Abstract
Inadequate production of cortisol, known as hypocortisolemia, has been a challenging condition to diagnose especially when it comes to adrenal insufficiency. Most practitioners utilize the morning serum cortisol levels when conducting screening processes. We investigate the clinical utility of using a cutoff point of 7 μg/dL on afternoon serum cortisol level for ruling out hypocortisolemia. Based on available patient records, those above this threshold may eliminate the concern of adrenal insufficiency due to the high sensitivity and specificity. Furthermore, this method is convenient as compared to conventional stimulation tests for afternoon cortisol assessment. This is a new approach for initial screening that is non-invasive.
Materials and methods: The study included 60 patients exhibiting hypotension and fatigue. All participants underwent afternoon basal serum cortisol measurements, followed by an ACTH-stimulated cortisol test one hour post-injection.
Results: The results shows that basal afternoon cortisol of 7μg/dL or more can exclude hypercortisolemia and afternoon basal cortisol 7 μg/dL correspond to acth stimulated cortisol 18 mcg/dl as a cutoff value excluding hyporcortisolemia.
Conclusion: The data indicates that serum cortisol concentration exceeding 7μg/dL in the afternoon can be used as reliable exclusion criteria in specimens collected from low-risk individuals thereby eliminating the need for additional confirmatory tests. More in-depth studies need to be done to refine diagnostic thresholds and make crucial clinical decisions better.
Keywords:Hypocortisolemia; Afternoon Cortisol; Acth Stimulated Cortisol
Abbreviations: HPA: Hypothalamic-Pituitary-Adrenal; CRH: Corticotropin-Releasing Hormone ACTH: Adrenocorticotropic Hormone; SCN: Suprachiasmatic Nucleus; GR: Glucocorticoid Receptors
Introduction
Feeling perpetually fatigued, possessing minimal energy, your blood pressure decreases upon standing, and even a minor stressor can leave you entirely drained. These could suggest hypocortisolemia, a condition in which the body fails to produce sufficient cortisol [1]. Commonly known as the “stress hormone,” cortisol is generated by the adrenal glands in response to cues from the pituitary and hypothalamus. It is crucial for maintaining homeostasis and affects blood pressure, metabolism, immune response, and most significantly the body’s reaction to stress [2]. One of cortisol’s main functions is to prepare the body for a fight-or-flight reaction. It stimulates gluconeogenesis, a process where the liver generates glucose from non-carbohydrate sources, ensuring a steady energy supply and optimal brain function during stressful situations [3].
Along with stress management, cortisol plays a vital role in metabolism. It controls the equilibrium of proteins, fats, and carbohydrates by affecting how the body metabolizes and reserves these macronutrients. Cortisol aids in fat decomposition for energy use and supports stable blood sugar levels by opposing insulin’s effects. Yet, prolonged elevated cortisol levels can result in adverse effects, including weight increase, insulin resistance, and metabolic issues [4]. Cortisol is vital for the immune system, having anti-inflammatory qualities that assist in managing immune reactions and avoiding excessive inflammation. This function is essential for stopping autoimmune diseases and preserving overall immune health [5]. Another important function of cortisol is in managing blood pressure and heart health. It aids in regulating blood vessel narrowing and fluid equilibrium, guaranteeing adequate circulation and oxygen distribution across the body. Insufficient cortisol levels can result in dangerously low blood pressure, which may cause adrenal insufficiency [6].
Cortisol release occurs in a natural cycle called the circadian rhythm, reaching its highest levels in the early morning and steadily decreasing during the day. This rhythm assists in managing sleep-wake patterns, energy levels, and general bodily operations. Interruptions to this pattern like inconsistent sleep, shift employment, or ongoing stress can cause hormonal imbalances and health issues [7]. The hypothalamic-pituitary-adrenal (HPA) axis controls the release of cortisol, where the hypothalamus secretes corticotropin-releasing hormone (CRH) that prompts the pituitary to generate adrenocorticotropic hormone (ACTH). ACTH stimulates the adrenal cortex to secrete cortisol into the blood [8]. This increase in cortisol enhances alertness, activates energy reserves, and facilitates metabolic functions essential for everyday tasks. As the day wears on, cortisol levels decrease, hitting their minimum in the late evening, which helps facilitate sleep onset. This daily rhythm is controlled by the body’s internal biological clock, mainly regulated by the hypothalamus’s suprachiasmatic nucleus (SCN). Exposure to light is vital for aligning this rhythm, since disturbances in sleep cycles, shift work, or ongoing stress can change typical cortisol release [9].
Multiple elements affect cortisol release, such as stress, age, health condition, and lifestyle choices. Acute stress can lead to a temporary rise in cortisol levels, whereas chronic stress might disrupt the HPA axis, leading to consistently high cortisol or reduced daily fluctuations. This imbalance is associated with conditions such as metabolic disorders, cardiovascular issues, mood disorders, and immune dysfunction. Aging influences cortisol production, frequently resulting in a diminished daily rhythm, which correlates with cognitive deterioration and heightened susceptibility to stress-related health issues [10]. Conversely, disorders like Addison’s disease cause inadequate cortisol synthesis, resulting in symptoms including fatigue, weight loss, and low blood pressure. Grasping the daily variations in cortisol levels and the processes that govern its regulation is essential for creating therapies for stress-related and endocrine disorders. Practices such as sticking to a consistent sleep routine, handling stress effectively, and adjusting light exposure can aid in regulating cortisol levels and enhancing overall health [11].
Cortisol functions by attaching to glucocorticoid receptors (GR) in specific tissues, affecting gene expression and the production of proteins. It stimulates gluconeogenesis in the liver, releases fatty acids, and dampens immune responses by blocking pro-inflammatory cytokines. Chronic cortisol imbalance, such as in Cushing’s syndrome (high cortisol) or Addison’s disease (low cortisol), may lead to significant health issues [12]. The feedback system maintains homeostasis by decreasing CRH and ACTH secretion when cortisol levels are sufficient. A thorough comprehension of cortisol’s function is crucial for diagnosing and treating HPA axis disorders and for enhancing glucocorticoid therapies [13]. Hypocortisolemia, defined as insufficient cortisol levels, may occur due to primary adrenal insufficiency (Addison’s disease), secondary adrenal insufficiency (pituitary issues), or tertiary adrenal insufficiency (hypothalamic issues). Precise diagnosis is essential, since unmanaged hypocortisolemia may result in life-threatening adrenal crises. Different diagnostic tests exist, each possessing its own advantages and drawbacks. Evaluating these tests aids in identifying the most suitable option according to clinical symptoms, accessibility, and diagnostic precision [14].
The morning serum cortisol test is typically the initial step in diagnosing adrenal insufficiency because it is easy to perform and non-invasive. As cortisol exhibits a daily pattern with its highest level occurring in the early morning, a low morning cortisol level (<3 μg/dL or 83 nmol/L) strongly suggests adrenal insufficiency. Nevertheless, values that fall within the borderline range (3- 15 μg/dL) might necessitate additional testing, since they are ambiguous. This test by itself is not conclusive, as elements like stress, illness, and differences in individual baseline levels may affect outcomes [15]. The ACTH stimulation test, often referred to as the short Synacthen test, is regarded as the gold standard for diagnosing primary adrenal insufficiency. It assesses baseline cortisol levels prior to and following the administration of synthetic ACTH (cosyntropin). A typical response is an increase in cortisol to ≥18-20 μg/dL (500-550 nmol/L). An insufficient rise indicates adrenal insufficiency. This examination is especially valuable for identifying primary adrenal insufficiency, where the adrenal glands fail to react to ACTH [16]. In secondary and tertiary adrenal insufficiency, prolonged ACTH deficiency can result in adrenal atrophy, making early stage results seem normal and necessitating further testing [17].
Alternative tests like the salivary cortisol test and the 24- hour urinary free cortisol test provide non-invasive options but are mainly employed for diagnosing hypercortisolism (Cushing’s syndrome) instead of adrenal insufficiency [18]. Assessing plasma ACTH together with cortisol can aid in distinguishing between primary and secondary adrenal insufficiency, since high ACTH indicates primary adrenal failure, whereas low or improperly normal ACTH implies a central origin [19]. An Innovative Concept offers the Benefits of an Afternoon cortisol examination. What if one afternoon blood sample could remove the necessity for additional testing? Recent research indicates that it is possible. Here’s the reason: In healthy individuals, cortisol’s natural levels decrease by 50% by the late afternoon. Currently, afternoon cortisol levels are evaluated alongside diagnostic tests like the ACTH Stimulation Test (the primary method for assessing cortisol response to synthetic ACTH) and serum cortisol concentration when baseline values are uncertain, facilitating an evaluation of its clinical significance [20].
Morning cortisol levels under 3 μg/dL (83 nmol/L) reliably indicate adrenal insufficiency, whereas levels above 15 μg/dL (414 nmol/L) usually rule it out. Intermediate values necessitate additional examination, like the ACTH stimulation test [21]. As cortisol levels drop throughout the day, adjustments to the thresholds for afternoon values are necessary [22]. Recent studies have investigated the diagnostic significance of cortisol measurements taken in the afternoon: Research showed that without adrenal dysfunction, afternoon cortisol levels stay adequately elevated [23]. Clinical guidelines indicate that although afternoon cortisol levels should not substitute morning ones, values exceeding 7 μg/dL can reliably rule out hypocortisolemia [24]. Afternoon basal cortisol assists in preventing redundant ACTH stimulation tests for patients who have sufficiently adequate cortisol levels, enabling testing at times outside of the morning when necessary [25].
Materials and methods
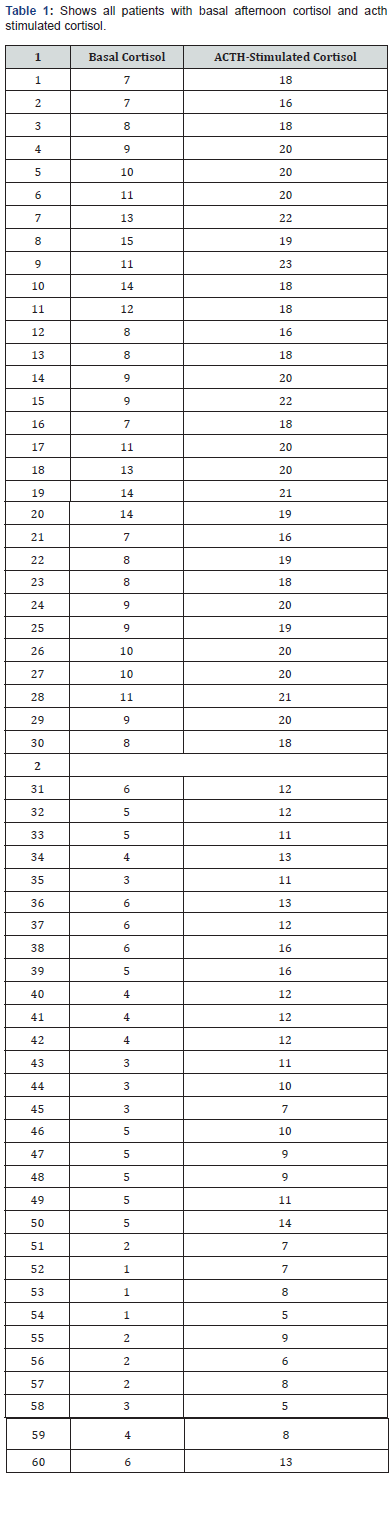
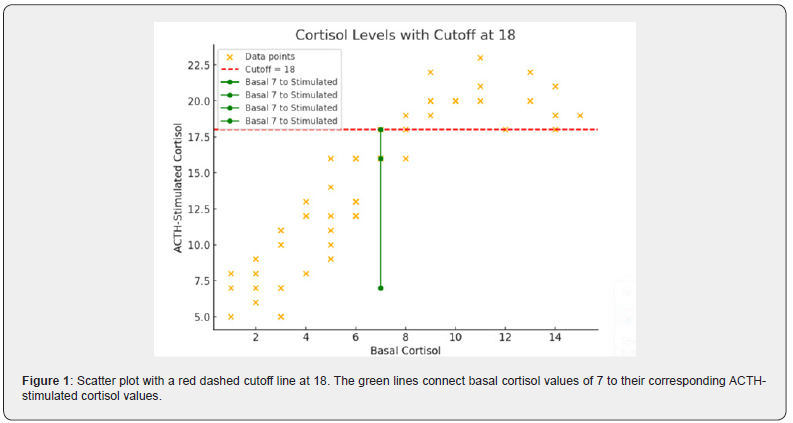
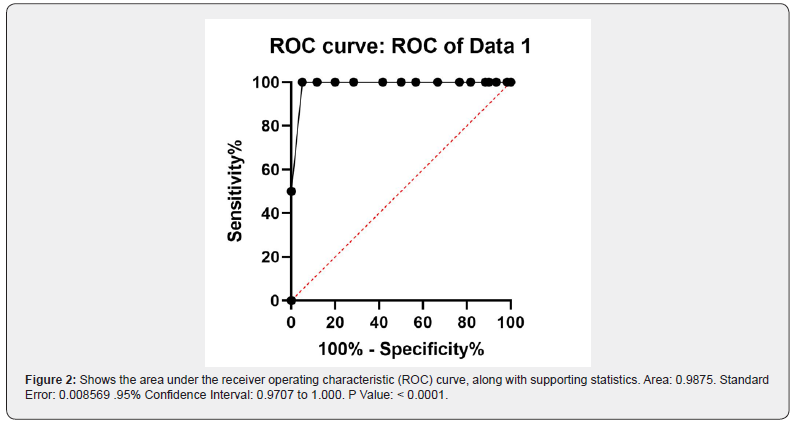
The study included 60 patients exhibiting hypotension and fatigue. All participants underwent afternoon basal serum cortisol measurements, followed by an ACTH-stimulated cortisol test one-hour post-injection. We analyzed the relationship between baseline afternoon cortisol levels and cortisol levels measured 1 hour after ACTH stimulation. The dataset consists of 60 entries with two numerical variables, basal cortisol and ACTH-stimulated cortisol. Using a diagnostic threshold of 18 μg/dL to rule out hypocortisolemia, we evaluated whether afternoon basal cortisol levels could predict stimulated cortisol values reaching this cutoff. Statistical analyses involved comparing ACTH-stimulated cortisol results against the 18 μg/dL threshold and assessing correlations between basal and stimulated cortisol levels. Normality testing via the Shapiro-Wilk test revealed non-normal distribution for ACTHstimulated cortisol data, prompting the use of the non-parametric Wilcoxon signed-rank test for paired comparisons. The results shows that basal afternoon cortisol of 7μg/dL or more can exclude hypercortisolemia and afternoon basal cortisol 7 μg/dL correspond to acth stimulated cortisol 18 μg/dL as a cutoff value excluding hyporcortisolemia as shown in Tables 1-3 and Figures 1 and 2.
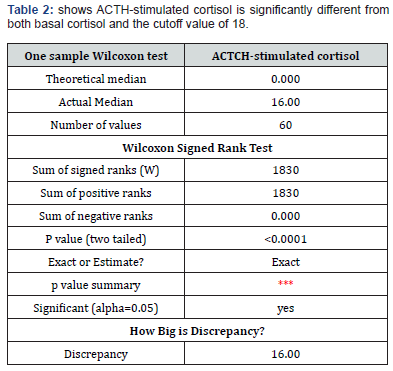
Results
I used the Wilcoxon signed-rank test
Basal vs. ACTH-stimulated cortisol:
p=1.49×10−11 → Significant difference.
ACTH-stimulated cortisol vs. Cutoff (18):
p=4.69×10−5 → Significant difference.
ACTH-stimulated cortisol is significantly different from both basal cortisol and the cutoff value of 18μg/dL.
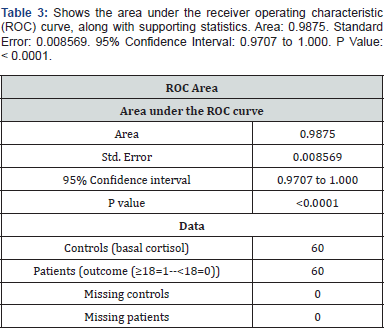
Discussion
Our aim was to determine whether afternoon serum cortisol levels exceeding 7 μg/dL could serve as a reliable cutoff for excluding hypocortisolemia. The findings suggest that this threshold provides a dependable method for ruling out adrenal insufficiency, offering a practical and less burdensome alternative to traditional stimulation tests [26]. Typically, cortisol levels are evaluated using morning serum cortisol measurements, which reflect peak secretion in the early hours due to circadian rhythms. Morning cortisol levels below 3 μg/dL are strongly indicative of adrenal insufficiency [27]. Morning cortisol levels above 15 μg/dL often rule out the condition [26]. However, many patients undergo cortisol evaluations at different times of the day, necessitating the establishment of appropriate cutoff values that accommodate physiological fluctuations. Our results align with existing research indicating that an afternoon cortisol measurement above a certain threshold can effectively exclude hypocortisolemia [28].
The conventional approach for diagnosing adrenal insufficiency often requires an ACTH stimulation test, which can be resource-intensive and inconvenient [29]. Our study suggests that in cases where afternoon serum cortisol levels exceed 7 μg/ dL, additional confirmatory testing may be unnecessary, reducing the frequency of unnecessary stimulation tests and optimizing healthcare resources [30]. This threshold may also be particularly useful in outpatient settings where patients may not always be available for morning cortisol evaluations. Having a reliable afternoon reference point allows for greater scheduling flexibility, while maintaining diagnostic accuracy [31].
Despite these promising results, our study has limitations:
a) Small sample size: Larger studies are needed to validate
these findings across diverse populations.
b) Unaddressed variables: Factors such as coexisting
medical conditions, medication use (e.g., exogenous steroids,
oral contraceptives), and individual circadian variations were not
comprehensively analyzed. Future research should examine how
these variables influence cutoff values.
c) Additional biomarkers: While this study focuses on a
single cortisol measurement, incorporating ACTH levels or salivary
cortisol could enhance diagnostic precision. Future studies should
also evaluate whether this criterion remains valid across different
assay methodologies and laboratory environments. Our research
backs the use of an afternoon serum cortisol level of and greater
than 7 μg/dL as an effective diagnostic standard for excluding
hypocortisolemia. This method could lessen dependence on
dynamic testing while offering a more accessible diagnostic route
for patients. Nonetheless, additional confirmation in larger groups
and various clinical environments is necessary to strengthen these
recommendations.
Conclusion
Afternoon cortisol levels above 7 μg/dL can provide a practical diagnostic threshold for excluding hypocortisolemia, offering a useful alternative when morning testing is not possible. While this threshold is not definitive on its own, it can help streamline clinical decision-making and reduce unnecessary testing. Further research is needed to refine the cutoff values and enhance the accuracy of afternoon cortisol measurements in different patient populations.
References
- Wieling W, Kaufmann H, Claydon VE, van Wijnen VK, Harms MPM, et al. (2022) Diagnosis and treatment of orthostatic hypotension. Lancet Neurol 21(8): 735-746.
- Stephens MA, Wand G (2012) Stress and the HPA axis: role of glucocorticoids in alcohol dependence. Alcohol Res 34(4): 468-83.
- Kuo T, McQueen A, Chen TC, Wang JC (2015) Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol 872: 99-126.
- Angelousi A, Margioris AN, Tsatsanis C (2020) ACTH Action on the Adrenals. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, de Herder WW, Dhatariya K, Dungan K, Hofland J, Kalra S, Kaltsas G, Kapoor N, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, New M, Purnell J, Sahay R, Shah AS, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (editors.), Endotext [Internet]. MDText.com, Inc.; South Dartmouth (MA).
- Yeager MP, Guyre CA, Sites BD, Collins JE, Pioli PA, et al. (2018) The Stress Hormone Cortisol Enhances Interferon-υ-Mediated Proinflammatory Responses of Human Immune Cells. Anesth Analg 127(2): 556-563.
- Ramamoorthy S, Cidlowski JA (2016) Corticosteroids: Mechanisms of Action in Health and Disease. Rheum Dis Clin North Am 42(1): 15-31.
- Mohd Azmi NAS, Juliana N, Azmani S, Mohd Effendy N, Abu IF, et al. (2021) Cortisol on Circadian Rhythm and Its Effect on Cardiovascular System. Int J Environ Res Public Health 18(2): 676.
- Houngbadji MSTS, Niang B, Boiro D, Mbaye A, Seck A, et al. (2018) [Adrenocorticotropic hormone (ACTH) insensitivity syndrome: about a case]. Pan Afr Med J 30: 244.
- Hannibal KE, Bishop MD (2014) Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther 94(12): 1816-1825.
- Knezevic E, Nenic K, Milanovic V, Knezevic NN (2023) The Role of Cortisol in Chronic Stress, Neurodegenerative Diseases, and Psychological Disorders. Cells 12(23): 2726.
- Froehlich E, Madipakkam AR, Craffonara B, Bolte C, Muth AK, Park SQ (2021) A short humorous intervention protects against subsequent psychological stress and attenuates cortisol levels without affecting attention. Sci Rep 11(1): 7284.
- Oakley RH, Cidlowski JA (2013) The biology of the glucocorticoid receptor: new signaling mechanisms in health and disease. J Allergy Clin Immunol 132(5): 1033-1044.
- Lightman SL, Birnie MT, Conway-Campbell BL (2020) Dynamics of ACTH and Cortisol Secretion and Implications for Disease. Endocr Rev 41(3): bnaa002.
- Lee SC, Baranowski ES, Sakremath R, Saraff V, Mohamed Z (2023) Hypoglycaemia in adrenal insufficiency. Front Endocrinol (Lausanne) 14: 1198519.
- Kumar R, Carr P, Wassif W (2022) Diagnostic performance of morning serum cortisol as an alternative to short synacthen test for the assessment of adrenal reserve; a retrospective study. Postgrad Med J 98(1156): 113-118.
- Birtolo MF, Antonini S, Saladino A, Zampetti B, Lavezzi E, et al. (2023) ACTH Stimulation Test for the Diagnosis of Secondary Adrenal Insufficiency: Light and Shadow. Biomedicines 11(3): 904.
- Neary N, Nieman L (2010) Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes 17(3): 217-223.
- Ceccato F, Barbot M, Zilio M, Frigo AC, Albiger N, et al. (2015) Screening Tests for Cushing's Syndrome: Urinary Free Cortisol Role Measured by LC-MS/MS. J Clin Endocrinol Metab 100(10): 3856-3861.
- Abraham SB, Abel BS, Sinaii N, Saverino E, Wade M, et al. (2015) Primary vs secondary adrenal insufficiency: ACTH-stimulated aldosterone diagnostic cut-off values by tandem mass spectrometry. Clin Endocrinol (Oxf) 83(3): 308-314.
- El-Farhan N, Tennant S, Rees SE, Evans C, Rees DA (2024) Salivary Cortisol Response to ACTH Stimulation Is a Reliable Alternative to Serum Cortisol in Evaluating Hypoadrenalism. J Clin Endocrinol Metab 109(2): e579-e588.
- Gasco V, Bima C, Geranzani A, Giannelli J, Marinelli L, et al. (2021) Morning Serum Cortisol Level Predicts Central Adrenal Insufficiency Diagnosed by Insulin Tolerance Test. Neuroendocrinology 111(12): 1238-1248.
- Weinrib AZ, Sephton SE, Degeest K, Penedo F, Bender D, et al. (2010) Diurnal cortisol dysregulation, functional disability, and depression in women with ovarian cancer. Cancer 116(18): 4410-4419.
- Henry M, Thomas KGF, Ross IL (2021) Sleep, Cognition and Cortisol in Addison's Disease: A Mechanistic Relationship. Front Endocrinol (Lausanne) 12: 694046.
- Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, et al. (2016) Diagnosis and Treatment of Primary Adrenal Insufficiency: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 101(2): 364-389.
- Manosroi W, Phimphilai M, Khorana J, Atthakomol P (2019) Diagnostic performance of basal cortisol level at 0900-1300h in adrenal insufficiency. PLoS One 14(11): e0225255.
- Javorsky BR, Raff H, Carroll TB, Algeciras-Schimnich A, Singh RJ, et al. (2021) New Cutoffs for the Biochemical Diagnosis of Adrenal Insufficiency after ACTH Stimulation using Specific Cortisol Assays. J Endocr Soc 5(4): bvab022.
- Manosroi W, Phimphilai M, Khorana J, Atthakomol P, Pipanmekaporn T (2020) Predictive Factors of Adrenal Insufficiency in Outpatients with Indeterminate Serum Cortisol Levels: A Retrospective Study. Medicina (Kaunas) 56(1): 23.
- Rousseau E, Joubert M, Trzepla G, Parienti JJ, Freret T, et al. (2015) Usefulness of Time-Point Serum Cortisol and ACTH Measurements for the Adjustment of Glucocorticoid Replacement in Adrenal Insufficiency. PLoS One 10(8): e0135975.
- Ospina NS, Al Nofal A, Bancos I, Javed A, Benkhadra K, et al. (2016) ACTH Stimulation Tests for the Diagnosis of Adrenal Insufficiency: Systematic Review and Meta-Analysis. J Clin Endocrinol Metab 101(2): 427-434.
- Sacks DB, Arnold M, Bakris GL, Bruns DE, Horvath AR, et al. (2011) National Academy of Clinical Biochemistry; Evidence-Based Laboratory Medicine Committee of the American Association for Clinical Chemistry. Guidelines and recommendations for laboratory analysis in the diagnosis and management of diabetes mellitus. Diabetes Care 34(6): e61-99.
- Gurubhagavatula I, Barger LK, Barnes CM, Basner M, Boivin DB, et al. (2021) HPA Guiding principles for determining work shift duration and addressing the effects of work shift duration on performance, safety, and health: guidance from the American Academy of Sleep Medicine and the Sleep Research Society. J Clin Sleep Med17(11): 2283-2306.






























