Autoantibodies Associated with Rheumatoid Arthritis: Rheumatoid Factor and Anti-Citrullinated Peptide Antibodies
Christian Blas La Rosa Fabian*
Department of Clinical Pathology and Pathology, National Hospital Dos de Mayo, Peru
Submission: March 20, 2019;Published: May 08, 2019
*Corresponding author: Christian Blas La Rosa Fabian, Area of Autoimmunity- Department of Clinical Pathology and Pathology. National Hospital Dos de Mayo, Lima Peru, South America
How to cite this article: Christian Blas La Rosa Fabian. Autoantibodies Associated with Rheumatoid Arthritis: Rheumatoid Factor and Anti-Citrullinated Peptide Antibodies. Ortho & Rheum Open Access J 2019; 14(1): 555880. DOI: 10.19080/OROAJ.2019.14.555880
Abstract
Rheumatoid Arthritis (RA) is a systemic autoimmune chronic inflammatory disease. Progress in understanding the pathogenesis of RA processes increased the interest in studying the biomarkers involved in different stages of the disease. The current classification criteria for RA include two different types of biomarkers that represent inflammatory processes, such as C-reactive protein and erythrocyte sedimentation rate or immune processes that include autoantibodies, such as rheumatoid factor (RF) and antibodies against citrullinated proteins. (ACPA). In this article, we review both autoantibody methods for detection and utility in the diagnosis, prognosis and follow-up of RA.
Introduction
Rheumatoid arthritis (RA) is the most common cause of inflammatory arthritis, affecting about 1% of the general population. RA is a common autoimmune systemic disease characterized by synovial inflammation, which potentially leads to weak articulation and irreversible destruction. A combination of multiple factors, such as genetic, environmental and hormonal, leads to the development of certain auto induced processes and characteristic damage to the disease [1]. Progress in understanding the pathogenesis of RA processes increased the interest in studying the biomarkers involved in different stages of the disease. There are at least two distinct subsets of AR. The most common type is associated with certain HLA DRB1 haploptypes, the production of antibodies against citrullinated peptides and has a more aggressive course of disease [2,3]. The detection of autoantibodies such as rheumatoid factor (RF) and citrulinated cyclic peptide antibodies (Anti-CCP) is part of the diagnostic criteria of the European League against Rheumatism / American College of Rheumatology (EULAR / ACR) [4] and it can guide the choice of treatments aimed at preventing or delaying the development of symptomatic RA [5].
Early diagnosis and immediate effective therapy are crucial for the prevention of joint deterioration, functional disability and the unfavourable and even fatal outcome of the disease. The new concept „window of opportunity“ shows that the identification of RA in the early stages is essential to prevent erosion and to stop the progression of radiological changes. The optimal and current treatment of RA requires that appropriate therapy be initiated within 3 to 6 months after the onset of the disease. In this context, the attention given to the identification of biomarkers with a diagnostic role in the early stages of the disease continues to be a subject of great interest [6,7]. In this article the main biomarkers in AR will be reviewed.
Rheumatoid Factor
The RF was discovered by Waaler in 1940 as an antibody directed against serum gamma globulins that promoted the agglutination of sheep red blood cells sensitized by subaglutinating doses of rabbit antibodies [8]. In 1952 Pike, Sulkin, and Coggeshale named this autoantibody „rheumatoid factor“ because of its association with rheumatoid arthritis (RA) disease. RFs are autoantibodies directed against epitopes located in the Fc fragment of IgG, at the junction of the CH2 and CH3 domains of the heavy gamma chain. They are usually IgM class antibodies, although there are IgG, IgA and IgE isotypes. The isotypic antigens bound by RF are IgG1, IgG2, IgG4 and a subset of IgG3 lacking the G3m (u) allotype.
IgGs are the predominant serum immunoglobulins, particularly IgG1, followed by IgG2, G3 and G4. The availability of this protein at high concentration induces tolerance of T cells against the Fc fragment of IgG under physiological conditions. IgG3 have been found in higher levels in synovial fluid in patients with RA than in normal blood. In addition to the different subclasses, the modified IgG structure may also be involved in the triggering of RF production. It is known that chronic inflammation, which is a hallmark of RA, and subsequent oxidative stress, contribute to post-translational modifications.
Detection Methods
Agglutination
The RF can be measured by different methods, the classical agglutination techniques were initially used due to the ability of the IgM to induce agglutination. The first screening test was based on the fact that the RF agglutinates red blood cells sensitized with rabbit IgG, which is the classic Waaler-Rose test. In addition, since the substrate used is a rabbit IgG, as compared to human IgG, the sensitivity is lower and the specificity for RA is higher. The agglutination with latex that detects in the presence of gamma-immunoglobulin or fraction II of Cohn adsorbed on an inert support of latex-polystyrene, this antigen binds to the RF producing an agglutination of the polystyrene latex particles, visible macroscopically [9]. The amount of RF IgM is usually expressed as the highest dilution of the serum that produces detectable agglutination. The concentration of RF IgM can be expressed in IU / mL based on the reference standards of the World Health Organization (WHO).
ELISA (Enzyme Linked Immunosorbent Assay)
Quantitative techniques, such as enzyme-linked immunosorbent assay (ELISA), have replaced the tests mentioned above, offering more reproducible results compared to semiquantitative tests. The ELISA has the ability to detect RF IgM, IgG and IgA isotypes [10].
Nephelometry and Turbidimetry
The latex particles coated with added human IgG serve as a capture agent in these methods [11]. The fixation of RF is determined by nephelometric or turbidimetric analysis based on precipitation. In recent years, these methods tend to replace latex tests [12]. Turbidimetry measures RF levels in human serum using heat inactivated human IgGs bound to latex particles. No difference between RF isotypes. Nephelometry is a latex particle agglutination test that uses polystyrene particles coated with IgG. The precipitation of aggregated IgG is measured by a nephelometer, usually a laser nephelometer based on the principle that a diluted suspension of small particles will scatter the light that passes through it [13].
Multiple Tests
Multiplex Assay are relatively new high-throughput techniques such as microarrays and flow cytometry for the quantitative detection of multiple analytes from a single biological sample [14]. Although they have not yet been standardized and validated, multiple trials can reduce analytical time and improve accuracy.
RFs in non-Rheumatic Diseases and Healthy People
RF can be detected in many non-rheumatic conditions and healthy people (Table 1). Infections and chronic diseases may be characterized by the presence of RFs, but unlike those detected in RA patients, the RFs produced during infections are usually transient and not detrimental. For example, in hepatitis C (76%), Epstein-Barr infection (20%), cytomegalovirus infection (20%) and subacute bacterial endocarditis (40%) [15,16], as well as in many inflammatory conditions and malignant neoplasms [17] The frequency of RF in other conditions limits its specificity in AR.
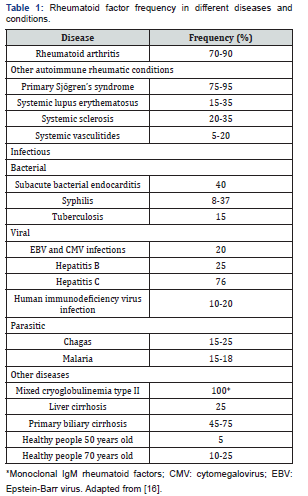
RF positivity has also been reported in the healthy population [18,19], and up to 4% of young Caucasians may be RF positive, with a similar distribution between the two genders. RFs found in healthy subjects are different from those present in RA patients as their titres are low/moderate and they are likely to be produced by CD5-expressing B cells as low- affinity, poly-reactive IgMs without any signs of maturation affinity [20].
RF in Autoimmune Diseases
Frequently RFs are detected in patients with systemic autoimmune diseases, such as systemic lupus erythematosus, mixed connective tissue disease, polymyositis, and dermatomyositis. High titers of RF are associated more frequently with RA, Sjögren‘s syndrome (SS) and mixed cryoglobulinemia of type II and III (CM), generally related to HCV.
RF in Rheumatoid Arthritis
Studies conducted during the last decade have indicated that it can have a diagnostic, prognostic and therapeutic value. The RF is part of the diagnostic criteria of the European League against Rheumatism / American College of Rheumatology (EULAR / ACR) [4]. The positivity of RF supports the diagnosis of RA (seropositive patients), while a negative RF test does not exclude the diagnosis (seronegative patients). The IgM RF are the isotype detected most frequently, but IgG, IgA, IgE and IgD RF can also be observed [21]. A combined increase in the titers of both IgM and IgA RF is almost exclusively present in patients with RA [22,23]. It has been shown that three RF isotypes IgM, IgA, and IgG are detected in up to 52% of RA patients, but in fewer than 5% of patients with other connective tissue diseases. Moreover, the presence of IgA and IgG RF isotypes in the absence of IgM-RF is more prevalent in patients with connective tissue diseases than in RA patients. The specificity of IgM-RF increases considerably in high titers [24]. RF is found in the serum of 70- 80% of RA patients. On the other hand, up to 30% of RA patients are seronegative for RF, and this proportion can exceed 50% in the early phase of the disease [25]. However, there is some evidence that individuals without arthritis may also present with RF seropositivity [26].
The specificity of RF varies from 70% to 90%. The specificity of IgM-RF increases considerably in high titers. The RF show a low positive predictive value (20-30%), but a high negative predictive value (NPV) (93-95%). The sensitivity RF is much lower in juvenile idiopathic arthritis (JIA) than in RA; no more than 5% of patients are positive, which makes their detection not very useful as a diagnostic tool in JIA [22]. In addition to its role in the diagnosis of RA, it also seems to have a prognostic role. The highest levels of RF are associated with the presence of aggressive disease, rheumatoid nodules and extra-articular manifestations [27]. It has been reported that persistently high IgM-RF titers or the presence of the IgA-RF isotype are strongly associated with a more rapid progression of the disease, with extra-articular manifestations such as rheumatoid nodules and vasculitis, and a worse outcome. Several studies have shown that the treatment of RA patients can reduce RF levels. The detection of IgM RFs is also helpfulas a prognostic index, and some studies have shown that immunosuppressive therapy with infliximab, etanercep, adalimumab, rituximab, and abatacept and tocilizumab can decrease serum RF levels. However, the clinical usefulness of RFs in monitoring disease activity [28] and treatment response is limited [29].
Autoantibodies against Citrullinated Proteins (ACPA)
Before the importance of citrullinated protein epitopes in RA was discovered, three autoantibodies were known already, which later were found to be members of the ACPA family. These were the anti-perinuclear factor (APF), anti-keratin antibodies (AKA) and anti-Sa. APF, described first in 1964, targets keratohyalin granules around the nuclei of buccal mucosal epithelial cells [30]. AKA, described in 1979, reacts with stratum corneum epithelial cells of the rat esophagus [31]. The specificities of both markers are similar (90% or higher), and the sensitivities are also in the same range (35-70%). Both autoantibodies may serve as early diagnostic markers, since they are detectable before classic clinical symptoms appear [32]. In 1995, it was shown that AKA and APF largely recognize the same autoantigens, filaggrin (filament aggregating protein) or pro-filaggrin [33], and three years later, it was revealed that the target epitopes were all citrullinated [34] Anti-Sa is an RA-specific autoantibody discovered in 1994, which recognized a 50 kDa protein in immunoblots of spleen and placental extracts. The diagnostic sensitivity was only 43%, but the specificity was 99%. A decade after its discovery, anti-Sa was shown to target [35].
Citrullination of proteins in RA
Post-translational modifications are chemical changes that proteins undergo after being synthesized. One of these modifications is citrullination, which is the conversion of the arginine residue to citrulline. The modification of proteins can generate new epitopes, different from those to which the immune system is tolerant, thus leading to a possible immune reaction against the citrullinated antigens. Citrulinacion is catalysed by Ca2 + dependent enzymes denoted as peptidylarginine deiminases (PAD), from which 5 isoforms of PAD have been identified with differential expression in tissues and organs. PAD1 is predominantly expressed in the epidermis and uterus; PAD2 is the most ubiquitous member of the family and is expressed in skeletal muscle, spleen, brain, salivary glands, uterus, etc; PAD3 is expressed in follicles; PAD4 is expressed in neutrophils and eosinophils, whereas PAD6 has been detected in ovaries, testes and peripheral blood leukocytes.
Citrullination participates in various processes, both physiological and pathological. Within the physiological processes, it includes the terminal differentiation of epithelial cells, the regulation of gene expression and apoptosis. In inflammatory processes, when there is cell death, there is a flow of calcium from the interior to the outside of the cell, activating the PAD. PAD4 contributes to genetic regulation through the citrullination of histones and, what is more important, this isoform is responsible for the generation of new autoantigens in RA. When the system of depuration of apoptotic cells by macrophages or active cells is inadequate, the citrullinated proteins can be exposed to the immune system. During synovial inflammation, when many cells die by apoptosis, it is possible to detect these proteins. Their presence does not necessarily lead to the generation of ACPA but depends on the genetic susceptibility of each individual. ACPA are locally produced in RA joints, where proteins are citrullinated during the inflammatory process [36]. The major citrullinated protein in the joint was found to be fibrin [37]. Additionally, various other synovial and non-synovial proteins (type II collagen, vimentin, nuclear proteins and stress proteins) have been shown to be targets of citrullination in vivo [38]. Immune complex formation between ACPAs and citrullinated proteins and subsequent complement fixation were recently demonstrated to occur in RA synovium and are thought to perpetuate RA synovial inflammation, causing a vicious cycle [39].
Methods of Detection
The difficulties of working with proteins as a substrate for high performance assays, including the cost and the presence of antibodies in the non-citrullated regions, have led many researchers to work with ELISA based on immunodominant peptides of these citrullinated autoantigens. Several commercial tests using citrullinated specific antigens are available to determine ACPA positivity. First generation citrullinated cyclic proteins (CCP-1) detect antibodies against peptides derived from the prophylagrin/filaggrin protein. The second-generation citrulline cyclic proteins (CCP2) are the most widely used substrates in these tests. They were selected from a selection of citrullinated peptides for their strong reactivity with sera from patients with RA. In addition to anti-CCP2, a third generation anti-CCP test (anti-CCP3) designed from peptides with multiple antigenic epitopes with determined conformational structure has been developed. Thus, a better exposure and antigenicity of citrulline was obtained, which managed to increase the sensitivity a little more. The latter still maintain high specificity (95-98%), higher than that of RF, and a sensitivity comparable to that of the latter (67-81%). Additionally, a commercial house adds CCP3.1; the antigen used is the same as that of CCP3 but contains a conjugate that allows the detection of anti-CCP3 IgA class antibodies in addition to the usual anti-CCP3 IgG antibodies.
Clinical Utility
ACPA most specific serological markers of RA, with a specificity of 98-99% in some studies (Table 2). It should be noted, however, that these high specificities are obtained when the population with RA is compared with a healthy population; that is, only 1-2% of healthy individuals have ACPA. However, the specificity, although very high, is somewhat lower when compared with patients with other rheumatic diseases that can be considered in the differential diagnosis of RA, as is the case of psoriatic arthritis, systemic lupus erythematosus (SLE). Sjogren’s syndrome and other systemic diseases.
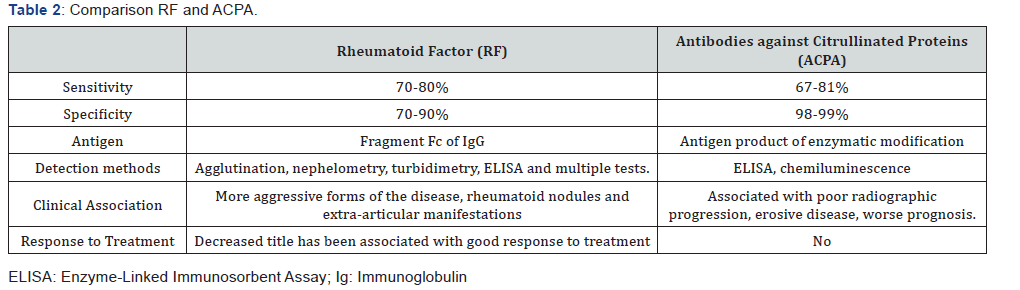
ACPA levels can also be important. This is clearly reflected in the new criteria of the RA (ACR / EULAR 2010), where it is considered that the high levels of RF and ACPA weigh more (3 points) than the low levels (2 points) [4]. ACPAs have an important prognostic importance, their production may precede the onset of the symptoms of RA for several years [40]. People with ACPA positive with initial and undifferentiated arthritis have a higher risk of subsequently developing RA.
In patients with RA, the presence of positive ACPA develop more bone erosions and experience a more severe progression of the disease, so ACPA has an important prognostic role during the progression of RA and is associated with pronounced radiographic progression [41]. ACPA production has been associated with several genetic predisposing factors, including the HLA-DRB1 and PTPN22 1858T alleles, as well as with environmental and lifestyle-related factors, primarily smoking, periodontitis and possibly the use of oral contraceptives and caffeine intake [42]. Thus, the assessment of ACPA, in addition to the clinical, radiographic and genetic outcome measures, can be important to assess disease prognosis and aids to design effective, early therapeutic strategies. Based on the clinical and laboratory findings, ACPA isotype distribution does not expand during disease progression from the undifferentiated arthritis stage to RA, and is relatively stable over time; moreover the baseline ACPA isotype profile is a significant predictor of disease severity, with more isotypes indicating a higher risk of radiographic damage [43]. Observations reporting association between the presence of ACPA and joint damage, as well as extra-articular manifestations suggest that ACPA are most likely pathogenic autoantibodies in RA. Interestingly, high ACPA (anti- CCP) level predicts the good clinical response to certain biologics (rituximab, abatacept), thus assessing this factor in clinical practice can help with personalized medicine and selecting the best candidates for biologic drug treatment [44].
Although, the exact roles of ACPAs in the pathogenesis of RA is not fully understood, in vitro studies demonstrated that ACPAs induce macrophage TNF-α production, osteoclastogenesis and complement activation [45]. The anti-mutated citrullinated vimentin (anti-MCV) is a key autoantibody of the ACPA family, where vimentin is secreted and citrullinated by macrophages in response to apoptosis, or by proinflammatory cytokines, such as tumor necrosis factor -α [46]. The occurrence of anti-MCV is between 21-43% in RA, while in other systemic autoimmune diseases only 1%, which makes the autoantibody having low sensitivity, but a very high specificity. Thus, determination of anti-MCV seems to be useful as a test of line 2 in patients suspected RA but negative for both RF and anti-CCP.
Conclusion
RF and ACPA are the two most remarkable autoantibodies in RA and provide different clinical and pathophysiological information. In general, ACPAs are a better diagnostic guide than RF due to their greater sensitivity and specificity for RA. Both RF and ACPA are factors of poor prognosis of joint destruction, while RF is also associated with extra-articular manifestations. RF in RA patients are of high affinity and high titer, which indicates that RF may contribute to disease perpetuation by potentiating immune complex formation and complement fixation. The responses of these two antibodies to treatment vary, reflecting their different mechanisms of participation in the pathogenesis of RA.
References
- IB McInnes, G Schett (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23): 2205-2219.
- NA Daha, REM Toes (2011) Rheumatoid arthritis: Are ACPA-positive and ACPA-negative RA the same disease? Nat Rev Rheumatol 7(2011): 202-203.
- DPC De Rooy, A Willemhe, B Mertens, TW Huizinga, AH Van der Helm-van Mil, et al. (2011) Can anti-cyclic citrullinated peptide antibody-negative RA be subdivided into clinical subphenotypes? Arthritis Res. Ther 13: R180.
- Aletaha D, Neogi T, Silman, Funovits J, Felson DT, et al. (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis 62(9): 1580-1588.
- Robinson WH, Lindstrom TM, Cheung RK, Sokolove J (2013) Mechanistic biomarkers for clinical decision making in rheumatic diseases. Nat Rev Rheumatol 9: 267-276.
- Schneider M, Kruger K (2013) Rheumatoid Arthritis-Early Diagnosis and Disease Management. Dtsch Arztebl Int 110(27): 477–484.
- Heidari B (2011) Rheumatoid Arthritis: Early diagnosis and treatment outcomes. Caspian J Intern Med 21(1): 161-170.
- Waaler E (1939) On the occurrence of a factor in human serum activating the speci c agglutination of sheep blood corpuscles. Acta Pathol Microbiol Scand 1: 172–188.
- Mafuku Y, Yoshida H (2001) Rheumatoid factor. Rinsho Buori 49(6): 580-584.
- Ulvestad E, Wilfred LL, Kristoffersen EK (2001) Measurement of IgM rheumatoid factor by ELISA. Scand J Rheumatol 30(6): 366-366.
- Hicks MJ, Heick M, Finley P, Gall EP, Minnich L, et al. (1982) Rheumatoid factor activity by rate nephelometry correlated with clinical activity in rheumatoid arthritis. Am J Clin Pathol 78(3): 342–345.
- Wolfe F, Cathy MA, Roberts FK (1991) The latex test revisited: Rheumatoid factor testing in 8,287 rheumatic disease patients. Arthritis Rheum 34(8): 951-960.
- Anuradba V, Chopra A (2005) In the era of nephelometry, latex aglutination is still good enough to detect rheumatoid factor. J Rheumatol 32(12): 2343-2344.
- Abreu I, Laroche P, Bastos A, Issert V, Cruz M, et al. (2005) Multiplexed immunoassay for detection of rheumatoid factors by FIDISTM technology. Annals of the New York Academy of Sciences 1050: 357–363.
- Y Renaudineau, C Jamin, A Saraux, P Youinou (2005) Rheumatoid factor on a daily basis, Autoimmunity 38(1): 11-16.
- F Ingegnoli, R Castelli, R Gualtierotti (2013) Rheumatoid Factors: Clinical Applications, Dis. Markers 35(6): 727-734.
- H Bartfeld (1969) Distribution of rheumatoid factor activity in non-rheumatoid states, Ann NY Acad Sci 168(1): 30-40.
- JF Simard, M Holmqvist (2012) Rheumatoid factor positivity in the general population,” British Medical Journal 345: e5841.
- Tasliyurt T, Kisacik B, Kaya SU, Yildirim B, Pehlivan Y, et al. (2013) The frequency of antibodies against cyclic citrullinated peptides and rheumatoid factor in healthy population: a field study of rheumatoid arthritis from northern turkey. Rheumatology International 33(4): 939-942.
- M Børretzen, C Chapman, JB Natvig, KM Thompson (1997) Differences in mutational patterns between rheumatoid factors in health and disease are related to variable heavy chain family and germ-line gene usage. European Journal of Immunology 27(3): 735-741.
- HW Schroeder Jr, L Cavacini (2010) Structure and function of immunoglobulins. Journal of Allergy and Clinical Immunology 125(2 Suppl 2): S41-S52.
- De Angelis V, Meroni PL (2007) Rheumatoid factors. In: Peters JB, Schoenfeld Y, editors. Autoantibodies. Amster- dam: Elsevier Science BV pp. 755–762.
- T Jonsson, K Steinsson, H Jonsson, AJ Geirsson, J Thorsteinsson, et al. (1998) Combined elevation of IgM and IgA rheumatoid factor has high diagnostic specicity for rheumatoid arthritis. Rheumatology International 18(3): 119–122.
- Deane KD, O'Donnell CI, Hueber W, Majka DS, Lazar AA, et al. (2010) The number of elevated cytokines and chemokines in preclinical seropositive rheumatoid arthritis predicts time to diagnosis in an age- dependent manner. Arthritis and Rheumatism 62(11): 3161–3172.
- Visser H (2005) Early diagnosis of rheumatoid arthritis. Best Pract Res Clin Rheumatol 19(1): 55-72.
- Visser H, Gelinck LB, Kampfraath AH, Breedveld FC, Hazes JM (1996) Diagnostic and prognostic characteristics of the enzyme linked immunosorbent rheumatoid factor assays in rheumatoid arthritis. Ann Rheum Dis 55(3): 157-161
- Vittecoq O, Pouplin S, Krzanowska K, Jouen-Beades F, Menard JF, et al. (2003) Rheumatoid factor is the strongest predictor of radiological progression of rheumatoid arthritis in a three-year prospective study in community-recruited patients. Rheumatology (Oxford) 42(8): 939-946.
- Tillmann T, Krishnadas R, Cavanagh J, Petrides KV (2013) Possible rheumatoid arthritis subtypes in terms of rheumatoid factor, depression, diagnostic delay and emotional expression: an exploratory case-control study. Arthritis Res Ther 15(2): R45.
- L Barra, V Bykerk, JE Pope (2013) Anticitrullinated protein antibodies and rheumatoid factor uctuate in early in amma- tory arthritis and do not predict clinical outcomes. Journal of Rheumatology 40(8): 1259–1267.
- RLF Nienhuis, E Mandema (1964) A New Serum Factor in Patients with Rheumatoid Arthritis: The Antiperinuclear Factor. Ann Rheum Dis 23: 302- 305.
- BJ Young, RK Mallya, RD Leslie, CJ Clark, TJ Hamblin (1979) Anti-keratinantibodies in rheumatoid arthritis. Br Med J 2: 97-99.
- Vencovský J, Machácek S, Sedová L, Kafková J, Gatterová J, et al. (2003) Autoantibodies can be prognostic markers of an erosive disease in early rheumatoid arthritis. Ann Rheum Dis 62(5): 427-430.
- Sebbag M, Simon M, Vincent C, Masson-Bessière C, Girbal E, et al. (1995) The antiperinuclear factor and the so-called antikeratin antibodies are the same rheumatoid arthritis-specific autoantibodies. J Clin Invest 95(6): 2672-2679.
- Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ, et al. (1998) Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest 101(1): 273-281.
- ER Vossenaar, N Després, E Lapointe, A Van der Heijden, M Lora, et al. (2004) Rheumatoid arthritis specific anti-Sa antibodies target citrullinated vimentin. Arthritis Res Ther 6(2004): R142-150.
- Reparon-Schuijt CC, van Esch WJ, van Kooten C, Schellekens GA, de Jong BA, et al. (2001) Secretion of anti-citrulline-containing peptide antibody by B lymphocytes in rheumatoid arthritis. Arthritis Rheum 44(1): 41–47.
- Masson-Bessiere C, Sebbag M, Girbal-Neuhauser E, Nogueira L, Vincent C, et al. (2001) The major synovial targets of the rheumatoid arthritis-specific antifilaggrin auto- antibodies are deiminated forms of the alpha- and beta- chains of fibrin. J Immunol 166(6): 4177–4184.
- Goëb V, Thomas-L'Otellier M, Daveau R, Charlionet R, Fardellone P, et al. (2009) Candidate autoantigens identified by mass spectrometry in early rheumatoid arthritis are chaperones and citrullinated glycolytic enzymes. Arthritis Res Ther 11(2): R38.
- Zhao X, Okeke NL, Sharpe O, Batliwalla FM, Lee AT, Ho PP, et al. (2008) Circulating immune complexes contain citrullinated fibrinogen in rheumatoid arthritis. Arthritis Res Ther 10(4): R94.
- Dekkers J, Toes RE, Huizinga TW, van der Woude D (2016) The role of anticitrullinated protein antibodies in the early stages of rheumatoid arthritis. Curr Opin Rheumatol. 28(3): 275-281.
- L Klareskog, M Widhe, M Hermansson, J Rönnelid (2008) Antibodies to citrullinated proteins in arthritis: pathology and promise. Curr Opin Rheumatol 20(3): 300-305.
- Szodoray P, Szabó Z, Kapitány A, Gyetvai A, Lakos G, et al. (2010) Anti- citrullinated protein/peptide autoantibodies in association with genetic and environmental factors as indicators of disease outcome in rheumatoid arthritis. Autoimmun Rev 9(3): 140-143.
- Van der Woude D, Syversen SW, van der Voort EI, Verpoort KN, Goll GL, et al. (2010) The ACPA isotype profile reflects long-term radiographic progression in rheumatoid arthritis. Ann Rheum Dis 69(6): 1110-1116.
- Gardette A, Ottaviani S, Tubach F, Roy C, Nicaise-Roland P, et al. (2014) High anti-CCP antibody titres predict good response to rituximab in patients with active rheumatoid arthritis, Joint. Bone Spine 81(5): 416-420.
- A Watad, H Amital (2016) ACPAs Are Much More Than Diagnostic Autoantibodies, Rambam. Maimonides. Med J 7(4).
- Soós L, Szekanecz Z, Szabó Z, Fekete A, Zeher M, et al. (2007) Clinical evaluation of anti-mutated citrullinated vimentin by ELISA in rheumatoid arthritis, J Rheumatol 34: 1658-1663.






























