Abstract
Several organic reactions e.g., alkylation, esterification, S- methylation, P-methylation etc have been achieved using iodomethane. The present review describes the several uses of methyl iodide to achieve O-methylation of organic compounds and the use of the resulting compounds for organic and natural product synthesis.
Keywords:Methyl Iodid; O-Methylation; Alcohol; Esters; Acids.
Introduction
The commercially available methyl iodide is a colorless transparent liquid which turns brown on exposure to light. It is easier to handle than methyl chloride and so is used a great deal as methylating agent in the laboratory of organic synthesis, but the chloride is used industrially because it is cheaper. Methyl iodide is stabilized by the addition of silver wire or copper beads. It is purified by passing through a column of silica gel or activated alumina and then distill. It is also purified by washing with dilute aqueous sodium thiosulfate, dilute aqueous sodium carbonate, water, dried over calcium chloride and distill.
The colorless distilled product should be stored in a brown bottle and kept out of sunlight. As a future protective measure a few drops of clean mercury can be added. Methyl iodide reacts faster than methyl bromide under SN2 conditions that have been studied [1]. In both laboratory and field trials methyl iodide is not less or better than methyl bromide in controlling the tested soil borne plant pathogens and weeds [2]. When compared with other alkyl iodides, methyl iodide is the most effective fumigant.
Preparation of Methyl Iodide
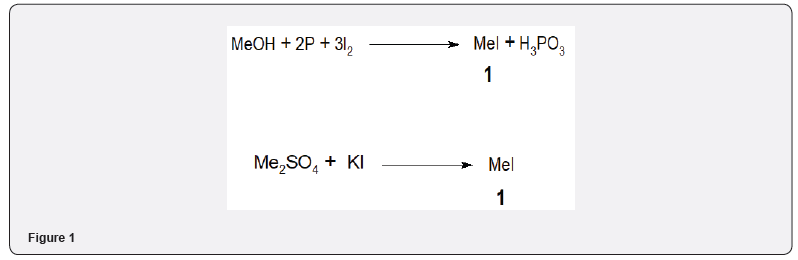
Methyl iodide 1 is prepared by warming a mixture of methanol, red phosphorous and iodine [3] Figure 1. Methyl iodide 1 can also be prepared by heating methyl sulfate on potassium iodide solution in water [4] Figure 1.
Use of Iodomethane
Methyl iodide has been extensively used for methylation of organic compounds. It includes O-methylation, C-methylation, S-methylation, N -methylation, P-methylation, and metal methylation. In addition, methyl iodide has been utilized for cleavage reaction, condensation reaction etc. The present review describes the use of methyl iodide for O-methylation of phenols, alcohols, and carboxylic acids.
O-Methyation of Phenols
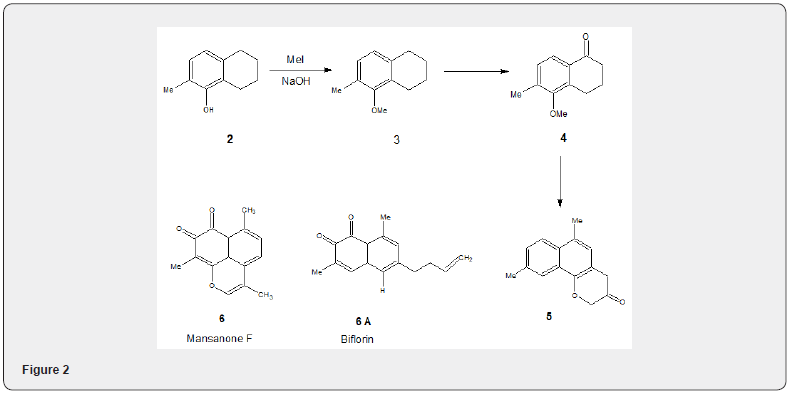
Methyl iodide has been highly used for methylation of alcohol. phenolic compounds and carboxylic acid. The phenolic hydroxyl groups are readily methylated with methyl iodide under basic conditions. Few examples have been illustrated in this review. The phenol 2 [5] on methylation with methyl iodide in presence of sodium hydroxide affords the o-alkylated compound 3 whose conversion to the tetralone 4 Figure 2 has been achieved by chromic acid oxidation. The transformation of the tetralone 4 to the lactone 5 is already reported [6]. The lactone 5 has been utilized for the total synthesis [7] of the naturally occurring o-naphthoquinones Mansonone F 6 and Biflorin 6A which exhibit remarkable biological activities [8].
Methyl iodide also has been used for selective methylation of phenolic compounds and the resulting monomethylated product has been utilized for organic synthesis. Some examples are cited below.
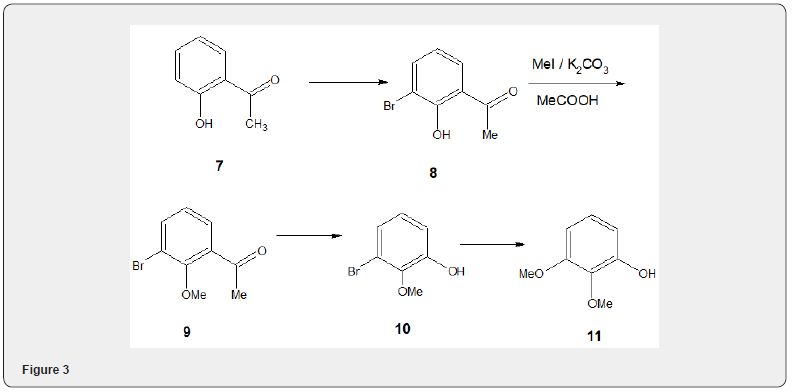
The bromophenol 8, prepared from 2-hydroxyacetophenone 7 by bromination, on alkylation with methyl iodide and potassium carbonate in acetone yields O-methylated compound 9 Figure 3 which is subjected to Baeyer-Villiger oxidation [9] followed by alkaline hydrolysis to obtain the phenol 10. Treatment of phenol 10 with sodium methoxide and cupric bromide in DMF furnishes iridiol 11 [10] (overall yield 90%), a useful intermediate in the synthesis of p-quinone.
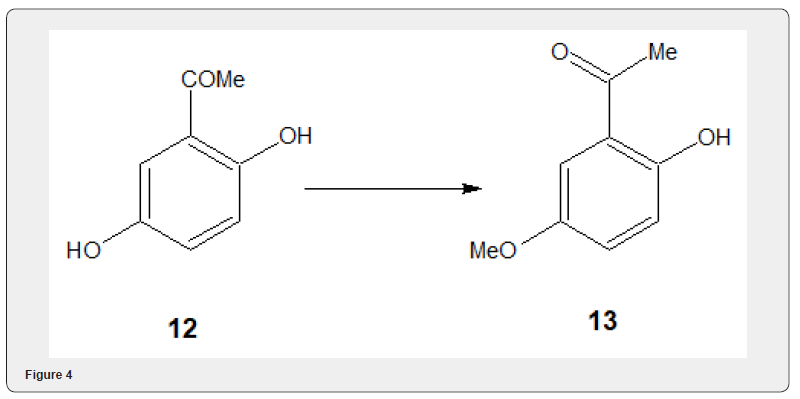
Fluoride salts aluminaon alumina have been widely used for alkylation of phenols and alcoholes with methyl iodide using Cs-F on alumina, (described in the following pages), Quinacetophenone monomethyl ether 13 has been prepared in 55-64% yield by selective methylation of quinaceto- phenone 12 using methyl iodide and potassium carbonate [11] Figure 4. The selective methylation has also been experimented [12] with more acidic C2 hydroxyl group of 2,3-dihydroxybenzaldehyde 14 Figure 5 by stirring the aldehyde 14 with 1 equiv of potassium carbonate, 1.3 equivalent of methyl iodide and dimethyl formamide at 25OC to obtain 15. The protection of the remaining hydroxyl group with MOM (methoxymethyl) group yields the compound 16 which has been utilized to accomplish the synthesis of phomazarin 17, widely studied aza anthraquinone.
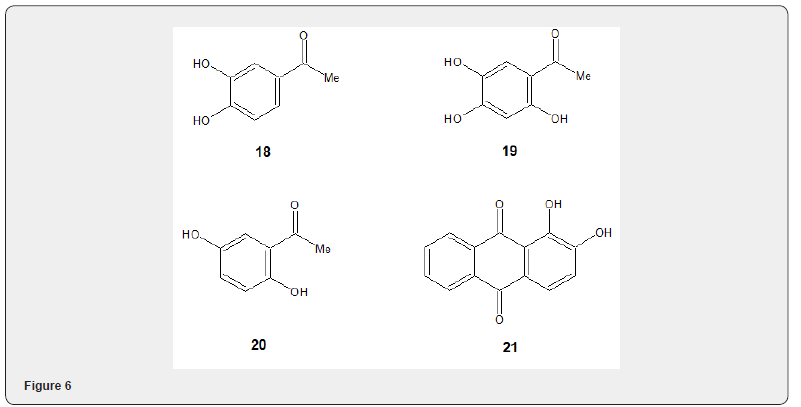
The selective methylation of phenols has also been studied with lithium carbonate and methyl iodide using dimethylformamide as solvent [13]. It has been observed that phenols which are more acidic than PhOH due to the presence of electron withdrawing groups can be alkylated efficiently. Treatment of o,m,p-hydroxy - acetophenones 18, 19, 20 and alizarin 21 with lithium carbonate, dimethylformamide and methyl iodide, at 40ºC, undergoes methylation at ortho and para position. m-isomer remained unaffected. DMF proved more suitable solvent than MeOH, MeCN, or THF. The use of more reactive base NaHCO3, K2CO3 decreased selectivity. The use of more reactive halides such as benzyl chloride or propargyl bromide did not increase the yield of the alkylated product.
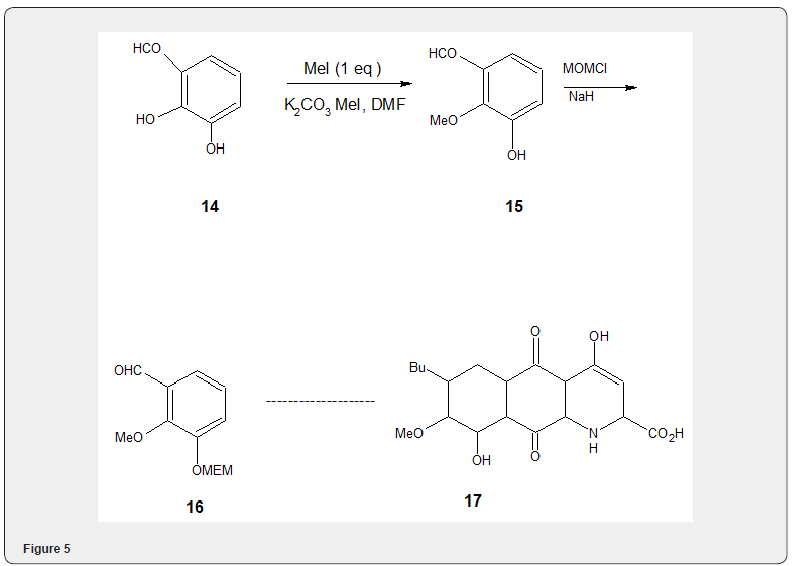
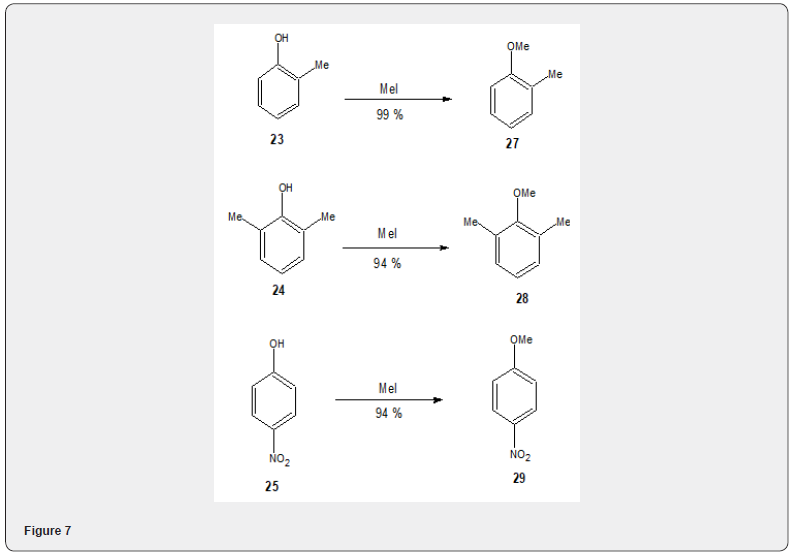
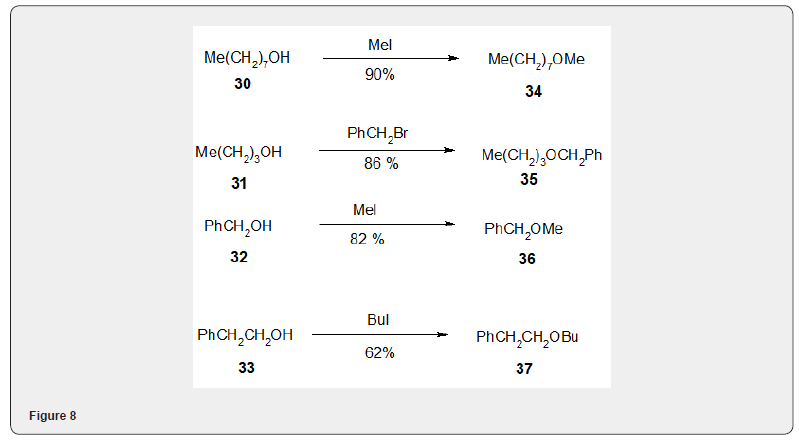
Figure 6 Fluoride salts on alumina[14] have been widely used for the alkylation of several phenoles and alcohols with methyl iodide.Thus the phenols 22, 23, 24, 25 on alkylation with MeI in presence of fluoridesalts yielded the akylated products 26, 27, 28, 29 respectively, Figure 7 Similarly the alcohols 30, 31, 32, 33 and on alkylation with methyl iodide yield the alkylated products 34, 35, 36 and 37 respectively Figure 8.
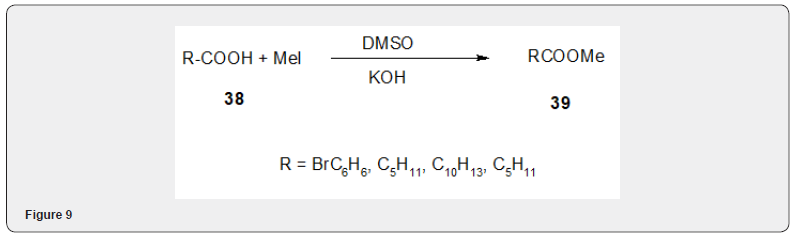
Recently an efficient method of the methylation of carboxylic acid with methyl iodide has been developed by AvilaZarraga and Martinez [15] The method consists in the methylation of carboxylic acid with potassium hydroxide, dimethylsulfoxide and methyl iodide. Though there are other methods for methylation [8 and related references] the present method [8] is carried out under mild condition using inexpensive reagents. The yield of the of the methylester is quite acceptable.
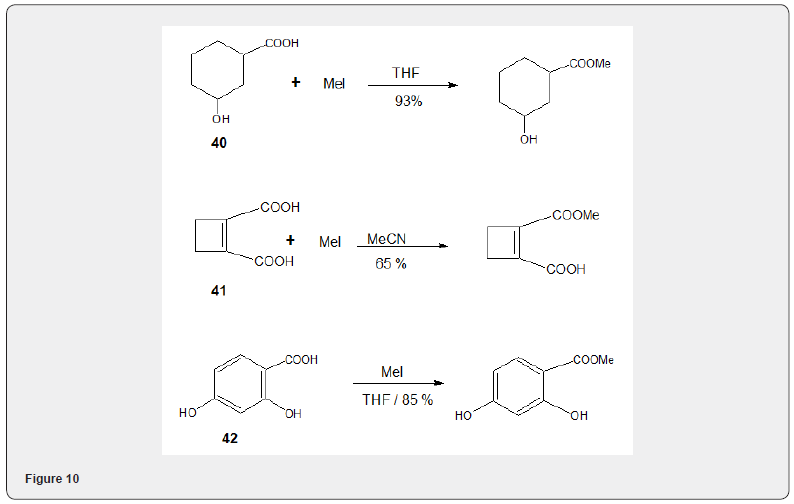
The present method consists in the addition of carboxylic acid to a slurry of potassium hydroxide in DMSO and iodomethane. Figure 9 Ionic lquids as solvents have been also been used for efficient esterification of carboxylic acids with alkyl halides [16] The coupling of number of phenols and alcohols with acyl and benzoylhalides in acetoniitrile. [17] have been studied to develop an effcient method for tfhe synthesis of esters and ethers. Chemo selective methylation of carboxylic acid group, in presence of phenolìc, alcoholic and also carboxylic acid can be accomplished in high yield [18] using DBU and methyl iodide Figure 10.
Conclusion
We have discussed the uses of methyl iodide in the o-methylation of alcohols, phenols, and carboxylic acid for organic synthesis.
References
- Schwarzenblack RP, Gschwand PM, Imboden RM (1993) Environmental Organic Chemistry. John Wiley & Sons, New York.
- Ohn HD, Sims JJ, Grech NM, Becker JO, McGiffen (1996) Methyl Iodide, an Ozone-Safe Alternative to Methyl Bromide as a Soil Fumigant Plant Disease 80: 731-735.
- King HS (1933) Methyl Iodide. Organic Synthesis CV: 2 399-403.
- Hartman WW Methyl iodide. Organic Synthesis CV: 2 404-409.
- Poon PS, Banerjee AK (2007) An alternative route for the synthesis of 5,6, 7,8-tetrahydro-2,5-dimethylnaphthalene-1-ol. Organic Chemistry an Indian Journal 3: 10-13.
- Poon PS, Banerjee AK (2006) Alternative procedure for the synthesis of Mansonone F and Biflorin precursor. Nat ProdRes 20(6): 629-635.
- Best WM, Wege D (1986) Intramolecular Diels-Alder addition of benzynes to furans. Application to the total synthesis of Biflorin and Mansonone E I and F Aus J Chem 39: 647-666.
- Suh YG, Kim SN, Shin D Y, Hyun SS, Lee DS, et al. (2006) The structure –activity relationship of monsonone F, a potent anti-MRSA sesquiterpenoid quinone: SAR studies on the C6 and C9 analogs. Bioorg Med Chem Lett 16(1): 142-145.
- Krow GR, The Baeyer–Villiger Oxidation of Ketones and Aldehydes. Organic Reactions 43: 251-798.
- Bovicelli P, Antonioletti R, Barontini M, Borionini G, Bernini R, et al. (2005) New convenient synthesis of Iridiol. An approach to the synthesis ubiquinones. Tetrahedron Lett 46: 1255-1257.
- Vyas GN, Shah NM (1944) Quinacetaphenone monomehyl ether. Org Syn Coll 4: 836-837.
- Boger DL, Hong J, HHikota M Ishida M (1999) Total Synthesis of Phomazarin. J Am Chem Soc 121: 2471-2477
- Wyman W, Davis R, John D, Patterson, Pfister JR (1988) Selective alkylation of certain phenolic and enolic functions with lithium carbonate and alkyl halide. Synth communs 18: 1379-1384.
- Ando T, Yamawaki J, Kawate T, Sumi S, Hanafausa T (1982) Fluoride salts on alumina as reagents for alkylation of phenols and alcohols. Bull Chem Soc Jpn 55(8): 2504-2507.
- Zarraga Avila Gustavo j Martinez R (2OO1) Efficient methylation of Carboxilic acids with poassium hydroxide-methyl -sulfoxide and iodomethane. Synth Communs 34: 2177-2183.
- Gok Y, Alici B, Cetinkaya E, Ozdemir I, Ozeroglu O (2O1O) Ionic liquids as solvent for efficient esterification of carboxylic acids with alkyl halides. Turk J Chem 34: 187-119.
- Shah STA, Heinrich Am, Choudhary, M.I, Voelter (2002) An efficent approach towards syntheses of ethers and esters using CsF-Celite as a solid base. Tetrahedron letts 43(47): 86o3-86o3.
- Mal D (1986) Chemoselective methylation of carboxylic acids using DBU and iodomethane. Synth Communs 16: 331-335.






























