Abstract
The annulation reaction developed by Robinson has been extensively used for the synthesis of ring compounds containing different substituents. The resulting compounds have proved very useful for the synthesis of many organic compounds and natural products especially related to steroids and terpenoids.
Keywords:Michale Reaction; Methyl Vinyl Ketone; Podocarpic Acid; Taxodione; Pisiferic Acid
Introduction
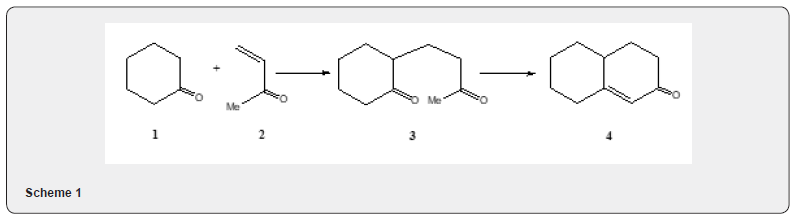
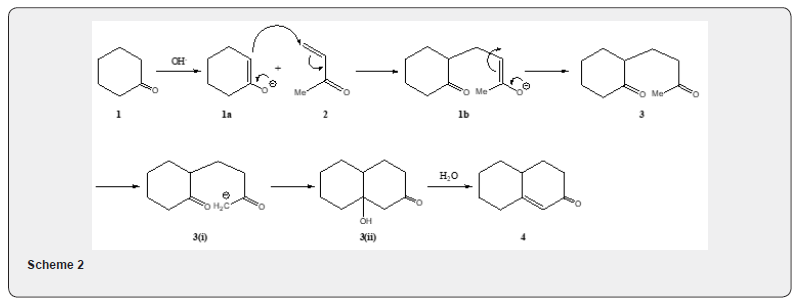
The conjugate addition of nucleophilic agent to alkene bond of an enone or related system has been termed as Michale reaction. The reaction of a cyclic ketone e.g. cyclohexanone 1 which with methyl vinyl ketone 2 in presence of base yields a bicyclic α,β-unsaturated ketone 4 via the ketone 3, in Scheme 1, is called the Robinson annulation reaction [1-4]. The Robinson annulation reaction consists of two selective reactions։ Michaele addition and aldol reaction. The mechanism of the reaction can be explained (Scheme 2) as follows։ The enolate anion 1a, reacts with methyl vinyl ketone 2 to yield 1b which affords the ketone 3. The ketone 3 is converted by base into the α,β- unsaturated ketone 4 via the intermediates 3(i) and 3 (ii). (Scheme 2).
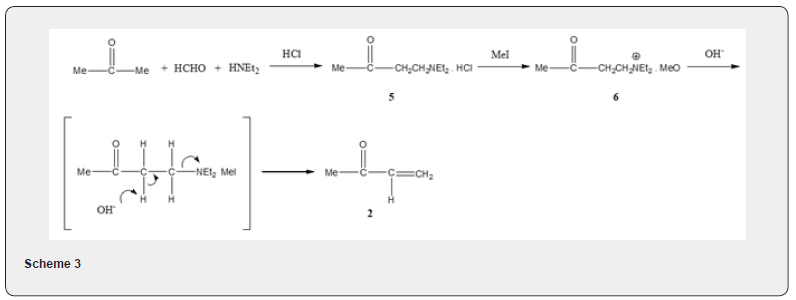
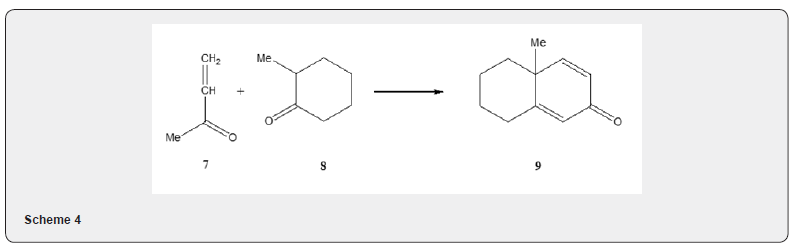
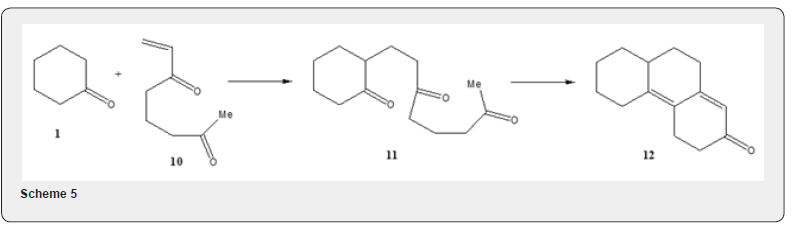
As the methyl vinyl ketone has a tendency to undergo polymerization, especially in the presence of base, the yield of the annulation product is often low. Therefore attempt has been made for its preparation in situ. The method consists in the treatment of acetone, formaldehyde and diethylamine hydrochloride to afford the hydrochloride salt of the Mannich base 5 which on quaternization with methyl iodide affords the salt 6. Treatment of the salt 6, with base yields methyl vinyl ketone 2 (Scheme 3). A judicious choice of base is important because it will serve to remove the alpha hydrogen from the cyclic ketone. Besides polymerization of methyl vinyl ketone, a double alkylation of the starting ketone by reaction with a second Michael acceptor molecule, may take place as side reaction and thus further reduce the yield. The polymerization of the enone 2 as well as double alkylation of the starting ketone can be avoided by using organotin triflate as catalyst [5]. When 3-butyne-2-one 7 is used [6] as a Michael acceptor component it reacts with the cyclic ketone 8 yielding 2,5-cyclohexadienone 9 (Scheme 4). A double annulation reaction is also possible, if the Michael acceptor like 10 is made to react with ketone 1. The product of the Michael addition 11 can undergo two subsequent aldol condensation to yield the tricyclic dienone 12 [4] (Scheme 5).
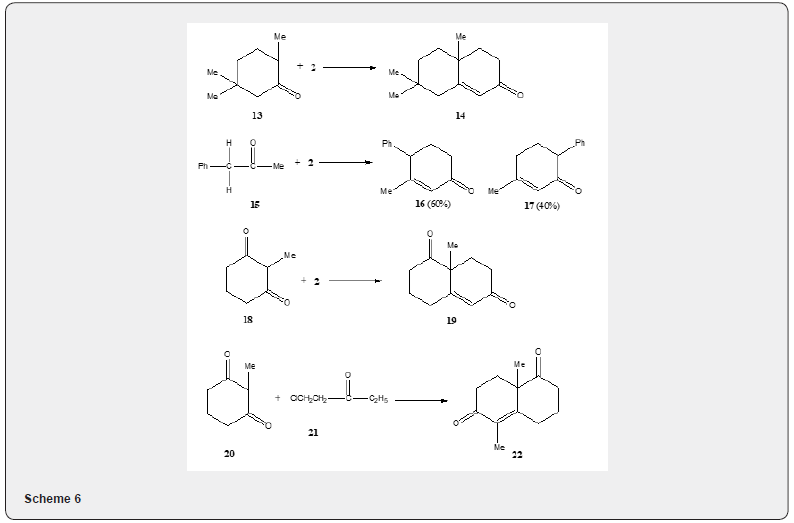
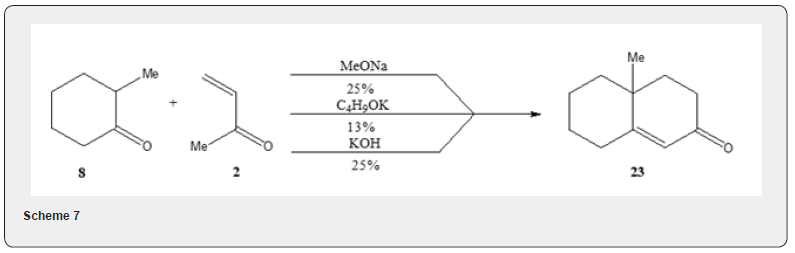
Acid catalyzed Robinson annulation has also been accomplished by heating in solution [7] (Scheme 6) for a long time. Thus, the ketones 13, 15 and 18 yield the bicyclic ketones 14, 16, 17 and 19 respectively. The modified procedure affords the cyclic adducts that are usually comparable to or better than those obtained in the normal base-catalyzed variation of the Robinson- Annulation reaction [8]. The process fails with ketones such as 2-butanone, 4-methyl-2-pentanone, cyclopentanone which do not enolize readily. Acid catalyzed Robinson annulation has also been observed by Pita and Banerjee [9]. The condensation of the ketone 20 with the chloroketone 21 using p-toluenesulfonic acid has been reported to yield the αβ -unsaturated ketone 22. The solvent free Robinson annulation reaction proceeds efficiently at room temperature. It is interesting to know that the two-step reaction such as Robinson annulation proceeds efficiently in the absence of solvent in one pot [10]. Thus ketone 8 reacts with vinyl ketone 2 in absence of solvent to yield the α,β-unsaturated ketone 23. The yield of the ketone 23 varies according to the base used as can be observed in Scheme 7.
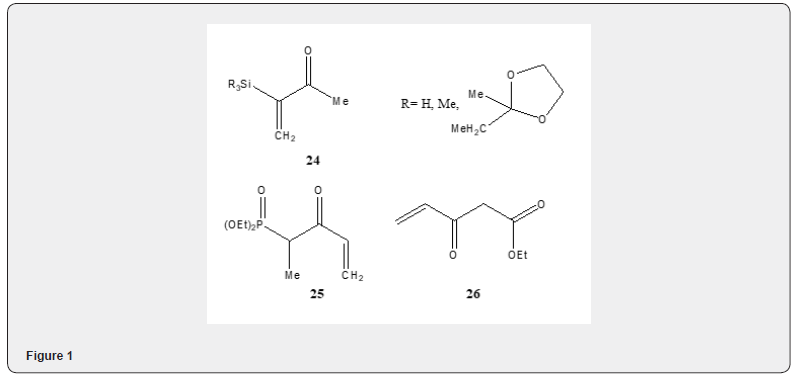
Enantioselective solvent free Robinson annulation reaction has been studied by Rajgopal, et al. [11]. The promotion of onepot Robinson annulation reaction has been achieved by gradual pressure and temperature manipulation under supercritical condition [12] by carbon dioxide in the presence of MgO catalyst. The method has been applied for various ketones to synthesize fused polycyclic compounds. The method is convenient, but the experimental technique is not simple, and the yield is not satisfactory. Robinson annulation has also been accomplished efficiently with alumina on heating with microwave irradiation [13]. Microwave irradiation has proved useful only after the addition of pyrrolidine. Other secondary amines e.g. piperidine, morpholine, diisopropylamine and diethylamine have also been used. The reaction performed by heating in an oil bath at 155oC affords a very poor yield of annulated product. The Robinson annulation reaction has been used in the synthesis of a wide range of compounds including terpenes and steroids. The use of methyl vinyl ketone suffers from low yields as it undergoes polymerization. Various compounds have been tested to replace methylvinyl ketone such as the precursor α-silyted vinyl ketone 24 [14]. 1-pentene-3-one-4-phosphonate 25 as kinetic ethyl vinyl ketone equivalent in the Robinson annulation [15]. The Nazarov reagent 26 [16] has been extensively used to achieve Robinson annulation Figure 1.
Applications for Robinson Annulation in synthesis of diterpenes
Robinson annulation has been utilized for the synthesis of natural products especially for the synthesis of terpenoids [17,18]. In this review the applications of Robinson annulation in synthesis of some selected diterpenes have been discussed Figure 2.
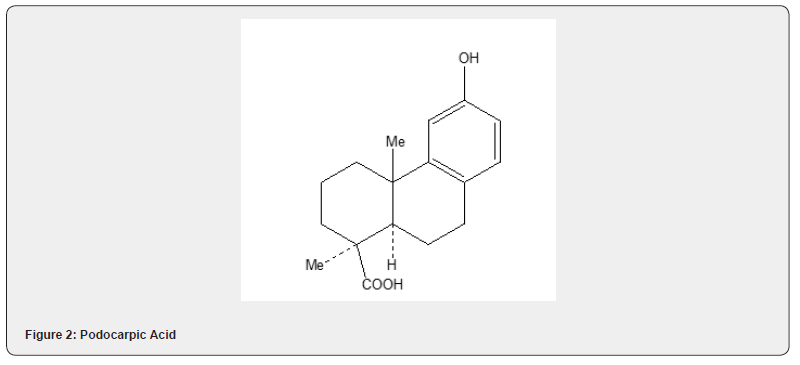
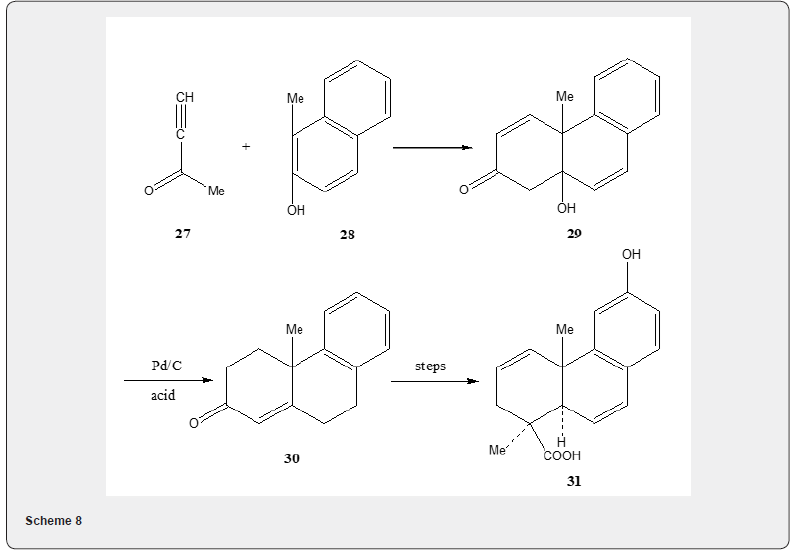
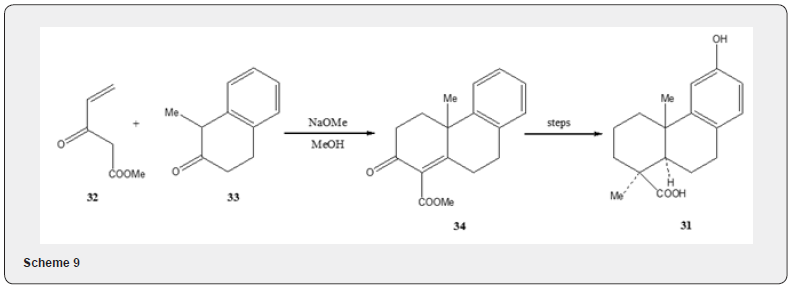
Podocarpic acid, a tricyclic diterpene acid, has been synthesized by several routes [17]. Wenkert and Tahara [19] utilized Robinson annulation reaction to achieve the first total synthesis of podocarpic acid. The synthetic details are described in Scheme 8. The annulation of 1-methyl-2-naphthol 28 with methyl ethnyl ketone 27 yields tricyclic alcohol 29. The catalytic hydrogenation of alcohol followed by acid catalyzed dehydration produces the α,β- unsaturated ketone 30 which is converted into podocarpic acid 31. The Robinson annulation has been utilized [20] by Wenkert and collaborators to achieve an alternative synthesis of podocarpic acid. The synthetic route is depicted in Scheme 9. The tetralone 33 is subjected to annulation reaction with unsaturated ester 32 to obtain the unsaturated ester 34 whose transformation into podocarpic acid 31 has been accomplished utilizing standard organic reactions Figure 3.
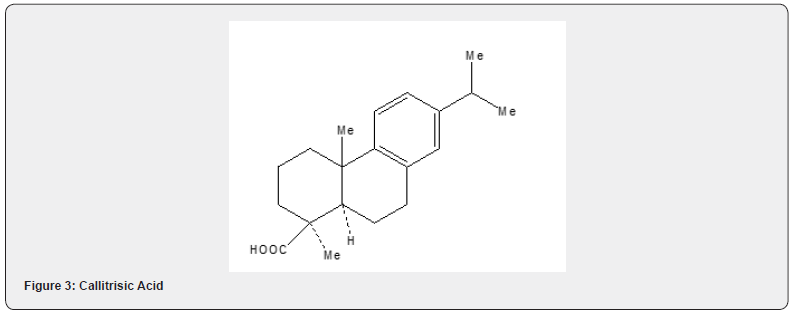
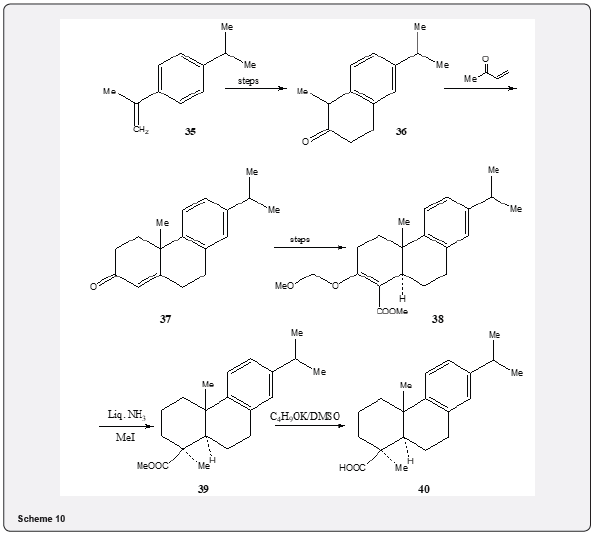
The application of Robinson annulation reaction can be observed in the synthesis of callitrisic acid by Welch and collaborators [21]. The synthetic details are described in Scheme 10. The ketone 36, prepared from isopropyl benzene 35, is converted into the tricyclic ketone 37 by Robinson annulation with methyl vinyl ketone. The conversion of the ketone 37 to an enolether 38 is achieved without any difficulty. Metal ammonia reduction followed by methylation affords tricyclic ester 39 which is converted to callitrisic acid 40 by hydrolysis with potassium t-butoxide in dimethylsulfoxide Figure 4.
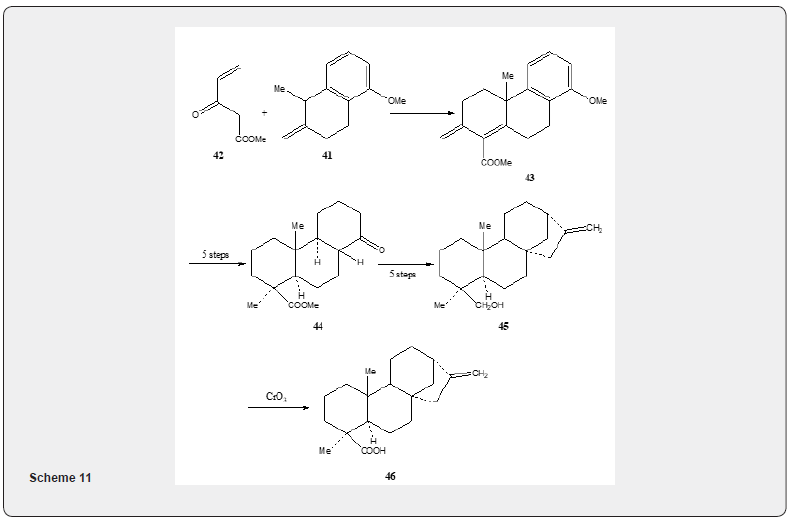
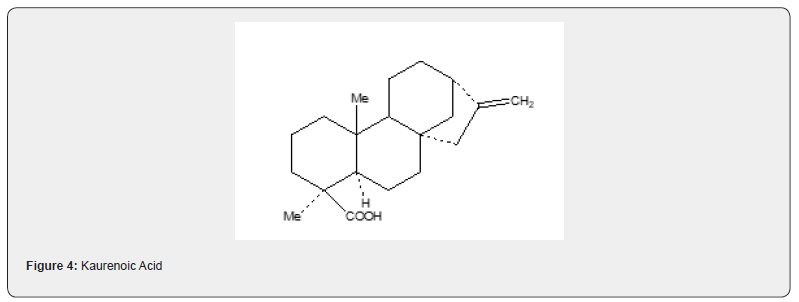
Mori and Matsui [22] utilized the Robinson annulation reaction to achieve the synthesis of kaurenoic acid 46, tetracyclic diterpenoid compound. The synthetic details are described in Scheme 11. The Robinson annulation reaction of β-tetralone 41 with methyl vinylketo ester 42 yields tricyclic enone ester 43 which is converted saturated ketoester 44 in five steps (alkylation, thioketalization, desulphurization, hydrogenation with rhodioplatinum, oxidation). The transformation of 44 into 45 is realized in five steps (alkylation of protected ketone, cleavage of allyl unit to give a keto aldehyde, aldol cyclization, Wolff-Kishner recuction, Wittig reaction). Oxidation of alcohol 45 leads to the formation of kaurenoic acid 46 Figure 5.
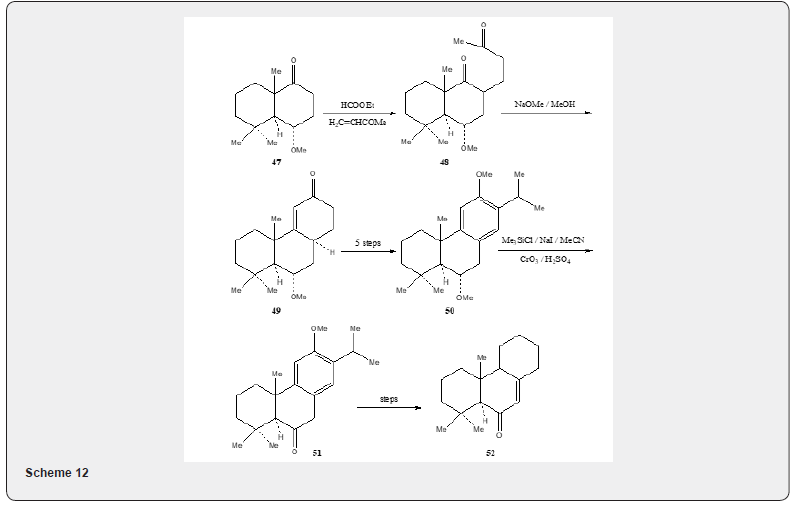
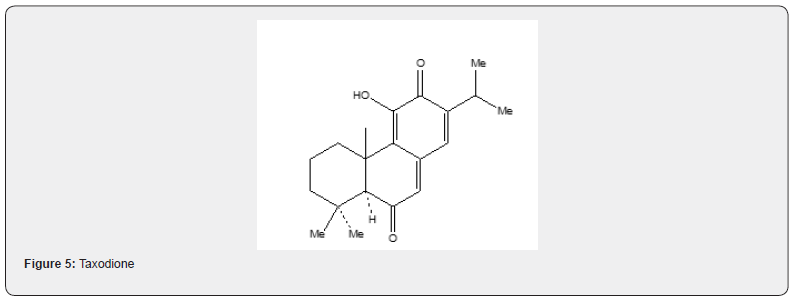
Taxodione 52, a diterpenoid quinone, isolated [23] from Taxodium Distichum Rich (Taxodiaceae) exhibits tumorinhibitory activity in vivo against the Walker Carcino sarcoma 256 in rats and in vitro against cells derived from human carcinoma of the nasopharynx (KB). Several syntheses [24] of taxodione 52 have been developed. The present review describes the synthesis of taxodione 52 developed by Banerjee and Carrasco [25]. The synthetic details are described in Scheme 12. The ketone 47 is formylated and the resulting formyl derivative is subjected to Robinson annelation with methyl vinyl ketone generated in situ by the procedure developed by Howell and Taylor [26] to obtain 48. The cyclization of the 48 with sodium methoxide in methanol affords α,β-unsaturated cyclic ketone 49. The transformation of the ketone 49 into the isopropylated tricyclic compound 50 is achieved in five steps (formation of the enolate anion with LDA in THF and treatment with acetone, dehydration of the resulting alcohol, cyclization with sulfuric acid and methylation with dimethyl sulfate and potassium carbonate). The conversion of the compound 50 to the compound 51 is achieved by treating a solution of the compound 50 in acetonitrile with trimethylsilyl chloride and sodium iodide. The resulting material without purification is oxidized with Jones reagent [27] to obtain compound 51.
As the compound 51 has already been converted into taxodione 52 [24] the present approach constitutes a formal total synthesis of Taxodione Figure 6. Pisiferic acid 61 an abietane type diterpene acid, isolated [28] from the methanolic extract of leaves and twigs of chamaecpyparis pisifera Endle, shows antimicrobial activity especially against all positive bacteria. An excellent synthesis of pisiferic acid has been reported by Zu and Tu [29]. The present review describes the synthesis of pisiferic acid 61 developed by Banerjee and collaborators [30]. The synthetic details are described in Scheme 13. Irradiation of alcohol 53 in cyclohexane with lead tetraacetate and iodine yields the cyclic ether 54 in 45% yield which on oxidation with Jones reagnt [27] provides the ketone 55. The Robinson annulation of the formyl derivative of 55 with methylvinyl ketone produced by the procedure of Howell and Taylor [26] yields the adduct 56 which on being heated with sodium methoxide in methanol undergoes cyclization and affords ketoether 57. The transformation of 57 into pisiferol 58 is achieved in five steps (reaction with diethyl carbonate, treatment with MeLi, aromatization with methanolic hydrochloric acid, methylation with dimethyl sulfate and sodium hydroxide, cleavage of the cyclic ether with zn, znI and acetic acid).
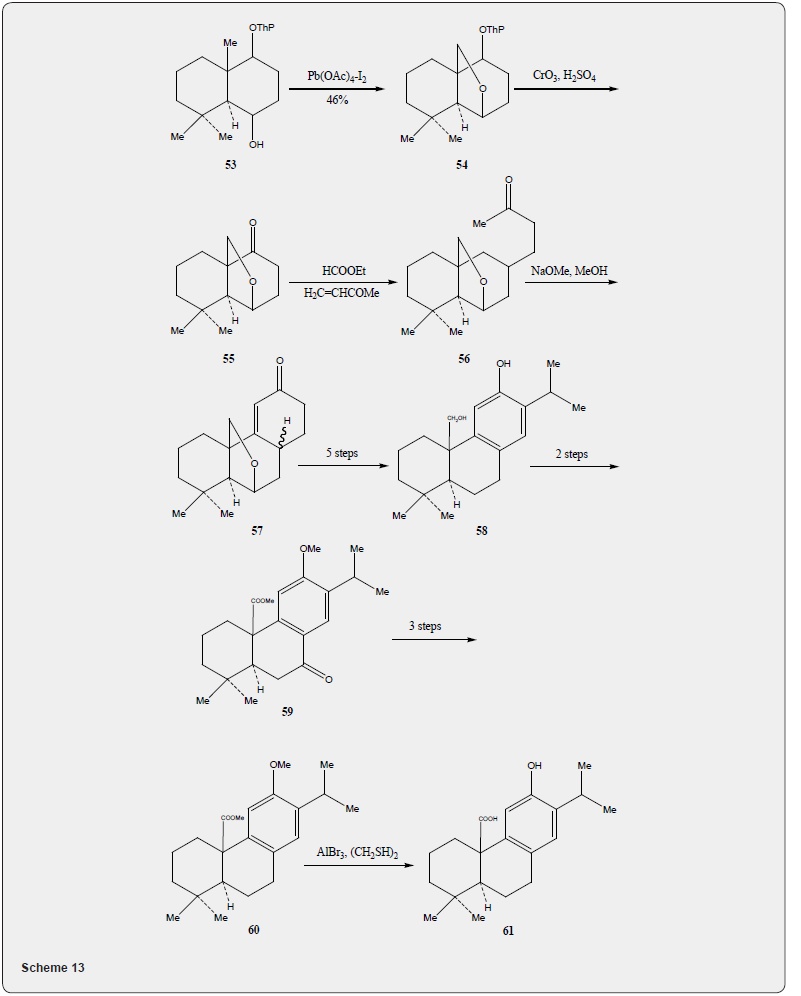
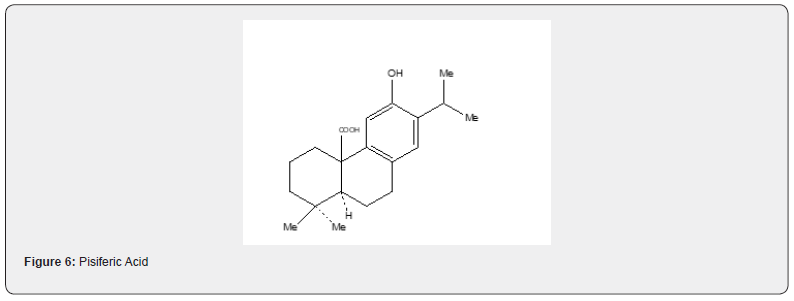
The pisiferol is converted into the ketoester 59 in two steps: (i) oxidation with Jones reagent and (ii) methylation with dimethylsulfate. The transformation of the ketoester 59 into the ester 60 has been achieved in three steps: (i) reduction of the carbonyl group with sodium borohydride and conversion of the resulting alcohol to its tosyl derivative (ii) elimination of the tosyl group by heating with sodium iodide and zinc dust in dimethoxyethane. The transformation of the ester 60 into pisiferic acid 61 has been achieved by heating with aluminium bromide and ethanethiol. It is important to mention that the synthesis of taxodione and pisiferic acid have not been described in detail in this review because the detailed descriptions have already been described [24,31]. Robinson annulation reaction have been utilized for the synthesis of several diterpenes (simple and complex). It is recommended to go through the published articles [17,18] to have more informations.
Conclusion
It can be observed that several modifications have been made to the original Robinson annulation reaction which has proved very useful for the synthesis of several diterpenes eg podocarpic acid, callitric acid, taxodione, pisiferic acid.
Acknowledgement
This was supported by FONACIT Foundation [project number IVIC 9043(238043)].
References
- Rapson WS, Robinson R (1935) Experiments on the synthesis of substances related to steroid. A new general method for the synthesis of substituted cyclohexenones. J Chem Soc pp։ 1285-1288.
- Du Feu EC, McQuillin FJ, Robinson R (1937) A simple synthesis of certain octalones and ketotetrahydrindenes which may be of angle-methyl-substituted type. A theory of the biogenesis of the sterols. J Chem Soc pp: 53-60.
- Jung ME (1976) A review of annulation. Tetrahedron 32: 3-31.
- Gawley RE (1976) The Robinson Annulation and related reactions Synthesis 12: 777-794.
- Soto T, Wakahara Y, Otera J, Nozaki H (1990) Tetrahedron Letts 31: 1581-1584
- Woodward RB, Singh T (1950) Synthesis and rearrangement of cyclohexadienones. J Am Chem Soc 72(1): 494-500.
- Heathcock CH, Ellis JE, McMurry JE, Coppolino A (1971) Acid-catalyzed Robinson annulation. Tetrahedron Letts pp: 4995-4999.
- Marshall J, Fanta WI (1964) The Synthesis of bicyclic ketol from cyclohexanones. J Org Chem 29(9): 2401-2405.
- Banerjee AKB, Pita-Boente MI (1985) A Synthetic Approach to Noreudesmanoid Phytoallexins. Heterocycles 23: 5-9.
- Miyamoto H, Kanetaka S, Tanaka K, Yoshizawa K, Toyota S, et al. (2000) Solvent-Free Robinson Annelation Reaction. Chemistry Letts 29(8): 888-889.
- Rajgopal D, Narayanan R, Swaminathan S (2001) Asymmetric one-pot Robinson annulations. Tetrahedron Letts 42(29): 4887-4890.
- Kawanami H, Ikushima Y (2004) Promotion of one- pot Robinson annelation achieved by gradual pressure and temperature manipulation under supercritical conditions. Tetrahedron Letts 45(26): 5147-5150.
- Takatori K, Nakayama M, Futaishi N, Yamada S, Hirayama S, et al. (2003) Solid-supported Robinson annulation under microwave irradiation. Chem Pharm Bull 51(4): 455-457.
- Stork G, Ganem B (1973) α-Silylated vinyl ketones. A new class of Reagents for the annelation of ketones. J Am Chem Soc 95918): 6152-6153.
- Kuo F, Fuchs PL (1986) Use of 1-penten-3-one-4- phosphonate as kinetic ethyl vinyl ketone equivalent in the Robinson annulation reaction. Synth Commun 16(14): 1745-1759.
- Audran G, Bremond P, Feuerstein M, Sylvain RA, Santelli M (2013) Nazarov reagents and their use in organic synthesis. Tetrahedron 69(39): 8325-8348.
- Smith D (1992) The total synthesis of tri- and tetracyclic diterpenes in the total synthesis of natural products, vol 8, edit J Apsimon, John Wiley& Sons, Inc, New York pp. 1-223.
- Heravi MH, Alipour B, Zadsirjan V, Malmir M (2023) Robinson annulation applied to the total synthesis of natural products. Asian J Org Chem 12(3): e202200668.
- Wenkert E, Tahara A (1960) Total synthesis of d-podocarpic acid. J Am Chem Soc 82(12): 3229.
- Wenkert E, Afonso A, Bredenberg JB-S, Kaneko C, Tahara A (1964) Synthesis of some resin acids. J Am Chem Soc 86(10): 2038-2043.
- Welch SC, Hagen CP, Kim JH, Chu PS (1977) Stereoselective total synthesis of diterpene resin acids. J Org Chem 42(17): 2879-2887.
- Mori K, Matsui M (1968) (±)-Kaur-16-ene-19-oic acid and Kaur-16-en-19-ol, monogynol and some oxygenated kauranes. Tetrahedron 24: 3095-3111.
- Kupchan SM, Karim A, Marcks C (1968) Taxodione and Taxodone, two novel diterpenoides quinone methio dides tumor inhibitors from Taxodium distichum. J Am Chem Soc 90(21): 5923-5924.
- Banerjee AK, Carrasco MC (1994) Taxodione, Synthetic Studies. Atta-ur-Rahman (Ed) Studies in Natural Products Chemistry 14: 677-702.
- Banerjee AK, Carrasco MC (1983) Synthetic approaches to (±) Taxodione. Synth Commun 13(4): 281-287.
- Howell FH, Taylor DAH (1958) The synthesis of some substituted hexahydro-12-methyl-2-oxophenanthrene. J Chem Soc: 1248-1254.
- Bowers, A, Halsall, TG, Jones ERH, Lemin AJ (1953) The chemistry of triterpenes and related compounds. Elucidation of the structure of polyporenic acid. J Chem Soc Perkin Trans 1: 2548-2555.
- Fukui H, Koshimizu K, Egawa H (1978) A new diterpene with antimicrobial activity from Chamaecyparis pisifera Endle. Agric Biol Chem 42(7): 1419-1423.
- Zhu H, Tu P (2005) A convenient strategy for the total synthesis of pisiferic acid type diterpenes. Synth Commun 35(1): 71-78.
- Banerjee AK, Hurtado H, Laya M, Acevedo JC, Alvarez J (1988) Total Synthesis of (±) Pisiferic Acid. J Chem Soc Perkin Trans 1: 931-938.
- Banerjee AK, Laya MS, Mora HD, Cabrera, EV (2008) The chemistry of bioactive diterpenes. Current Org Chem 12(13): 1050-1070.






























