The Incidence of Serious Infections among Rheumatoid Arthritis Patients using Biological Agents
Yaman Walid Kassab1*, Mohammed Salah Ayad1, Fares MS Muthanna2, Hiba Khaled Al Dahoul1, Aina Yazrin Ali1 and Ganesh Sritheran Paneerselvam3
1Department of Hospital and Clinical Pharmacy, Faculty of Pharmacy, Cyberjaya University College of Medical Sciences, 63000 Selangor, Malaysia
2Faculty of Pharmacy, University Teknologi MARA (UiTM), 42300 Bandar Puncak Alam, Selangor Darul Ehsan, Malaysia
3School of pharmacy, Taylor’s University, 47500 Subang Jaya, Selangor Darul Ehsan, Malaysia
Submission: June 17, 2019; Published: July 10, 2019
*Corresponding author: Yaman Walid Kassab, Department of Hospital and Clinical Pharmacy, Faculty of Pharmacy, Cyberjaya University College of Medical Sciences, Persiaran Bestari, Cyber 11, 63000 Cyberjaya, Selangor Malaysia
How to cite this article: Yaman W K, Md Salah A, Fares M M, Hiba K A D, Aina Y A, et al. The Incidence of Serious Infections among Rheumatoid Arthritis Patients using Biological Agents . Ortho & Rheum Open Access J 2019; 14(3): 555887. DOI: 10.19080/OROAJ.2019.14.555887
Abstract
Objective: To assess the risk of serious infections (SI) in rheumatoid arthritis patients receiving biological agents.
Methodology: This retrospective cohort study was conducted at a tertiary hospital in Malaysia. The medical records of RA patients aged ≥ 18 years and receiving biological agents from January 2010 until August 2018 were included. Each enrolled medical record was traced for up to 1 year starting from date of receiving biologics until an infection took place.
Results: A total of 57 patients’ medical records were included in this study. A total of 8 serious infections (Sis) were identified, the most frequent was respiratory tract infection (RTI). The overall incidence rate (IR) of SI was 14 per 100 PYs (person-years): 6.7 per 100 PYs in adalimumab group, 13.6 per 100 PYs in etanercept group and 20 per 100 PYs in tocilizumab group. There was no significant difference in risk of SI between treatment groups, the adjusted HR (95% CI) for adalimumab was 0.348 (0.039 – 3.118), p=0.346 and for etanercept was 0.669 (0.150 – 2.992), p=0.599 compared with tocilizumab. Only lung diseases was determined as a true predictor in the adjusted model, lung diseases adjusted OR (95% CI) was 7.498 (1.043 – 53.903), p=0.045, but adjusted OR (95% CI) for long term use of steroids was 3.076 (0.42 – 22.543), p=0.269, and for age was 0.875 (0.123 – 6.203), p=0.894.
Results: A total of 57 patients’ medical records were included in this study. A total of 8 serious infections (Sis) were identified, the most frequent was respiratory tract infection (RTI). The overall incidence rate (IR) of SI was 14 per 100 PYs (person-years): 6.7 per 100 PYs in adalimumab group, 13.6 per 100 PYs in etanercept group and 20 per 100 PYs in tocilizumab group. There was no significant difference in risk of SI between treatment groups, the adjusted HR (95% CI) for adalimumab was 0.348 (0.039 – 3.118), p=0.346 and for etanercept was 0.669 (0.150 – 2.992), p=0.599 compared with tocilizumab. Only lung diseases was determined as a true predictor in the adjusted model, lung diseases adjusted OR (95% CI) was 7.498 (1.043 – 53.903), p=0.045, but adjusted OR (95% CI) for long term use of steroids was 3.076 (0.42 – 22.543), p=0.269, and for age was 0.875 (0.123 – 6.203), p=0.894.
Keywords: Rheumatoid Arthritis; Incidence; Serious Infections; Biological Agents
Introduction
Rheumatoid arthritis (RA) is a chronic, progressive inflammatory condition that can lead to significant disability and joint pain. Serious infections are frequent in patients with rheumatic diseases and this may be related to the disease itself or the drugs used to treat it (particularly immunosuppressants and steroids). Many trials have shown that biological agents significantly control signs and symptoms, slow progression and improve function and quality of life of patients with early or long-standing RA [1]. However, biological agents also play an important role in host defense and may be associated with increased risk of serious infections (SI) [2-6]. The aim of this study was to evaluate the risk of SI among patients with RA treated with the three biological agents licensed in Malaysia namely Tocilizumab, Etanercept (ETN) and Adalimumab (ADA).
Methods
Patients and Study Design
This study was designed to compare the risk of SI between biological agents used to treat RA. We used all electronic medical records of RA patients following up at medication therapy adherence clinic (MTAC) at a tertiary hospital in Malaysia. All medical records of RA patients on biologics from the period of 1st January 2010 to 31st August 2018 were screened and reviewed. Each enrolled patient’s medical record was traced for up to one year starting from date of receiving biologics until the clinical outcome (serious infection) takes place. The treatment effect of the following three biological agents on the incidence of SI was compared in this study: tocilizumab, adalimumab, and etanercept. An outcome was the first incidence of SI during the follow-up periods. Serious infection is defined as an infection requires hospitalization and/or IV antibiotics or leading to significant disability/incapacity [7]. If a patient was switched to another biological agent, the subsequent follow-up will be considered for the new biological agent. Events were attributed to the most recent drug [8].
Statistical Analysis
Data were analysed using the Statistical Package for Social Sciences (SPSS) software version 22. The Fisher’s Exact test was used for categorical variables. The incidence rates (IRs) of serious infection were calculated by dividing the number of incidence cases by the number of corresponding biologic-exposed personyears (PYs) for each treatment group. Cumulative infection-free time during 1 year of follow-up period for each treatment group was computed from life tables using the Kaplan-Meier approach. Log rank test was used to compare estimated infection-free time for the three biological drugs. Cox regression hazard model was used to calculate the adjusted HR (hazard ratio) for each biological agent. Binary Logistic Regression test was used to calculate adjusted odds ratios (ORs) for potential confounders. P-value < 0.05 was used for significance Table 1.
Results
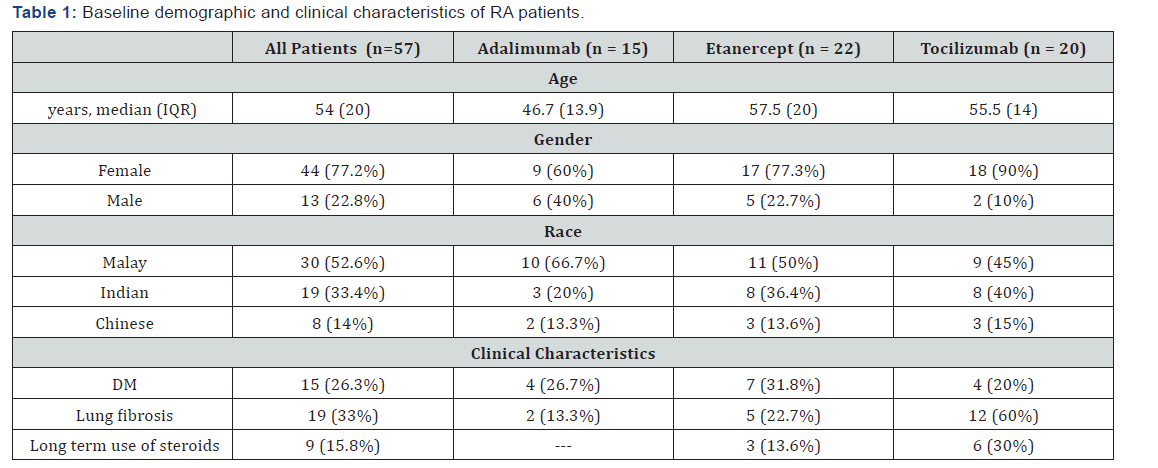
Demographic and Clinical Characteristics of RA Patients at the Start of Biological Agents
Our study findings showed that the median age of the RA patients was 57 (± 20) years with majority of them less than 60 years old (66.7%). In addition, most of RA patients were females and represent the highest percentage in tocilizumab group (90%) followed by ETN group and ADA group respectively. Ethnic distribution showed that more than half of the patients were Malay (52%), followed by Indian patients (19%) and Chinese patients (8%) respectively. It was noted that more than half of the patients in tocilizumab group had lung fibrosis and about one third of the patients in etanercept group had DM.
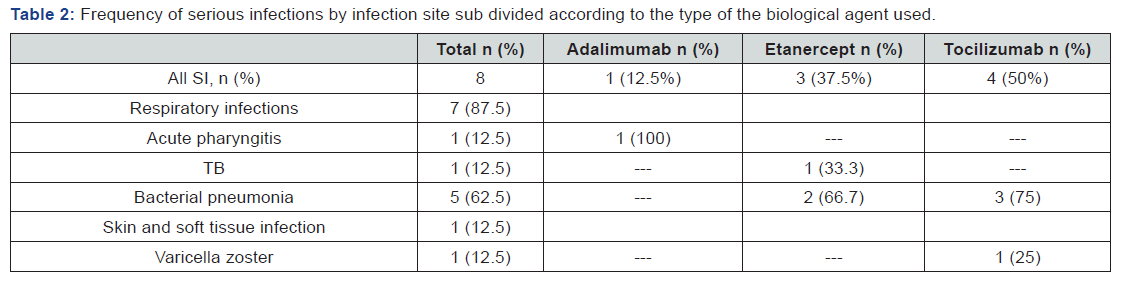
Type and Frequency of Serious Infections among the Three Biological Agents
A total of 8 serious infections were identified during one year of follow-up after start of biological agents. The most common SI was respiratory tract infections (87.5%, including 5 cases of bacterial pneumonia, one case of acute pharyngitis and one case of TB) followed by SSTI (12.5% including one case of varicella zoster infection). Tocilizumab was associated with higher rate of SI (50% of SI cases including 3 cases of URTI and one case of SSTI) followed by etanercept (37.5% including one case of TB and 2 cases of bacterial pneumonia) and adalimumab (12.5% including one case of acute pharyngitis) respectively as shown in Table 2.
Incidence and Risk of Serious Infections among the Three Biological Agents
The total incidence of SI was 8 events, yielding an IR of 14 per 100 PYs (person-years), while the censored cases were 49 (86%). The IRs across the different three biological agents were 6.7 per 100 PYs in adalimumab group, 13.6 per 100 PYs in etanercept group and 20 per 100 PYs tocilizumab group. A total of 7 RTI were identified yielding a crude IR of 12.3 per 100 PYs: adalimumab IR was 6.7 per 100 PYs, 13.6 per 100 PYs in etanercept group and 15 per 100 PYs in tocilizumab group. The crude HR (95% CI) for adalimumab was 0.348 (0.039 – 3.118), p=0.346 and for etanercept is 0.669 (0.150 – 2.992), p=0.599 compared with tocilizumab. Although the crude HR of etanercept was higher than crude HR of adalimumab when compared with tocilizumab, but this difference of HR among the three biologics was not statistically significant as shown in Table 3. Note: aCompared with tocilizumab using Cox regression hazard model.

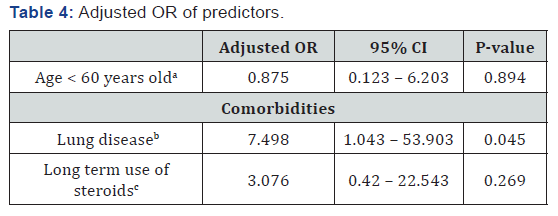
Adjustment of Predictors
Our study showed that lung disease was determined as a true predictor in the adjusted model, adjusted OR (95% CI) was 7.498 (1.043 – 53.903), p=0.045. Other potential confounders not
identified as true predictors, adjusted OR (95% CI) for long term use of steroids was 3.076 (0.42 – 22.543), p=0.269, and for age was 0.875 (0.123 – 6.203), p=0.894, as shown in Table 4.
Notes: Binary logistic regression
aCompared with ≥ 60 years old,
bCompared with patients with no lung disease,
cCompared with patients not given steroids for long term.
Discussion
Type of SI among the three biological agents. Our study identified 8 serious infections among RA patients taking biologics during one year of follow up. The most frequent type of serious infections was respiratory tract infections (87.5%) followed by skin and soft tissue infections (12.5%). These findings are consistent with the results of a systematic review [2], which concluded that the most common sites of infection are the respiratory tract (including pneumonia), skin and soft tissues, and the urinary tract. The pattern of infection in a prospective cohort study in UK was reported as the respiratory infections were the most frequently documented class of infections followed by skin and soft tissue infections [9]. The most commonly reported site of SI consistently across studies is lower respiratory tract infection followed by skin and soft-tissue infections. Other common infection sites are bone and joint infections and urinary tract infections [10]. In a study comparing risk of hospitalized infections between TNF inhibitors and non- TNF inhibitors, it addressed that the most common infections were pneumonia (37%), skin/soft tissue (22%), urinary tract (9%), and bacteremia/sepsis (7%) [11].
Furthermore, our study showed significant association (p=0.013) between lung diseases and the incidence of SI. The reason can be explained by genetic factors, or variations in human leukocyte antigen (HLA) associated with interstitial lung disease (ILD) in RA patients which increase the risk of RTI [12]. Also, this association of genetic factors with ILD was tested in another study [13], which suggested that the variation in HLA was associated with increased risk for ILD in Japanese patients with RA. Another study suggests the association of the high levels of anti-citrullinated autoantibodies with pulmonary manifestations of RA-ILD [14]. The chronic inflammation in RA affects pulmonary airways, vasculature, pleura, parenchyma, all can be affected with variable degrees of pathologic inflammation [15]. Lower respiratory tract infections are more frequent in patients with bronchiectasis associated RA who are receiving biologics [16].
In a systematic literature review evaluated the association between newer biologics and development of lung diseases, its results highlighted an association between the use of newer biologic agents (tocilizumab), and the development of noninfectious parenchymal lung disease in patients with RA [17]. Our study showed that a total of 7 RTI were identified yielding a crude IR of 12.3 per 100 PYs: adalimumab IR was 6.7 per 100 PYs, 13.6 per 100 PYs in etanercept group and 15 per 100 PYs in tocilizumab group. There are conflicting evidences between studies addressing the association between the type of infection and different biological agents. A study reported that adalimumab had a higher risk of respiratory SI compared with tocilizumab, while the risk of serious SSTI in tocilizumab group was higher than adalimumab group [4].
Another study conducted in Italy [7], it concluded that adalimumab had a higher risk of respiratory SI and SSTI than etanercept. In a study [18], conducted in Germany using the Dutch Rheumatoid Arthritis Monitoring registry showed that ADA was associated with higher rate of lower RTI and SSTI than ADA. On the contrary, other studies suggesting that tocilizumab has higher rate of serious respiratory tract infections than ADA and ETN, like this prospective cohort study conducted in UK [9], which reported that tocilizumab had a higher rate of serious respiratory tract infections than ADA and ETN, but these findings were no longer statistically significant in the fully adjusted model. Tocilizumab had superior efficacy compared with adalimumab suggesting that it may be a more potent drug which could provide a biologically plausible link to a higher infection rate as shown in this RCT [19].
Risk of SI between the three Biological Agents
Our study showed that tocilizumab was associated with the highest IR of SI (20% per 100 PYs) among the three biologics followed by ETN IR (13.6% per 100 PYs) and ADA IR (6.7% per 100 PYs) respectively. Although crude HR (95% CI) for etanercept is 0.669 (0.150 – 2.992), p=0.599 was higher than that of adalimumab 0.348 (0.039 – 3.118), p=0.346 when compared with tocilizumab, but this difference was not statistically significant. These results are consistent with the results of this meta-analysis [20], which included 163 RCTs, it identified that the risk of SI between etanercept and adalimumab were statistically insignificant compared to control. Another systematic review and meta-analysis [21] concluded that tocilizumab has higher IR of SI 5.45 (4.26, 6.96), than TNFi 4.90 (4.41, 5.44). The same results from ADACTA RCT [19] which compared tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis, it reported that tocilizumab had a higher risk of SI compared with adalimumab, but this difference was not statistically significant. It also founded that tocilizumab had superior efficacy compared with adalimumab suggesting that it may be a more potent drug which could provide a biologically plausible link to a higher infection rate.
In a recent prospective cohort study conducted in UK [9] reported that tocilizumab had a higher rate of SI than etanercept and adalimumab, but these findings were not statistically significant in the fully adjusted model. The results from a large retrospective cohort study [22] compared the risk of SI between specific biologic agents. It concluded that the adjusted HR (95% CI) for etanercept was 1.24 (1.07–1.45) and had higher significant risk than tocilizumab and adalimumab. In contrast, the results of this multicenter cohort study conducted in Japan [4], which identified that adalimumab was significantly associated with a greater risk of serious infection compared with tocilizumab, which is consistent with the results of a meta-analysis [23], which compared safety of TNF inhibitors, concluded that adalimumab, was associated with higher risk of SI.
In an observational study conducted in Taiwan using the national registry of RA patients [24] concluded that adalimumab has a higher risk of serious infection than etanercept. Another observational study conducted in Germany [18] using the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry of RA patients to compare risk of serious infection in RA patients treated with etanercept, adalimumab and infliximab. This study founded that the risk of serious infections in patients with RA treated with adalimumab or infliximab was similar, while the risk of serious infections in patients with RA treated with etanercept was lower than with adalimumab. Another prospective cohort study conducted in Japan [25] using the registry of Japanese RA patients on biologics for long-term safety (REAL) registry to evaluate the risk of serious infections in RA patients treated with tocilizumab compared with TNF inhibitors adjusting for covariates using Cox regression hazard analysis. This study concluded that no significant difference between TCZ and TNFIs regarding the incidence of serious infection.
This risk has been closely studied over the last 18 years and there are conflicting evidences about the risk of serious infections between different biological agents. The inconsistent results of studies may be due to lack of adequately powered head-to head randomized clinical trials evaluating serious infections as the outcome. The results of studies using registries and administrative databases to assess the risk of serious infections among RA patients treated with different biological agents are inconsistent. For example, one study [18] using the Dutch Rheumatoid Arthritis Monitoring registry showed that the risk of serious infection in RA patients treated with etanercept was lower than that in patients treated with adalimumab. In contrast, another previous study [9] using the British Society for Rheumatology Biologics Register did not find significant differences between adalimumab and etanercept, [22].
Predictors that may Affect Incidence of SI
Although our study showed significant association between lung diseases (p=0.013), long term use of steroids (p=0.016) and the incidence of SI, but only lung diseases was determined as a true predictor in the adjusted model, lung diseases adjusted OR (95% CI) was 7.498 (1.043 – 53.903), p=0.045, while other potential confounders not identified as true predictors, adjusted OR (95% CI) was 3.076 (0.42 – 22.543), p=0.269 for long term use of steroids, and was 0.875 (0.123 – 6.203), p=0.894, for age. Other studies identified some predictors like DM, advanced age, long term use of steroids, and lung diseases, which were not determined as true confounders in our study, may be due to small sample size. In a study in Japan [26] evaluating the risk of SI in RA patients treated with TNF inhibitors over 3 years, it reported that the increasing age, chronic pulmonary diseases (include interstitial pneumonia, chronic obstructive pulmonary disease, bronchial asthma, prior pulmonary tuberculosis, and bronchiectasis) and prednisolone use 10 mg/day or more were significantly associated with SI.
Another study [11] reported that older age, chronic obstructive pulmonary disease (COPD), and higher prednisone dosage (>7.5 mg/day) were associated with a significantly increased risk for infection. In a multicenter retrospective cohort study in Japan [4] founded that BMI, prednisolone use, diabetes mellitus, and chronic lung disease were also significant factors associated with the risk of pulmonary hospitalized infection in RA patients taking biologics. In this study [7], the results confirmed that the true confounders are advanced age at the start of treatment and steroid use were both associated with a higher risk of adverse events, but didn’t identify that the comorbidities like DM and chronic lung disease as true confounders which didn’t increase the risk of SI in this study. With regards to lung diseases as a strong predictor identified in most of the studies, the genetic changes in RA contribute significantly to the development of interstitial lung disease (ILD). As a result, the lung diseases increase the risk of SI in RA patients [12].
Another study [13] identified the association of genetic factors with ILD in Japanese patients with RA, which resulted in increased risk of SI in RA patients. The high levels of anticitrullinated autoantibodies were associated with pulmonary manifestations of RA-ILD as documented in this study [14]. The chronic inflammation in RA affects pulmonary airways, vasculature, pleura, parenchyma, all can be affected with variable degrees of pathologic inflammation [15]. Lower respiratory tract infections are more frequent in patients with bronchiectasis associated RA who are receiving biologics [16]. Also, MTX induced pneumonitis and other MTX induced pulmonary toxicities increase risk of respiratory infection [27].
With regards to DM, in a case control study [28] describing the association of DM with developing skin and soft tissue infections in RA patients taking TNF inhibitors, it concluded that patients with diabetes mellitus and those with a history of prior skin infection were significantly more likely to have SSTI. Diabetes mellitus is considered a risk factor, because the specific defects in innate and adaptive immune function which have been identified in diabetic patients [29] contribute to increased risk of infection. Hyperglycemic environment that favors immune dysfunction such as damage to the neutrophil function, depression of the antioxidant system, and humoral immunity, leading to decrease in the antibacterial activity, and consequently high risk of infections [30].
With regards to advanced age as a risk factor, physiological ageing is accompanied by decline in immune system function and immune alteration during ageing increases susceptibility to infections. This is due to progressive declines in the percentage of total lymphocytes and absolute numbers of T and B cells. The NK cells declined from infancy to adulthood but increased in elderly patients [31]. The decline in immature, innate and adaptive immune system in old age is considered as high risk of different types of infection, autoimmune disease and malignancy [32]. This study [8] addressed that the advanced age is an important risk factor for developing SI in RA patients and may be due to steroid use. The long term use of steroids put the RA patients at high risk of SI as the immunomodulatory effect, alteration in gene transcription, and the power of steroids to induce lymphocyte apoptosis, all explain steroids’ immunosuppressive activity [33]. Long term use of steroids is associated with high risk of infection due to gene targeting alteration which enhance apotosis of some immune cells [34].
Limitations of the Study
A retrospective study cannot assess causality, under powered study due to small sample size, and the effect of different doses of biological agents on incidence of SI was not included in the study.
Conclusion
The incidence of SI was not determined by the type of biological agents. Lung disease was a significant factor associated with the risk of RTI.
References
- Wiens A, Venson R, Correr CJ, Otuki MF, Pontarolo R (2010) Meta-analysis of the efficacy and safety of adalimumab, etanercept, and infliximab for the treatment of rheumatoid arthritis. Pharmacotherapy 30: 339–353.
- Furst DE (2010) The risk of infections with biologic therapies for rheumatoid arthritis. Seminars in Arthritis and Rheumatism 39(5): 327-346.
- Komano Y, Tanaka M, Nanki T, Koike R, Sakai R, et al. (2011) Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for Longterm Safety. Journal of Rheumatology 38(7):1258-1264.
- Mori S, Yoshitama T, Hidaka T, Sakai F, Hasegawa M, et al. (2017) Comparative risk of hospitalized infection between biological agents in rheumatoid arthritis patients: A multicenter retrospective cohort study in Japan. PLoS One 12(6): e0179179.
- Ramiro S, Sepriano A, Chatzidionysiou K, Nam JL, Smolen JS, et al. (2017) Safety of synthetic and biological DMARDs: a systematic literature review informing the 2016 update of the EULAR recommendations for management of rheumatoid arthritis. Annals of the Rheumatic Diseases 76(6): 1101-1136.
- Singh JA, Saag KG, Bridges SL, Akl EA, Bannuru RR, et al. (2016) 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatology 68(1): 1-26.
- Atzeni F, Sarzi-Puttini P, Botsios C, Carletto A, Cipriani P, et al. (2012) Long-term anti-TNF therapy and the risk of serious infections in a cohort of patients with rheumatoid arthritis: comparison of adalimumab, etanercept and infliximab in the GISEA registry. Autoimmunity Reviews 12(2): 225-229.
- Galloway JB, Hyrich KL, Mercer LK, Dixon WG, Fu B, et al. (2011) BSRBR Control Centre Consortium; British Society for Rheumatology Biologics Register. Anti-TNF therapy is associated with an increased risk of serious infections in patients with rheumatoid arthritis especially in the first 6 months of treatment: updated results from the British Society for Rheumatology Biologics Register with special emphasis on risks in the elderly. Rheumatology (Oxford) 50(1): 124-131.
- Rutherford AI, Subesinghe S, Hyrich KL (2018) Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Annals of the Rheumatic Diseases 77(6): 905-910.
- Lahiri M, Dixon WG (2015) Risk of infection with biologic antirheumatic therapies in patients with rheumatoid arthritis. Best Practice and Research: Clinical Rheumatology 29(2): 290-305.
- Curtis JR, Yang S, Patkar NM, Chen L, Singh JA, et al. (2014) Risk of hospitalized bacterial infections associated with biologic treatment among US veterans with rheumatoid arthritis. Arthritis Care and Research (Hoboken) 66(7): 990-997.
- Zulma X Yunt, Joshua J Solomon M (2015) Lung Disease in Rheumatoid Arthritis. Rheumatic Disease Clinic of North America 41(2): 225–236.
- Furukawa H, Oka S, Shimada K, Sugii S, Ohashi J, et al. (2012) Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PLoS One 7(5): e33133.
- Harlow L, Rosas IO, Gochuico BR, Mikuls TR, Dellaripa PF, et al. (2013) Identification of citrullinated hsp90 isoforms as novel autoantigens in rheumatoid arthritis-associated interstitial lung disease. Arthritis and Rheumatism 65(4): 869-879.
- Kevin KB (2007) Rheumatoid Lung Disease. Proceedings of the American Thoracic Society 4(5): 443–448.
- Geri G, Dadoun S, Bui T, Castillo D, Pinol N, et al. (2011) Risk of infections in bronchiectasis during disease-modifying treatment and biologics for rheumatic diseases. BMC Infectious Diseases 11: 304.
- Hadjinicolaou A, Nisar M, Bhagat S, Parfrey H, Chilvers E, et al. (2011) Non-infectious pulmonary complications of newer biological agents for rheumatic diseases--a systematic literature review. Rheumatology (Oxford) 50(12): 2297-2305.
- Van Darte SA, Fransen J, Kievit W, Flendrie M, den Broeder AA, et al. (2013) Difference in the risk of serious infections in patients with rheumatoid arthritis treated with adalimumab, infliximab and etanercept: results from the Dutch Rheumatoid Arthritis Monitoring (DREAM) registry. Annals of Rheumatic Diseases 72(6): 895-900.
- Gabay C, Emery P, van Vollenhoven R, Dikranian A, Alten R, Pavelka K, et al. (2013) ADACTA Study Investigators. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet 381(9877):1541-1550.
- Singh JA, Wells GA, Christensen R, Tanjong GE, Maxwell L, et al. (2011) Adverse effects of biologics: a network meta-analysis and Cochrane overview. Cochrane Database Systematic Review (2): CD008794.
- Vibeke S, Sima A, Jonathan F, Jamie G, Sriram K, et al. (2015) Systematic review and meta-analysis of serious infections with tofacitinib and biologic disease-modifying antirheumatic drug treatment in rheumatoid arthritis clinical trials. Arthritis Research and Therapy 17: 362.
- Yun H, Xie F, Delzell E, Levitan EB, Chen L, et al. (2016) Comparative Risk of Hospitalized Infection Associated with Biologic Agents in Rheumatoid Arthritis Patients Enrolled in Medicare. Arthritis & Rheumatology 68(1): 56-66.
- Michaud TL, Rho YH, Shamliyan T, Kuntz KM, Choi HK (2014) The comparative safety of tumor necrosis factor inhibitors in rheumatoid arthritis: a meta-analysis update of 44 trials. American Journal of Medicine 127(12):1208–1232.
- Chiu YM, Lang HC, Lin HY, Yang MT, Fang CH, et al. (2014) Risk of tuberculosis, serious infection and lymphoma with disease-modifying biologic drugs in rheumatoid arthritis patients in Taiwan. International Journal of Rheumatic Diseases 3: 9-19.
- Sakai R, Cho SK, Nanki T, Watanabe K, Yamazaki H, et al. (2015) Head-to-head comparison of the safety of tocilizumab and tumor necrosis factor inhibitors in rheumatoid arthritis patients (RA) in clinical practice: results from the registry of Japanese RA patients on biologics for long-term safety (REAL) registry. Arthritis Research and Therapy 17:74.
- Sakai R, Komano Y, Tanaka M, Nanki T, Koike R, et al. (2012) Time-dependent increased risk for serious infection from continuous use of tumor necrosis factor antagonists over three years in patients with rheumatoid arthritis. Arthritis Care & Research (Hoboken) 64(8): 1125-1134.
- Kim YJ, Song M, Ryu JC (2009) Mechanisms underlying methotrexate-induced pulmonary toxicity. Expert Opinion on Drug Safety 8(4): 451-458.
- Wasson NJ, Varley CD, Schwab PF, Winthrop K (2013) Serious skin & soft tissue infections in rheumatoid arthritis patients taking anti-tumor necrosis factor alpha drugs: a nested case-control study. BMC Infectious Diseases 13: 533.
- Peleg AY, Weerarathna T, McCarthy JS, Davis TM (2007) Common infections in diabetes: pathogenesis, management and relationship to glycaemic control. Diabetes/Metabolism Research and Reviews 23(1): 3-13.
- Casqueiro J, Alves C (2012) Infections in patients with diabetes mellitus: A review of pathogenesis. Indian Journal of Endocrinology and Metabolism 16(11): S27–S36.
- Valiathan R, Ashman M, Asthana D (2016) Effects of Ageing on the Immune System: Infants to Elderly. Scandinavian Journal of Immunology 83(4): 255-266.
- Katharina A Simon, G A Hollander, Andrew M (2015) Evolution of the immune system in humans from infancy to old age. Proceedings of the Royal Society and Biological Science 282(1821): 20143085.
- Herold MJ, McPherson KG, & Reichardt H M (2006) Glucocorticoids in T cell apoptosis and function. Cellular and Molecular Life Sciences 63(1): 60–72.
- Coutinho AE, Chapman KE (2011) The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Molecular and Cellular Endocrinology 335(1): 2–13.






























