used three occasions for analysis: before start treatment, one year and five years after the start of treatment.
The software used for analysis was enCORE 2007, being evaluated, mineral density of the lumbar spine (L1-L4), femoral neck and total femur. The mean standard deviation for young adults (T-score) of all before and after the start of AI was analyzed and thus divided into 3 groups according to the World Health Organization (WHO): osteoporosis, patients with lower values to -2.5, osteopenia when the T index was between -1 and -2.5 and values greater than -1, considered normal. To assess bone density loss, a calculation was performed (previous bone mineral density subtracted from the current one and the difference divided by the previous bone mineral density and multiplied by one hundred), values lower than -2% were considered significant.
Blood tests to measure serum calcium, ionic calcium, vitamin D and parathyroid hormone (PTH) were performed in the HSPE laboratory. Calcium was analyzed by the colorimetric method with a reference value (RV) between 8.8-10.6 mg/dL. Ionic calcium was measured by ion selective electrode (VR 1.17-1.43 mmol/L).
PTH was measured by chemiluminescence (VR 4 - 58 g/mL) and 25-hydroxyvitamin D, also determined by chemiluminescence, results lower than 30 ng/mL were considered insufficient. All patients with insufficient vitamin D were treated with 7000 IU daily (loading dose) for eight weeks followed by 1000 IU daily maintenance every 6 months.
All patients, in all visits, were instructed on a balanced diet, adequate exposure to the sun and encouragement of physical activity [11]. Information was also collected on current age, menopausal status, immunohistochemical type, serum vitamin D value (measured by the plasma value of 25-hydroxyvitamin D), body mass index (BMI), ethnicity the patient considered herself to be, bone mineral density and T-score before starting treatments, six months and 5 years later in lumbar spine (L1- L4), femoral neck and femur, calculation of bone mineral density loss or gain at six months and five years. For data analysis, we used the Statistical Package for the Social Sciences (SPSS) 17.0 for Windows, where, initially, the KS Test was carried out, to verify the normality of the sample, later carried out the Kruskal- Wallis and Mann-Whitney test for Density and T -Score; and the Chi-Square Test for the Classification of Bone Mass, considering the significance level used for the tests was 5%.
Results
A total of 276 women were followed up, two (5.5%) being excluded for suspending medication due to pain, 20 (55.5%) for not having BMD within the time required for the study, seven (19.5%) for not having all relevant data for comparison, six (17%) for presenting osteoporosis before the investigation and one (2.5%) for progressing to death, leaving 240 divided into 4 groups with 60 in each. When compared in terms of age and body mass index (BMI) there were no statistically significant differences, demonstrating the homogeneity of the sample (p≥0.05) (Table 1).
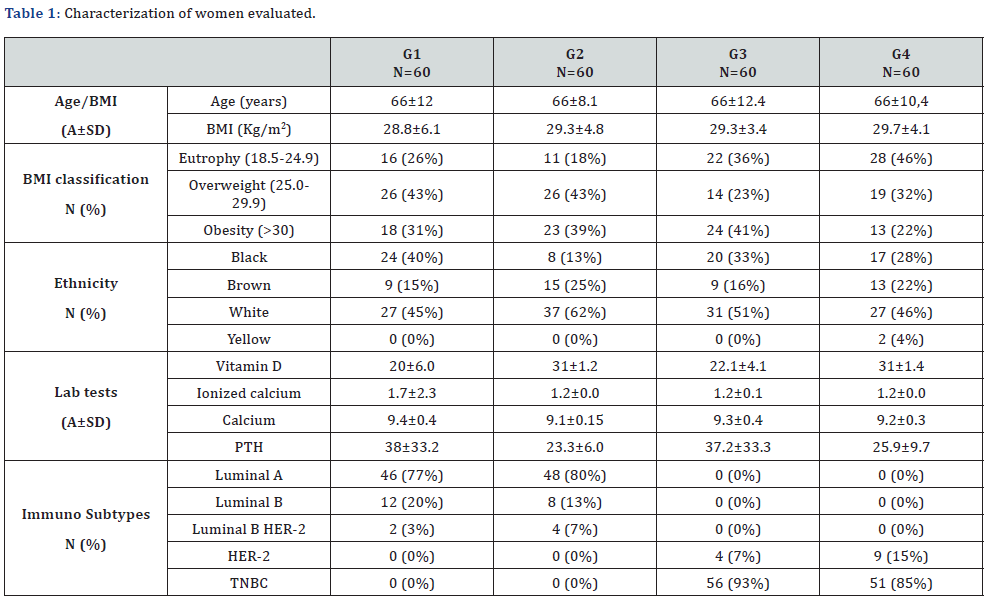
G1: aromatase inhibitors’ group with insufficient vitamin D; G2: aromatase inhibitors’ group with sufficient vitamin D; G3: control group with insufficient vitamin D; G4: control group with sufficient vitamin D; A: Average; SD: Standart deviation; N: number; PTH: parathyroid hormone.
For the distribution of the bone mass classification, we observed that there is a relationship, that is, a difference between the groups at all times, respectively (p=0.007, 0.001 and 0.006). In the evolution to osteoporosis in 1 year, we observed a rate of 12% in G1, 4% in G2, 5% in G3 and 0% in G4, that is, G1 presented a statistically significant difference when compared to G4 (p= 0.006). After 5 years there was no significant difference between the groups. When analyzing the evolution to osteopenia in 5 years, we observed a rate of 55% in G1, 42% in G2, 73% in G3 and 50% in G4, that is, G3 presented a statistically significant difference when compared to G1, G2 and G4 respectively (p=0.036, <0.001 and 0.009), being the one that changed the most over the years (Table 2).
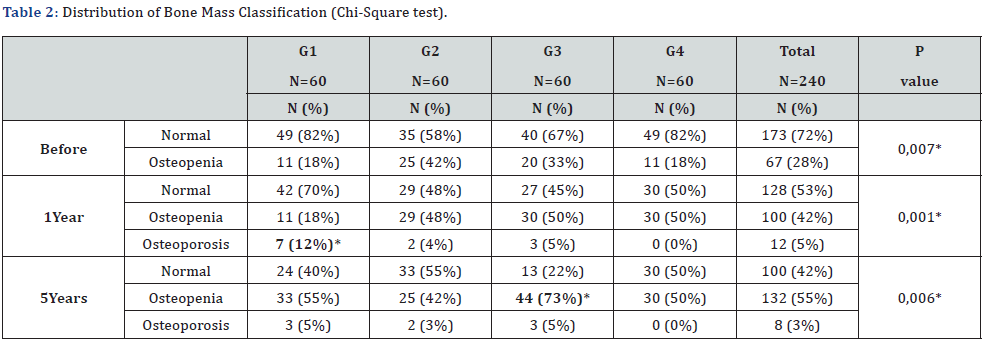
G1: aromatase inhibitors’ group with insufficient vitamin D; G2: aromatase inhibitors’ group with sufficient vitamin D; G3: control group with insufficient vitamin D; G4: control group with sufficient vitamin D; N: number.
In the T-Score evaluation, considering the column after 1 year, we found an average of -0.40 in G1, -0.82 in G2, -0.66 in G3 and -0.46 in G4 (p=0.04). When evaluated between groups, G2 showed a statistically significant difference compared to G1 (p=0.015) and G4 (p=0.017). When evaluated after 5 years, we found in the column an average of -0.56 in G1, -0.84 in G2, -1.02 in G3 and -0.54 in G4 (p=0.04), when evaluated between the groups, G3 showed a statistically significant difference compared to G1 (p=0.02) and G4 (p=0.003). In relation to the femur after 5 years, we found an average of -0.48 in G1, -0.47 in G2, -1.12 in G3, -0.62 in G4 (p=0.001), when evaluated between the groups G3 showed a statistically significant difference compared to G1 (p=0.002), G2 (p=0.002) and G4 (p<0.001). In the femoral neck at 5 years, we found an average of -0.91 in G1, -0.88 in G2, -1.31 in G3 and -0.65 in G4 (p=0.005), when evaluated between the groups G3 showed a statistically significant difference compared to G1 (p=0.004), G2 (p=0.007) and G4 (p<0.001) (Table 3).
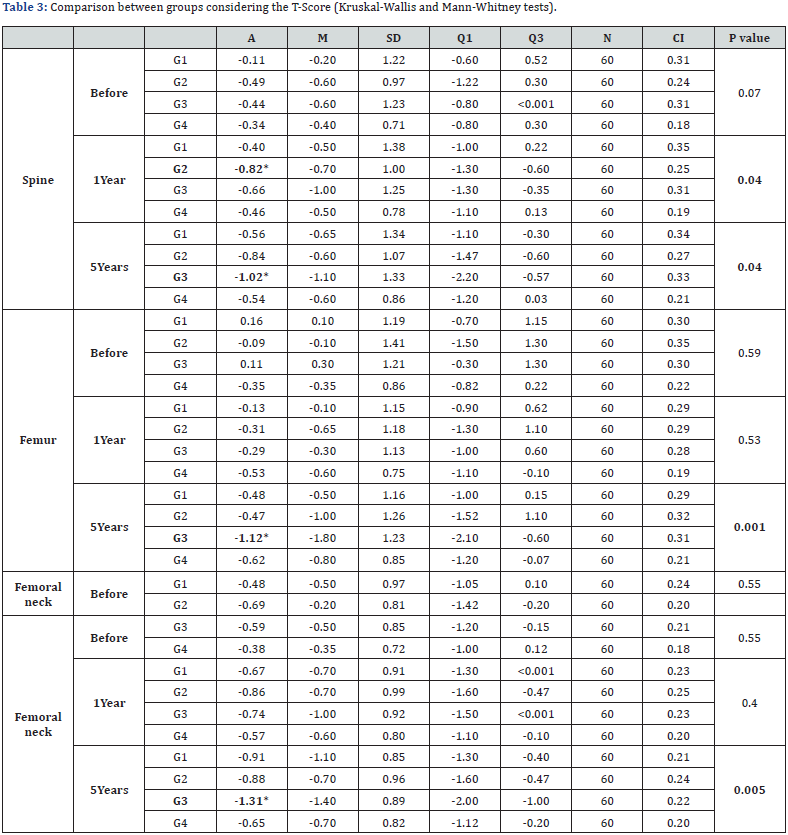
G1: aromatase inhibitors’ group with insufficient vitamin D; G2: aromatase inhibitors’ group with sufficient vitamin D; G3: control group with insufficient vitamin D; G4: control group with sufficient vitamin D; A: Average; M: Median; SD: Standart deviation; Q1: First quartile; Q3: Third quartile; N: number; CI: Confidence interval.
Considering the evaluation of density in the column over a period of 1 year, we observed an average of 1.14 in G1, 0.99 in G2, 1.10 in G3 and 0.91 in G4 (p<0.001), confirming the existence of differences between the groups. When analyzing between the groups, we found a statistically significant difference in G4 comparing to G1 (p<0.001), G2 (p<0.002) and G3 (p<0.001). After 5 years, we found a mean of 1.10 in G1, 0.97 in G2, 1.05 in G3 and 0.86 in G4 (p=0.001). Likewise, when analyzing between the groups, we found a statistically significant difference in G4 comparing to G1 (p<0.001), G2 (p<0.001) and G3 (p<0.001).
In the femur in the time of 5 years, we observed a mean of 0.98 in G1, 0.91 in G2, 0.90 in G3 and 0.83 in G4 (p=0.03), confirming that there is a difference between the 4 groups. When analyzed between groups, we observed statistically significant differences in G4 in relation to G1 (p<0.001), G2 (p=0.032) and G3 (p=0.009).
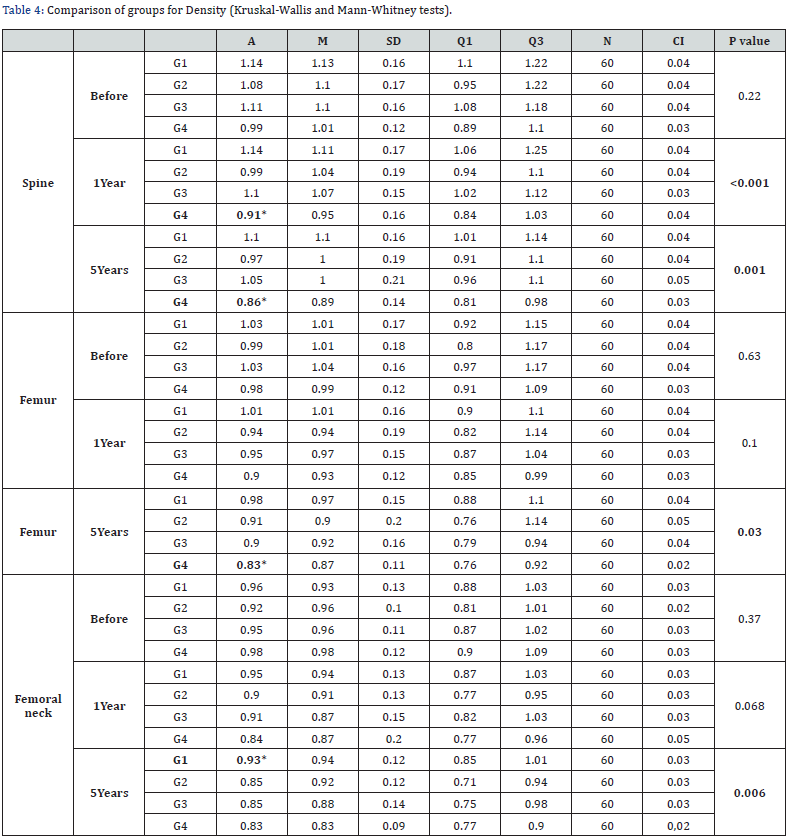
G1: aromatase inhibitors’ group with insufficient vitamin D; G2: aromatase inhibitors’ group with sufficient vitamin D; G3: control group with insufficient vitamin D; G4: control group with sufficient vitamin D; A: Average; M: Median; SD: Standart deviation; Q1: First quartile; Q3: Third quartile; N: number; CI: Confidence interval.
Finally, in the femoral neck over 5 years, where we had an average of 0.93 in G1, 0.85 in G2, 0.85 in G3 and 0.83 in G4 (p= 0.006), we confirmed that there is a difference between the 4 groups. And when analyzed between groups, we observed a statistically significant difference in G1 compared to G2 (p=0.003), G3 (p=0.013) and G4 (p<0.001) (Table 4).
Considering bone mass loss when comparing 1 year with 5 years, we observed that all groups presented statistically significant differences between themselves (spine p<0.001; femur p=0.012; femoral neck p=0.046), that is, the loss of mass bone was significant in all groups, being in the spine respectively G1 (-3.2), G2 (-2.2), G3 (-4.3) and G4 (-5.2); in the femur, G1 (-3.2), G2 (-2.2), G3 (-5.0) and G4 (-7.0) and in the femoral neck G1 (-1.6), G2 (-4.0), G3 (-5.8) and G4 (-1.4). It is worth mentioning that the greatest loss was found in the column, where G1 showed an 8-fold increase in loss after 5 years (-0.4% to -3.6%) and G3 showed a 43-fold increase (from -0, 1% to -4.4%) (Table 5).
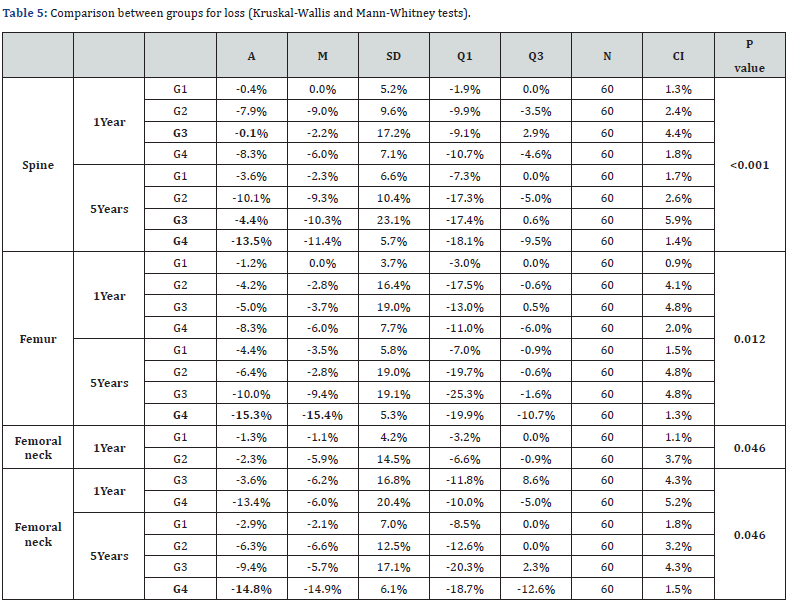
G1: aromatase inhibitors’ group with insufficient vitamin D; G2: aromatase inhibitors’ group with sufficient vitamin D; G3: control group with insufficient vitamin D; G4: control group with sufficient vitamin D; A: Average; M: Median; SD: Standart deviation; Q1: First quartile; Q3: Third quartile; N: number; CI: Confidence interval.
Discussion
Vitamin D has a fundamental and well-established role in the regulation of calcium and phosphate homeostasis. Demonstrating an increasingly important role not only in the normal metabolism of bones, but also in the proper function of other tissues and systems. It can play protective factors in the development of chronic, autoimmune, oncological, cardiovascular and infectious diseases, and its monitoring in the aging process of women is extremely important [12].
Low circulating estradiol levels in postmenopausal women are associated with increased bone turnover, low bone mineral density (BMD) and increased risk of fractures [6], the bone loss rate being an important factor of morbidity and mortality in this age group [8-10]. Endocrine therapy with aromatase inhibitors (AI) despite being the gold standard in the treatment of breast cancer in postmenopausal women with hormone receptor positive, raises the discussion regarding its impact on bone mass. This discussion has become relevant, as it is known that the use of AI in non-osteoporotic women negatively affects bone mineral density, and this long-term use may cause disorders in bone quality, thus increasing the chances of fractures [13].
The literature discusses whether AI are in fact the villains for the development of osteoporosis in these women in long-term treatment. Failure to adequately monitor protective factors such as; vitamin D in adequate amounts, balanced diets and incentives for the practice of physical activities throughout the treatment are reasons for discussion according to Hong et al. [13], early monitoring of these variables before the use of AI and during the course of treatment is of paramount importance in preventing these conditions. It was observed in this research that women, regardless of the type of treatment obtained, who had sufficient vitamin D, had significantly less progression to osteoporosis and osteopenia, compared to groups with insufficient vitamin D. Furthermore, the present study found an initial prevalence of osteopenia of 28%, which is lower than reported in the literature. Our patients from both treatments presented after 5 years, 55% of osteopenia and 8% of osteoporosis, corroborating the data in the literature.
A cross-sectional study carried out in São Paulo, with 4,332 postmenopausal women, found the prevalence of osteoporosis in 33% and of osteoporotic fractures in 11.5% [14]. Another study that evaluated 1871 aged over 50 years showed prevalence’s of osteoporosis and osteopenia of 13.7 and 48.8%, respectively [9]. Considering the treatments of choice for this research, the AI groups (12%) showed development of osteoporosis at higher rates than the control group (5%) after 1 year. However, when we evaluated the evolution to osteopenia, the control group showed a significant change in 73% of the women evaluated after 5 years of treatment, presenting these changes in the groups with insufficient vitamin D. AI seem to be related to the further development of osteoporosis, but only when vitamin D is insufficient. The evolution to osteopenia was observed as something inevitable, occurring in both groups, being even greater in the control group with insufficient vitamin D. It is known that in the menopausal period this situation is characterized as normal; bone mass loss in women with a history of early menopause is 2% per year and when we add endocrine therapy, this loss can reach up to 2.6% per year in women [15]. The evolution to osteoporosis and the loss of bone mineral density is not normal and must be prevented in those treatments where these risk factors exist, thus avoiding pathological fractures in this population [16].
Considering the evaluation of bone mass density in the spine, femur and femoral neck, the loss was greater in the control group with insufficient vitamin D after 5 years; the control group with sufficient vitamin D also showed significant losses after 5 years in the spine and femur. It is important to discuss the issue of changes that may occur in the lives of these patients over the years [12], since those who had enough Vitamin D were not supplemented at the beginning of treatment, being only oriented about a balanced diet, adequate exposure to the sun and physical activities. We also emphasize the condition that participants who had sufficient Vitamin D at the beginning may become insufficient during the treatment, requiring closer monitoring to the point of mitigating these failures [4], identifying risk factors as quickly as possible.
The AI group that received vitamin D replacement at the beginning because it was insufficient, may have presented a protective factor for the evaluation of density over the years, as we can even notice an increase in this variable in the femoral neck after 5 years (0.93), compared to the other groups. The question remains whether there is a more attentive view for patients using AI, since this loss of bone mass is already widely discussed scientifically. Charlson et al. [17] report in their study that 24% of women who started AI therapies without any bone mass assessment in the age groups from 67 to 70 years old, increasing this rate to 40% over 86 years old, making it a worrying fact. in this research.
It is worth mentioning that considering the normal values of annual bone mass loss (1.5% per year), the control group with sufficient vitamin D presented, after 5 years, losses above the desired values for all regions (-13.5%= column, -15.3%=femur, -14.8%=femoral neck). Thus, we identified that the participants who had sufficient vitamin D developed less progression to osteopenia and osteoporosis over the years of treatment, but when we evaluated the loss, the groups that needed Vitamin D replacement at the beginning of the interventions developed smaller losses over the course of 5 years.
In the present study, it was possible to observe a statistically significant loss in BMD in the femur after 5 years considering Group IA with insufficient Vitamin D and after 6 months in the Control Group with sufficient Vitamin D, in the Spine after 1 year and 5 years in the Control Group with Vitamin D enough. Corroborating our findings, the ATAC2 study, performed only to assess BMD during the 5 years of follow-up, showed significant loss only in the spine, hip and trabecular bones, as these are more influenced by the absence of estrogen than the others. It is also important to note that no woman with normal densitometry became osteoporotic.
In addition, the ATAC2 study carried out with 9366 women showed that, at 68 months of follow-up, the incidence of fracture was higher in the AI group compared to the tamoxifen group, which we did not find in our research, no osteoporotic patient had a fracture at follow-up of 5 years comparing the therapies used. It is important to emphasize the need for regular monitoring of BMD and the implementation of strategies to prevent this loss, such as the association of physical activity during the period in treatment with AI, for protection in women who mainly have other risk factors such as smoking, age and use concomitant corticosteroids [6,18].
Large clinical trials on breast cancer show association with increased risk of fractures with use of third-generation AI such as anastrozole [2,6], letrozole [17,19] and examestano [17]. In the present study, we could see the deleterious effects of AI on BMD after 6 months, and especially after 5 years. This is most likely due to the fact that bone loss is more pronounced in the first 2 years of medication use [19-21]. A study carried out in São Paulo in 2015 [9] proves that there is an inverse correlation between BMI and the risk of osteopenia and osteoporosis, that is, the literature demonstrates that low body weight (less than 58kg) is associated with an increased risk of osteoporosis and fractures [12]. In our study, we could see that 67.9% of the patients were classified between overweight and obesity. We did not find any with low weight, thus presenting a low rate of osteoporosis and the absence of fracture in this period evaluated, in agreement with what is reported in the literature that weight especially reduces this risk [4-6].
Conclusion
Bone loss in postmenopausal women at 5-year followup may be related to aromatase inhibitor use. Vitamin D supplementation, regardless of the type of treatment in breast cancer, seems to have a protective factor in the progression to osteoporosis.
References
- Global Burden of Disease 2019 Cancer Collaboration, Jonathan M Kocarnik, Kelly Compton, Frances E Dean, Weijia Fu, et al. (2022) Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA oncol 8(3): 420-444.
- Baum M, Budzar AU, Cuzick J, Forbes J, Houghton JH, et al. (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomized trial. Lancet 359(9324): 2131-2139.
- Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, et al. (2013) Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27--a randomized controlled phase III trial. J Clin Oncol 31(11): 1398-404.
- Eghbali T, Abdi K, Nazari M, Mohammadnejad E, Ghane RG (2022) Prevalence of Osteoporosis Among Iranian Postmenopausal Women: A Systematic Review and Meta-analysis. Clinical Clin Med Insights Arthritis Musculoskelet Disord 15: 11795441211072471.
- Karlamangla AS, Burnett-Bowie SAM, Crandall CJ (2018) Bone Health during the Menopause Transition and Beyond. Obstet Gynecol Clin North Am 45(4): 695-708.
- Cha C, Lee SJ, Hong H, Choi YY, Chung MS (2021) Adverse effects of adjuvant tamoxifen treatment on bone mineral density in premenopausal breast cancer patients: A systematic review and meta-analysis. Journal of Clinical Oncology 39(15_suppl): e12500-e12500.
- Jorde R, Kamilla AS, Kubiak J, Joakimsen R, Grimnes G, et al. (2019) Effects of vitamin D supplementation on bone turnover markers and other bone-related substances in subjects with vitamin D deficiency. Bone 124: 7-13.
- Silva BCC, Camargos BM, Fujii JB, Dias EP, Soares MMS (2008) Prevalência de deficiência e insuficiência de vitamina D e sua correlação com PTH, marcadores de remodelação óssea e densidade mineral óssea, em pacientes ambulatoriais. Arq Bras Endocrinol Metab 52(3): 482-488.
- Silva ACV, da Rosa MI, Fernandes B, Diniz RM, Damiani MEFR (2015) Factors associated with osteopenia and osteoporosis in women undergoing bone densitometry. Rev Bras Reumatol 55(3): 223-228.
- Marzullo P, Mele C, Mai S, Nardone A, Scacchi M, et al. (2021) Obesity and Bone Loss at Menopause: The Role of Sclerostin. Diagnostics 11(10): 1914.
- Instituto Nacional de Cancer Jose Alencar Gomes da Silva (2020) Dieta, nutrição, atividade física e câncer: uma perspectiva global: um resumo do terceiro relatório de especialistas com uma perspectiva brasileira/Instituto Nacional de Cancer Jose Alencar Gomes da Silva. – Rio de Janeiro, INCA, pp. 140.
- Federacao Brasileira das Associacoes de Ginecologia e Obstetricia (FEBRASGO) (2017) Importância da vitamina D na saúde da mulher. São Paulo (Série Orientações e Recomendações FEBRASGO, no. 14/Comissão Nacional Especializada em Osteoporose). vi, p. 82.
- Hong AR, Kim JH, Lee KH, Kim TY, Im AS, et al. (2017) Long-term effect of aromatase inhibitors on bone microarchitecture and macroarchitecture in non-osteoporotic postmenopausal women with breast câncer. Osteoporos Int 28(4): 1413-1422.
- Pinheiro MM, Reis N, Machado ET, Felipe FS, Yang O, et al. (2010) Risk factors for osteoporotic fractures and low bone density in pre and postmenopausal women. Rev Sau Pub 44(3): 479-485.
- Rachner TD, Gobel A, Jaschke NP, Hofbauer LC (2020) Challenges in Preventing Bone Loss Induced by Aromatase Inhibitors. J Clin Endocrinol Metab 105(10): dgaa463.
- Pineda-Moncusi M, Servitja S, Casamayor G, Cos ML, Rial A, et al. (2018) Bone health evaluation one year after aromatase inhibitors completion. Bom 117: 54-59.
- Charlson J, Smith EC, Smallwood AJ, Laud PW, Neuner JM (2016) Bone Mineral Density Testing Disparities Among Patients with Breast Cancer Prescribed Aromatase Inhibitors. J Natl Compr Canc Netw 14(7): 875-880.
- Agostini D, Zeppa SD, Lucertini F, Annibalini G, Gervasi M, et al. (2018) Muscle and Bone Health in Postmenopausal Women: Role of Protein and Vitamin D Supplementation Combined with Exercise Training. Nutrients 10(8): 1103.
- Shapiro, Charles L (2020) Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers vol 12(11): 3094.
- Ramchand SK, Cheung Y-M, Yeo B, Grossmann M (2019) The effects of adjuvant endocrine therapy on bone health in women with breast cancer. J Endocrinol 241(3): R111-R124.
- Stratton J, Hu X, Soulos PR, Davidoff AM, Pusztai L, et al. (2017) Bone Density Screening in Postmenopausal Women with Early-Stage Breast Cancer Treated with Aromatase Inhibitors. J Oncol Pract 13(5): e505-e515.






























