A Rare Case of Strokes, Diffused Cardiac Involvement and Thrombosis
Maryam Masoumi1,2*, Shokuofeh Mousavi2, Maryam Rastegarpanah2 and Mina Gheitani2
1Clinical Research of Development Center, Shahid Beheshti Hospital, Qom University of Medical Sciences, Qom, Iran
2Department of Internal Medicine, Faculty of Medicine, Qom University of Medical Sciences, Qom, Iran
Submission: January 16, 2019;Published: January 29, 2019
*Corresponding author: Maryam Masoumi, Clinical Research of Development Center, Shahid Beheshti Hospital, Qom University of Medical Sciences, Qom, Iran
How to cite this article: Maryam M, Shokuofeh M, Maryam R,Mina G. A Rare Case of Strokes, Diffused Cardiac Involvement and Thrombosis. Ortho & Rheum Open Access J 2019; 13(3): 555865865.DOI: 10.19080/OROAJ.2019.13.555865
Abstract
Catastrophic antiphospholipid syndrome (CAPS) is the fatal type of Antiphospholipid syndrome (APS), which is rare (< 1%). It generally leads to multiorgan failure with a worse prognosis and life-threating condition. The heart involvement is about 50% of the cases with CAPS, but whole cardiac layers involvement is very rare and unreported yet. This is a case of a 19-year- old woman with acute thrombosis. The diagnosis of CAPS was confirmed within 12 weeks. So, the best treatment strategy is the primary diagnosis, aggressive and combination therapies with anticoagulant, corticosteroid and plasma exchange.
Keywords: antiphospholipid syndrome, catastrophic antiphospholipid syndrome, cardiac involvement, stroke.
Abbrevations: CAPS: Catastrophic Antiphospholipid Syndrome; APS: Antiphospholipid Syndrome; TIA: Transient Ischemic Attack; TF: Tissue Factor; TLR: Toll Like Receptor; aPL: Antiphospholipid Antibody; TNF: Tumour Necrosis Factor
Introduction
Catastrophic antiphospholipid syndrome (CAPS) is the fatal type of Antiphospholipid syndrome (APS), that is rare (< 1%) [1]. It generally leads to multiorgan failure with a worse prognosis and life-threating condition, this situation, association with increased morbidity and mortality. The risk of cerebrovascular events and heart involvement are increased in CAPS. Stroke represents one of the most severe complications of it [2], and heart involvement is about 50%. Below we present a young woman with CAPS lead to brain and whole cardiac layers involvement (that is extremely rare and unreported yet).
Case Report
The patient we want to introduce is a 19-year-old woman who is due to abdominal pain, at rest Dyspnea (NYHAA class 4), weakness, lethargy, reducing urine output and hallucination referred to our hospital. The patient has occasional vomiting and has a delirium in several last months. The patient had no background disease in the past two months until last two month in an accident with head trauma and a major stressor caused by his brother‘s drowning occurred. Since then, she has frequent headaches and blurred vision that it‘s not seen any finding in favor of bleeding in the CT scan of the brain. The patient also suffered from a reduction in muscle strength and generalized weakness that she is treated with a diagnosis of ischemic brain infarction in another hospital. In the Brain MRI which is at that time, multiple cortical and subcortical areas and deeper areas are seen in the white matter in both cerebral hemispheres, which are more suggestive of multifocal acute embolic infarct and acute small vessels ischemia or vasculitis .
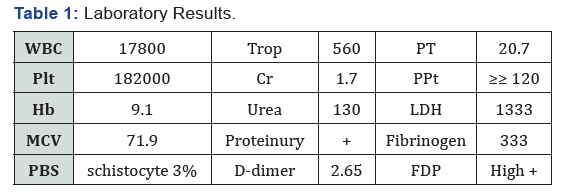

Also, in the patient‘s MRV indicate partial thrombosis. At the time of the visit, the patient‘s blood pressure was 152/82 mmHg and her heart rate were 120 beats/min and her body temperature were 37 degrees oral. She seemed confused. Her pupils were dilated and reactive to light. Oxygen saturation of her arterial blood without oxygen was 96%. Also, livedo reticularis lesions on the upper limb and mild pitting edema were in the lower limbs without any size differences in the limbs. The force of the four limbs is 3/5. Her pulmonary voices had fallen, and the diuresis was 300 cc in 12 hours. Patient‘s laboratory results are as follows: (Tables 1 & 2). In the ultrasonography of the abdomen and pelvic, a mild free fluid was reported in the abdominal cavity Also, in the patient‘s MRV indicate partial thrombosis. At the time of the visit, the patient‘s blood pressure was 152/82 mmHg and her heart rate were 120 beats/min and her body temperature were 37 degrees oral. She seemed confused. Her pupils were dilated and reactive to light. Oxygen saturation of her arterial blood without oxygen was 96%. Also, livedo reticularis lesions on the upper limb and mild pitting edema were in the lower limbs without any size differences in the limbs. The force of the four limbs is 3/5. Her pulmonary voices had fallen, and the diuresis was 300 cc in 12 hours. Patient‘s laboratory results are as follows: (Tables 1 & 2). In the ultrasonography of the abdomen and pelvic, a mild free fluid was reported in the abdominal cavity
In the chest X-ray due to the patient‘s distress, bilateral pleural effusion and cardiomegaly were observed. Under echocardiography further investigation was made. In the echocardiography, these results were obtained:
EF: 30-35%
LA dimension is dilated
RV is dilated
RV function is mildly reduced
RA is dilated
MV rheumatismal and severe MR
PAP: 55 mmHg
Minimal pericardial effusion
Two blood cultures were performed to rule out infective endocarditis. Negative blood culture was reported. Subsequently, TEE was performed for further investigation. The results are as follows:
LVEF: 34%
LA dimension and RA are enlarged
MV is very thick
Severe MR
AV is thick
A Multi-echogenic mobile mass was seen at the site of the catheter inside the SVC. According to the patient‘s bedside and negative blood cultures, the mass is more likely to clot. Given this evidence, Heparin started for the patient. The patient experienced an increase in the level of LDH (1752) decrease in fibrinogen level and increased schistocytes (1-2%) on the 10th day of admission. This indicates that the course of the patient‘s coagulopathy continues. Therefore, the patient became a candidate for plasma exchange and central vein catheter was placed on the left side of the patient‘s neck. During catheterization, the patient suffered from bleeding, ecchymosis on the arms, oozing in the catheter area, and her level of hemoglobin reached 9.7 to 5.3 the following day. Bleeding at the site of the catheter justifies the decrease of hemoglobin level. 1 unit of the washed packed cell was also injected. Two days after catheterization, the patient suffered from respiratory distress with 34 RR, 74% SpO2 and a decrease in pulmonary sound on the left side. In the CXR, a white lung was seen on the left lung. Due to the detection of vascular abnormalities during catheterization, a chest tube was placed on the left chest wall and two liters of blood were removed.
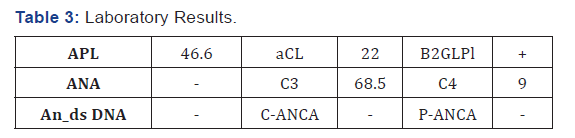
After embedding chest tube, the patient‘s distress was resolved and SpO2 reached 99%. During the admitted and treated with heparin, the patient suffered from colds and cyanosis in the upper left extremity in two occasions. For this reason, the color Doppler ultrasonography was performed on the upper left limb vessels. In this ultrasonography, basilic and cephalic vein thrombosis and in the next turn, the basilic and brachial vein thrombosis were seen. Due to the occurrence of repeated thrombosis and involvement of several organs, we suspected the APS. So, the following experiments were requested in Table 3. Regarding positive triple test and clinical signs of the patient (recurrent strokes, heart involvement, multiple venous thrombosis in the limb, renal involvement, pulmonary involvement due to PTE), CAPS was confirmed.
After confirming the CAPS, treatment by 16 U/Kg/h heparin, methylprednisolone pulse 1 g/d for 5 days and rituximab 500 mg weekly for 4 weeks and IVIg 20 g/d for 5 days began, then, after the completion of IVIG, the plasmapheresis was carried out. During the treatment, the patient suffered a platelet loss several times, so she was subjected to plasmapheresis. Patient symptoms improved by receiving anti-coagulant and immunosuppressant‘s Relative, except for psychosis and hallucinations, due to sustaining brain injuries is created and resistant to treatment. After a relative improvement in the patient, he was discharged and the plasmapheresis was followed up in an outpatient setting.
Discussion
This patient has CAPS (catastrophic antiphospholipid syndrome) with multifocal Brain strokes with what appears to be LA, RA & RV is dilated, and RV function is reduced and valvar thickness & regurgitation as cardiac manifestations. Catastrophic APS is a rare form of classic APS that has very severe variant clinical manifestation. Although catastrophic APS is observed in less than 1% of all patients with APS, the situation is commonly life-threatening [3,4] with 50% mortality rate [5]. CAPS is characterized by multiorgan failure due to thrombotic storm within small vessels, leading to progressive multiorgan dysfunction at a short time [6-8]. The precipitating factors for CAPS including infection (49%), neoplasms (17%), surgery (16%) [2], medications, withdrawal/low international normalized ratio, obstetric complications, trauma. Although in > 65% of the patients it does not you can find any precipitating factor [9-11]. In this case, trauma 2 months prior to the episode would be exited as a likely trigger.
In generally, CAPS definition by a rapid progressively multisystem disease with the most affected organs are kidneys (73%), lung (60%), brain (56%) and heart (50%) [2]. Our patient exhibited brain infarction, oliguria, respiratory failure, cardiac involvement, Serositis, all of which developed within a short period. Presentation of brain involvement may include as strokes (40%), encephalopathy and seizures. Cardiac failure
most commonly occurs while attributed to the SIRS response or myocardial infarction [2]. Levine JS et.al: when the SLE is associated with APS, there is a high prevalence of thrombosis. Arterial thrombosis occurs in the brain in 50% of cases and coronary occlusions in 23% [12]. Libman-sacks vegetation extends mainly on the mitral valve and the aortic valve but may affect any other valves, or even subvalvular apparatus [13]. Liebman-sacks endocarditis be displayed can from asymptomatic valvular pathology (regurgitation or thickening) to heart failure from valvular invalidism along with generalized symptoms such as fatigue, weight loss, night sweats and fever [14]. As in our case, TEE demonstrated severe MR, MV is very thick & AV is thick, LA dimension and RA is enlarged. There is an important point in our patient, he has endocarditis (valves involvement), myocarditis (LVEF: 34%) and pericarditis (minimal pericardial effusion) at the same time, that it‘s extremely rare and unreported yet.
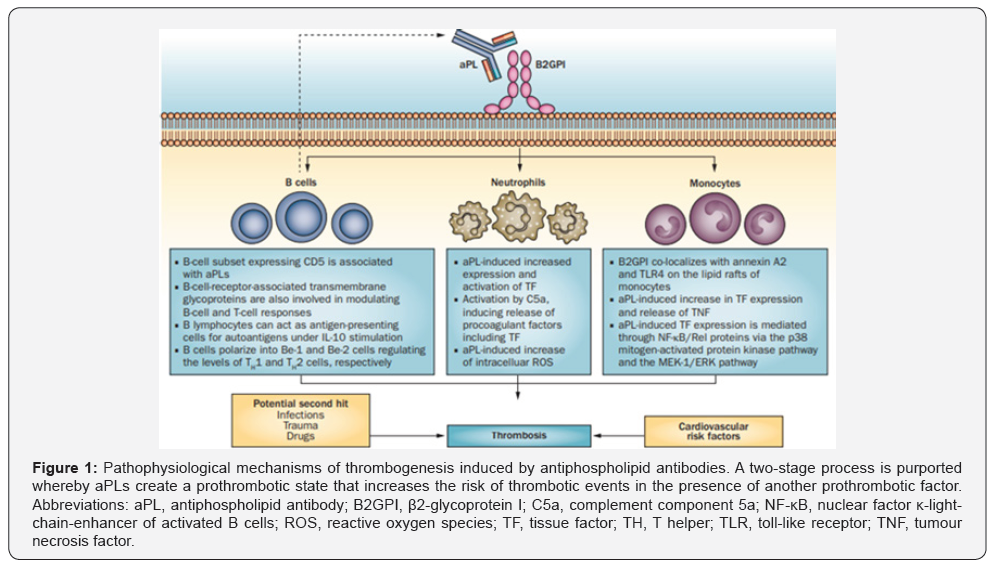
Catastrophic APS is most feared and often fatal complication. Secondary infective endocarditis should be considered with acute refractory heart failure [15]. Because determined in our case some mobile lesion in SVC while near the SVC catheter, we were gives blood cultures that were negative. The pathogenesis of thrombotic events and valve lesions in aPLs (and LSE) is aPLrelated prothrombotic state could be enforce by many various mechanisms, including: activated endothelial cells, platelets and monocytes, potentiating platelet aggregation, increases in fibrinolysis and the expression of regulatory proteins such as protein C, protein S and antithrombin [4,16-19]. in addition, there is evidence to show that the endothelium is activated by aPL, so firstly, begin the procoagulant activity, cellular injury [17,20] and disrupting procoagulant and anticoagulant reactions on cell membranes. Secondly, aPLs might communicate with special cell-surface receptors (lipids, proteins or both) and induce the expression of the signals that will upregulation of cell-surface proteins (Figure 1) [4,19]. Further deposition of complement components and immunoglobulins leads to formation clot and cusp fibrosis, scaring and thickening, and finally to valvular vegetation, fusion and rigidity causing deformation or dysfunction [19,21,22].
Traditional cardiovascular risk factors, which are present in more than 50% of patients which APS [23]. Importantly, Sciascia [24] repetition of aPL in young patients with CVE s (cerebrovascular event) and to determine whether aPL-positive young patients are at higher risk of CVE s when contrasted with patients without aPL [25]. Clinical manifestation of patients with stroke (acute, recent (within 2-4 weeks) or recurrent) or transient ischemic attack (TIA) represents an irreversible or partially reversible neurologic deficit due to vascular occlusion [17,26]. in the exposed case, it presents as a refractory headache, focal deficits, and psychosis. The acceptable guidelines for the management of patients with (C)APS are showed in Table 4 [27]. Anticoagulant can inhibit the thrombotic storm, immunomodulation can suppress the cytokine cascade, and plasma exchange can eliminate antiphospholipid antibodies, complement and cytokines, improving the survival rate. Therefore, we initiate our treatments with anticoagulation, corticosteroids, and plasmapheresis. However, as happened PTE in our patient at last. The APS patients need extreme care and avoid intravascular appliance due to new clot formation [28]. In the patients is quite well in the follow-up, but the psychosis is still left.

Conclusion
Cryptogenic stroke cases with high probable of underlying autoimmune disorders such as APS, Lupus or both. Early recognition of cardiac represent (even whole cardiac layers involvement), timely management, improvement of risk factors and combine aggressive therapies with anticoagulant, corticosteroid and plasmapheresis can reduce their morbidity and mortality.
References
- Li CZ, Li CC, Hsieh CC, Lin MC, Hueng DY, et al. (2017) Fatal antiphospholipid syndrome following endoscopic transnasal-transsphenoidal surgery for a pituitary tumor: A case report Medicine 96: 1.
- Rodriguez-Pinto I, Moitinho M, Santacreu I, Shoenfeld Y, Erkan D, et al. (2016) Catastrophic antiphospholipid syndrome (CAPS): Descriptive analysis of 500 patients from the International CAPS Registry. Autoimmunity reviews 15(12): 1120-1124.
- Fakhouri F, Noël LH, Zuber J, Beaufils H, Martinez F, et al. (2003) The expanding spectrum of renal diseases associated with antiphospholipid syndrome. American journal of kidney diseases 41(6): 1205-1211.
- Sciascia S, Cuadrado MJ, Khamashta M, Roccatello D (2014) Renal involvement in antiphospholipid syndrome. Nature Reviews Nephrology, 10(5): 279-289.
- Cervera R, Khamashta MA, Shoenfeld Y, Camps MT, Jacobsen S, et al. (2009) Morbidity and mortality in the antiphospholipid syndrome during a 5-year period: a multicentre prospective study of 1000 patients. Annals of the Rheumatic Diseases 68(9): 1428-1432.
- Cervera R, Rodríguez-Pintó I, Colafrancesco S, Conti F, Valesini G, et al. (2014) 14th international congress on antiphospholipid antibodies task force report on catastrophic antiphospholipid syndrome. Autoimmunity Reviews 13(7): 699-707.
- Gómez-Puerta JA, Espinosa G, Cervera R (2013) Catastrophic antiphospholipid syndrome: diagnosis and management in pregnancy. Clinics in laboratory medicine 33(2): 391-400.
- BG, SAK (2013) The pathogenesis of the antiphospholipid syndrome. N Engl J Med 368: 1033–1044.
- Ideguchi H, Ohno S, Ueda A, Ishigatsubo Y (2007) Catastrophic antiphospholipid syndrome associated with malignancies (case report and review of the literature). Lupus 16(1): 59-64.
- Obón BA, Ortas MN, Gutiérrez IC, Bustamante RR, Velilla CS, et al. (2008) Mortality due to heart involvement in the catastrophic antiphospholipid syndrome. Anales de medicina interna, Madrid, Spain 25(5): 229-230.
- Asherson RA (2005) Multiorgan failure and antiphospholipid antibodies: the catastrophic antiphospholipid (Asherson's) syndrome. Immunobiology 210(10): 727-733.
- Levine JS, Branch DW, Rauch J (2002) The antiphospholipid syndrome. New England Journal of Medicine 346(10): 752-763.
- Bai Z, Hou J, Ren W, Guo Y (2015) Diagnosis and surgical treatment for isolated tricuspid libman-sacks endocarditis: a rare case report and literatures review. Journal of cardiothoracic surgery 10(1): 93.
- Garcia D, Erkan D (2018) Diagnosis and management of the antiphospholipid syndrome. New England Journal of Medicine 378(21): 2010-2021.
- Lin GM, Chang FY, Wang WB (2015) Coagulase-negative staphylococcus infective endocarditis in a lupus patient with Libman-Sacks endocarditis. The Journal of heart valve disease 24(2): 236-238.
- Oosting JD, Derksen RH, Blokzijl L, Sixma JJ, de Groot PG (1992) Antiphospholipid antibody positive sera enhance endothelial cell procoagulant activity–studies in a thrombosis model. Thrombosis and haemostasis 68(03): 278-284.
- de Amorim L, Maia F, Rodrigues C (2017) Stroke in systemic lupus erythematosus and antiphospholipid syndrome: risk factors, clinical manifestations, neuroimaging, and treatment. Lupus 26(5): 529-536.
- Meroni PL, Riboldi P (2001) Pathogenic mechanisms mediating antiphospholipid syndrome. Current opinion in rheumatology. 13(5): 377-382.
- Cuadrado M, Buendia P, Velasco F, Aguirre M, Barbarroja N, et al. (2006) Vascular endothelial growth factor expression in monocytes from patients with primary antiphospholipid syndrome. Journal of Thrombosis and Haemostasis. 4(11): 2461-2469.
- Esdaile JM, Abrahamowicz M, Grodzicky T, Li Y, Panaritis C, et al. (2001) Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis & Rheumatism 44(10): 2331-2337.
- Ziporen L, Goldberg I, Arad M, Hojnik M, Ordi-Ros J, et al. (1996) Libman-Sacks endocarditis in the antiphospholipid syndrome: immunopathologic findings in deformed heart valves. Lupus 5(3): 196-205.
- Tenedios F, Erkan D, Lockshin M (2005) Cardiac involvement in the antiphospholipid syndrome. Lupus 14(9): 691-696.
- Cervera R, Tektonidou M, Espinosa G, Cabral A, González E, et al. (2011) Task Force on Catastrophic Antiphospholipid Syndrome (APS) and Non-criteria APS Manifestations (I): catastrophic APS, APS nephropathy and heart valve lesions. Lupus 20(2): 165-173.
- Sciascia S, Sanna G, Khamashta MA, Cuadrado MJ, Erkan D, et al. (2015) The estimated frequency of antiphospholipid antibodies in young adults with cerebrovascular events: a systematic review. Annals of the rheumatic diseases. 74(11): 2028-2033.
- Timlin H, Petri M (2013) Transient ischemic attack and stroke in systemic lupus erythematosus. Lupus 22(12): 1251-1258.
- Fernandez-Nebro A, Rúa-Figueroa Í, Lopez-Longo FJ, Galindo-Izquierdo M, Calvo-Alen J, et al. (2015) Cardiovascular events in systemic lupus erythematosus: a nationwide study in Spain from the RELESSER registry. Medicine 94(29): e1183.
- Teunisse C, Kalsbeek A, de Vries S, Huisman S, Boers J, et al. (2010) Reversible cardiac valvular disease in catastrophic antiphospholipid syndrome. 68(5): 215-220.
- Hartung HP, Mouthon L, Ahmed R, Jordan S, Laupland K, et al. (2009) Clinical applications of intravenous immunoglobulins (IVIg)–beyond immunodeficiencies and neurology. Clinical & Experimental Immunology 158(1): 23-33.






























