Abstract
Frozen cranial tissue sections were placed in the feature point inclusion domain of the 10X Genomics Visium chip, the sections were stained and imaged by HE for permeabilization, the oligo-dT probe with the specific address sequence in the chip recognized the mRNA in the cells, the spatial transcriptomics platform sequenced the intracranial sclerotic arterial blood cells, the mRNA was used as a template for cDNA synthesis, and the gene expression information was obtained by sequencing after the library was established. The 10X Genomics Visium array consists of a tissue optimization array for tissue permeabilization conditions and a gene expression array for formal sample space transcriptome experiments, with four 6.5 mm x 6.5 mm region size inclusion domains and 5,000 probe clusters composed of specific address sequences each.
Keywords:Spatial transcriptomics, intracranial arteriosclerosis
Abbreviations:CAM: Cell Adhesion Molecules; VCAM: Vascular Cell adhesion Molecule; LDL: Low-Density Lipoprotein; TCD: Transcranial Doppler Ultrasound, MRA: Magnetic Resonance Angiography, CTA: Computed Tomography Angiography; DSA: Digital Silhouette Angiography; FEA: Finite Element Method
Introduction
Intracranial arterial spatial transcriptomics
Frozen cranial tissue sections were placed in the feature point inclusion domain of the 10X Genomics Visium chip, the sections were stained and imaged by HE for permeabilization, the oligo-dT probe with the specific address sequence in the chip recognized the mRNA in the cells, the spatial transcriptomics platform sequenced the intracranial sclerotic arterial blood cells, the mRNA was used as a template for cDNA synthesis, and the gene expression information was obtained by sequencing after the library was established. The 10X Genomics Visium array consists of a tissue optimization array for tissue permeabilization conditions and a gene expression array for formal sample space transcriptome experiments, with four 6.5 mm x 6.5 mm region size inclusion domains and 5,000 probe clusters composed of specific address sequences each.
Transcription experimental assay process
Isopentane fixed fresh tissue samples, liquid nitrogen flash freezing and OCT embedding, frozen sections. Prepare 5-10 slides to extract RNA for quality evaluation (RIN value >7.0 is required), optimize the permeabilization conditions, set different permeabilization times for 6 of the 8 inclusion regions of the tissue optimization slide, and add RNA directly without tissue sections. Experimental procedure: Fixed→staining→ brightfield photography→ tissue permeabilization→ fluorescent cDNA synthesis → tissue removal → fluorescence scanning, and the optimal permeabilization time was judged according to the fluorescence intensity. The gene expression chip for the formal experiment has four inclusion regions, and the experimental process: staining and brightfield photography→ tissue permeabilization (performed with the optimized permeabilization time described above), cDNA synthesis→ library construction→ high-throughput sequencing. Cluster analysis was carried out according to the gene expression information of the microarray feature loci, the loci were matched with the tissue image according to the address sequence, the spatial position and time feature sequence of the three-dimensional tissue of each gene were located, the single-cell transcriptome data were anchored and integrated by spatial transcriptome technology, and the three-dimensional spatial transcriptome map of intracranial arterial vessels and their neuronal neighbors was observed, and the distribution of toxin cell types, the spatial organization of cell subsets with the same oxygen content and the grafting and copolymerization of internal physicochemical bonds were determined.
Expression of adhesion molecules ICAM-1 and VCAM-1 in intracranial arterial high-density sclerosis
The study of the phased copolymerization of cell adhesion molecules (CAMs) and diaphragms is crucial in intracranial arterial high-density sclerosis, and blood oxygen cell adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) are two types of adhesion molecules, which have a homologous structure with a variable region (V region) and a constant region (C region) with two cysteines in the domain linked by disulfide and 100 amino acid monomers. ICAM-1 contains 90-115 glycoproteins present in the inner and outer layers of arterial vessels, and the high-density sclerosis inflammation of intracranial arteries promotes the enhancement of ICAM-1 expression, and the major monomeric CD11a/CD18 of this region promotes the adhesion of neutrophils, monocytes and arterial vessels to the inner layer and the targeted therapy of medicinal molecules. The molecular weight of VCAM-1 in the cell membrane of the arterial vascular lining is 100-110ku, and the high-density sclerosis of the arterial blood vessel integrates VLA-4 to participate in the regulation of blood oxygen cells. Low internal stress damages plasma cells in the inner layer, promotes the secretion of ICAM-1 and VCAM-1, reduces intracranial nitric oxide levels and promotes low-density lipoprotein (LDL) oxidation, stabilizes vascular infiltration of plasma cells and molecules, and promotes the expression of adhesion molecules. Damage to the lining of the intracranial arteries prolongs LDL retention and promotes inflammation [1]. The oxidized LDL binds to the target receptor of the plantderived base diaphragm and arterial blood oxygen cells, and the diaphragm activates the secretory matrix metalloproteinase to digest the LDL to form soluble collagen fibrils and fluid lipid nuclei, and enzymatic factors infiltrate the fluid lipid nucleus. The complexity and heterogeneity of the internal components of the lipid nucleus were related to the phased course of treatment, the changes in the parameters of the local arterial vascular dynamics model were related to external factors, the density of the lipid nucleus accompanied by the increase of arterial pressure stress to colloidal crystals, and the combination of medicinal factors and antigenic determinants acted on the dissociation of toxins. Collagen accounts for more than 70% of the stable lipid crystals, and the hard crystals lead to the narrowing of the arterial lumen and shear stress deformation. Collagen accounts for 40% of the unstable lipid crystals, which is below 65 μm.
Imaging of hyperdense sclerosis of intracranial arteries
Intracranial arterial hyperdensity sclerosing stroke can be divided into perforating artery occlusion, arterial-arterial embolism, decreased hypoperfusion clearance and mixed mechanism according to the pathophysiological characteristics. Intracranial artery hyperdensity sclerosis image information processing methods include transcranial Doppler ultrasound (TCD), magnetic resonance angiography (MRA), computed tomography angiography (CTA) and digital silhouette angiography (DSA). Among them, DSA intracranial vascular stenosis assessment can show the various levels of intracranial arterial vascular branches, the degree of stenosis and occlusion of diseased blood vessels and determine the hemodynamic changes and compensation of distal collateral branches of diseased blood vessels. According to the intracranial artery angiography, the method of measuring intracranial artery stenosis is as follows: stenosis percentage = (1-D stenosis/D normal) ×100%, where D stenosis refers to the diameter of the artery at the most severe part of the stenosis, and D normal refers to the diameter of the normal artery proximal to the stenosis. The clinical types of high-density sclerotic stenosis of intracranial arteries include asymptomatic stenosis and symptomatic stenosis, which include ischemia in the area of stenosis vascular supply, ischemia in the area of collateral vessel supply due to stenosis, mixed or complex. It is subdivided into three subtypes, type A without infarct or lacunar infarct but without neurological deficit; type B small-area infarct or distal vascular tandem stenosis; Type C massive infarction or chronic occlusion of the distal trunk. In the process of targeted therapy, plant-derived basal diaphragms are treated with reference to the degree of stenosis of intracranial arteries and blood oxygen reserves, and targeted therapy is performed [2].
According to Mori’s prediction model of intracranial stenosis vascular structure, intracranial arteries were divided into stenosis concentric and moderately eccentric less than 5 mm. stenosis below 10 mm, extreme eccentricity with moderate angulation; Extreme angulation greater than 10mm. The vascular side of the intracranial artery should include the anterior and posterior arteries, the anterior middle artery and the posterior middle artery on the transpial artery side, the superior artery on the posterior artery side, and the posterior inferior artery on the anterior inferior artery side [2]. The blood oxygen source of intracranial arterial thrombolytic ischemic stroke samples should be predicted, DSA technology should be used to determine the arterial vascular flux, and TIMI should be used to determine the blood oxygen source, and the feasibility of targeted therapy should be correlated and analyzed. The correlation between the blood oxygen source and the model prediction was statistically analyzed (P=0.019), and there was no correlation between the arterial vascular healing and the model prediction (P=0.055). Highresolution MRI quantitatively measured the molecular weight and composition of low-density lipids: T1WI of the fiber cap was stable, PDWI was stable, 3DTOF was adjacent to the peak and valley, and T2WI was undulating, and fibrosis was accompanied by signal loss. The effect of the fiber cap and lipid nucleus on T1WI tissues under the fortifier was significantly different, the proximal and distal arc signal showed the bleeding status of the vascular flux model, the calcification showed the irregular trough signal, the fibrosis was mostly the stability maintenance signal, and the thrombus signal was wavering.
Intracranial artery vascular mechanical interception model and mathematical guidance
The finite element method (FEA) is the integration of finite elements into an infinite degree of freedom continuum to determine the boundary loads based on the mechanical analysis of the elements, and the basic processes include the establishment of geometric models, discretization of finite element networks, modification of element properties, debugging of boundary conditions, finite element interpretation, and domain processing [1]. Compressive stress (WSS) and strain are the mechanical model parameters of arterial high-density sclerosis, and compressive stress below 0.4 pa induces arterial high-density sclerosis. The arterial model consists of the inner, middle, and outer layers, and the lesion damages the inner layer of cells resulting in strain. The modulus of elasticity (E) is the ratio of stress to strain per unit domain, which is the definition of elasticity in a nonlinear relationship. The flaccid state of intracranial collagen fibers and smooth muscle affects arterial vascular anisotropy and continuous phase homeostasis. The linear modulus of elastic fibers is about 3×105-6×105N/m2, the elastic modulus of collagen fibers is 109N/ m2, and the smooth muscle modulus is one-tenth of that of elastic fibers. The outer diameter margin of the model is reciprocating and the elastic fibers are rigidly loaded by the collagen fibers. The Lagrangian method combined with the Euler method is used for finite element analysis of the watershed, and the incompressible equations are as follows:
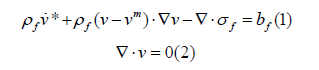
where ρf is the density, kg/m3; v is the velocity vector,
m/s; * 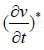
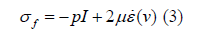
where p is the pressure, kg / (m.s)2,I is the second-order
unit modulus, and μ is the dynamic viscosity coefficient,
kg / (m.s) , ε (v) is the modulus of deformation rate,
s-1. In finite element analysis, initially, Ωf. is discretized
into a set of elements, 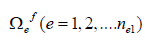
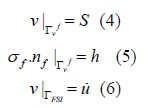
where s and h are given, nf is Γhf ‘s the unit outside normal vector, u is ΓFSI velocity on the particle, m/s. Initial:
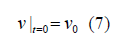
where v0 is a given function that satisfies the ∇v0 = 0 .
The finite element equation for incompressible fluids, let svh ,vvh and
sph = vph be finite dimensional parameters
and spaces with respect to velocity and pressure, respectively,
and for 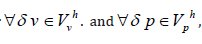
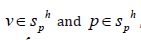 , such that:
, such that:
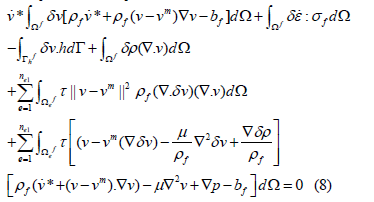
where δε is the imaginary variable rate modulus corresponding toδ v . The last two terms of Eq.(8) are the least squares term of the incompressible condition and the least squares term of the momentum equation. The straightedged triangular quadratic elements evenly distributed in the centering nodes have a stability coefficient τ in the form of
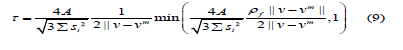
where si is the distance from the 3 vertices to the center of the straight-sided triangle. In the cell domain, the velocity vector V and the pressure vector P of Ω , δ v and δρ are their variations, and N and H are the parameter matrices of velocity and pressure. The finite element parameters are as follows:
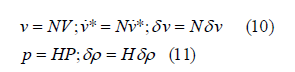
Combining the instability of δ v andδρ , the matrix operations of Eq.(10) and Eq.(11) are rewritten from Eq.(8) to obtain the finite element equation:
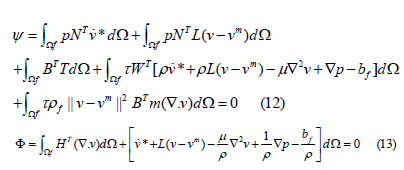
where L is the right gradient matrix of velocity, and B is the displacement-strain matrix of linear elastic finite elements. T is the stress tensor corresponding to B.
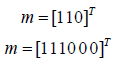
Ff is the equivalent nodal force vector of the external load in equation (14):
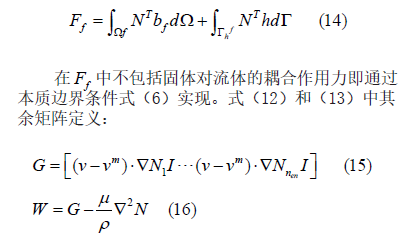
In the above formula, N1 is the basis function of velocity interpolation; Nnen is the number of nodes in the unit. Assuming that the t-moment parameter is determined, t+Δttime, the time domains of Eq.(12) and (13) are discrete, i.e:
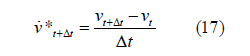
Microfluidics determines the nodal motion according to the interface ΓFSI.quantifies on, Γvf and Γhf and adds a specified displacement boundary on ΓFSI.
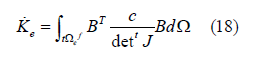
If the displacement at 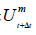
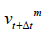 :
:
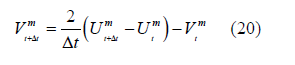
References
- Neuropsychiatric Circle. Evaluation and classification of intracranial atherosclerotic stenosis. Chinese Journal of Stroke.
- Chen Yubai (2013) Exploration and preliminary study of an individualized virtual model for predicting the risk of carotid atherosclerotic plaque rupture[D]. Chinese People's Liberation Army Medical College (09).






























