Abstract
This work produced propargylic dihydrofuroisoxazolines via Intramolecular Silyl Nitronate Cycloadditions (ISNC) from nitroethers formed during Michael Additions of alkenynyl oxides with nitroalkenes. These compounds underwent boron trihalide mediated ring openings to provide halogenated propargylic-isoxazolines. The propargylic-furoisoxazolines and isoxazolines were tested as antimicrobial agents against E. coli. The stereoselectivity and regioselectivity of the Lewis acid opened compounds were analyzed via NMR and computational modeling utilizing Spartan 2024.
Keywords: Furoisoxazoline; Intramolecular Silyl Nitronate Cycloaddition; Propargylic Nitroether; Fungicide
Introduction
Isoxazoles, isoxazolines, and their derivatives have been shown to possess a vast array of uses, such as antifungal [1-3] antibacterial [3,4], anticancer [3,5], analgesic [3], antiviral [3,4], anti-inflammatory [3], anti-tuberculosis [3], and ectoparasiticide [6] agents. The corresponding author has been investigating the regioselectivity and stereoselectivity of the Intramolecular Nitrile Oxide Cycloaddition [7] and the Intramolecular Silyl Nitronate Cycloadditions [8] along with many other researchers for the past three decades. The Duffy/Kurth and the Duffy-Matzner group demonstrated that propargylic nitroethers surprisingly don’t form furoisoxazoles but instead form a,b -unsaturated dihydrofuro carbaldehydes and ketones with propargylic systems. [9,10] Duffy-Matzner’s group recently investigated the chemoselectivity and stereoselectivity of allylic/propargylic nitroethers during Intramolecular Silyl Nitronate Cycloadditions (ISNC). [11] This paper explore the ring opening of the ether ring to form novel halogenated alkynyl isoxazolines. Small ring ethers (oxiranes, oxetanes) are easily opened by Lewis Acid Catalysts. Five and six membered ring ethers are much harder to open due to low ring strain. A very recent review examined Lewis Acid catalyzed ring opening of tetrahydrofurans and pyrans. [12] There are much fewer publications that consider the ring opening of furoisoxazoles and isoxazole derivatives. Kurth demonstrated that isoxazoles produced from the cleavage of furoisoxazoles were effective in treating Tomato Late Blight, Cucumber Gray Mold, Rice C Blast, and BLM in plants. Kim [13] found that 4H,6H-furo [3.4-c] isoxazole derivatives were also effective antimycotic agents. [2] These works noted that the ring opening was regioselective in that the benzylic C-O bond was cleaved but did not explain the selectivity or examine. This work examines Lewis Acid mediated ring opening of furoisoxazoline systems and inspects the antimicrobial properties of the novel propargylic furoisoxazoline and chlorinated propargylic isoxazoline compounds. Semi-empirical/HF/DFT calculations were used to explain any demonstrated regioselectivity and assist with NMR assignments.
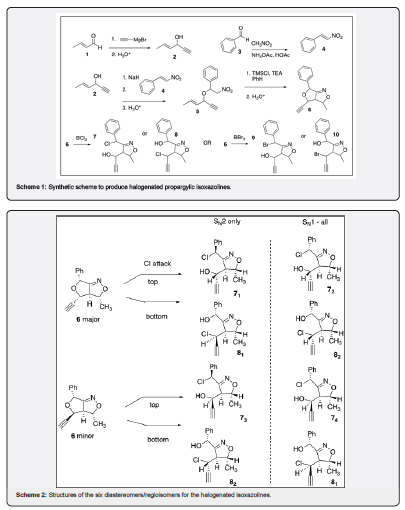
The Lewis acids: boron tribromide and boron trichloride, were employed to open the cyclic ethers. These reactions yield halogenated isoxazoline secondary alcohols with terminal alkyne moieties that could be used to add other functional groups in the future. Duffy-Matzner’s recent examination of the allylic/propargylic nitroether gave complete regioselectivity to the double bond over the triple bond via ISNC conditions. [11] This synthetic scheme is demonstrated below in Scheme 1. 4-Hexen-1-yn-3-ol (2) was prepared from a Grignard reaction with ethynylmagnesium bromide with crotonaldehyde (1). Nitrostyrene (4) was prepared in Henry reaction between nitromethane and benzaldehyde (3).10 Disappointingly, these allylic/propargylic systems do not react cleanly in the tandem one pot reaction of olefinic silyl nitronates reported by Hassner [14] and Cheng [15], instead a complex reaction mixture was obtained. Alternatively, a two-step process was examined. The nitroether (5) was produced Michael addition of a sodium alkoxide to a nitroolefin followed by column chromatography. [13] This was then treated with trimethylsilyl chloride and triethylamine for the ISNC reaction. Opening of the ether side of the resulting furoisoxazolines (6) utilized boron trichloride (or tribromide) to yield the two possible regioisomers of the halogenated propargylic isoxazolines (7-10).
Materials and Methods
Physical and Spectra Data
Proton and Carbon NMR were obtained on a JEOL (400 MHz) spectrometer. Listed proton NMR data are given in the following order: ppm (multiplicity, coupling constants, integrated number of protons and assignment). Listed carbon NMR data are given by chemical shift and assignment. All of the spectra were run in deuterated chloroform unless otherwise stated. The chemical shifts were determined as the distance in ppm from TMS. Infrared spectra were recorded on a Nicolet Avatar 361 FT-IR with Gateway 2000 data system. Samples were either run on NaCl plates.
Chromatography
TLC refers to thin layer chromatography, which was done on Sigma Chemical Co. plates made of 250 m silica gel on polyester with a 254 nm fluorescent indicator added. Visualization was performed via iodine chambers or UV lamp.
Reactions
Concentration under reduced pressure refers to a solvent removal on a Büchi RE 011 rotary evaporator connected to a water aspirator and an ethylene glycol cooling system. All reactions were run under a nitrogen atmosphere unless stated otherwise stated. Unless otherwise stated, all solvents were reagent grade and used without further purification.
Reagents
All reagents were purchased from Sigma-Aldrich and reagent grade unless stated otherwise.
Reported Methods
The syntheses of compounds 2-6a have been reported previously [11].
Synthetic Methods
Synthesis of (±)-5- [1-chloro-1-phenylmethyl]- 4-[1- hyroxyprop-2- yn-1- yl]-3-methyl-3,4-dihydroisoxazole (7): The same procedure as the synthesis of (±)-5-[1-bromo-1-phenylmethyl]-4-[1-hyroxyprop-2-yn-1-yl]- 3-methyl-3,4-dihydroisoxazole was followed. Furoisoxazoline (6, 0.106 g, 0.466 mmol) was treated with boron trichloride (1.2 mL 1M, 1.2 mmol) to give a green sappy solid with a crude yield of 43 mg (37.3%) as inseparable mixture of 4 diastereomers (4:3.6:1.4:1). The compounds were found to decompose with attempts to purify by column chromatography. Rf values could not be obtained. One replication reaction (never to be repeated) yielded mainly isomer one. This was used to help assign all the diastereomers. All attempts by chromatography or recrystallization resulted in decomposition of the products. This limited the ability to purify the product enough for elemental analysis. FTIR (neat) [3300 cm-1 (OH v), 3286 (H-C≡C v), 2970, 2926 (sp3 C-H v), 2117 (C≡C v), 1601 (C=C conj v), 1456 (C=N-O v), 1031 (C-O v), 801 (Ar δ), 699 C-Cl v].
Major Isomer
1H NMR (400 MHz, CDCl3) [δ= 1.36 ppm (d, J=6.4 Hz, 3H, CH3 I); 2.61 (d, J=2.4Hz, 1H, H-C≡C I); 3.32 (at, J=6.4Hz, 1H, CH-CHCH3 I); 4.77 (dd, J=6.4, 2.4Hz, 1H CH-C≡C I); 4.85 (pentet, J=6.4 Hz, 1H, CH-CH3 I), 5.95 (s, 1H, HC-Ar I); 7.44 (m, 5H, Ar-H).] 13C NMR (100 MHz, CDCl3): [δ= 21.1 ppm (CH3 I), 56.9 (CH-Ar I), 59.7, (CH-CH-CH3 I), 62.2 (CH-C≡C I), 75.9 (HC≡C I), 81.0 (C≡CH I), 81.2 (CH-CH3 I), 128.0, 128.6, 129.0, (Ar CH I), 136.2 (Ar C I), 156.8 (C=N I)].
Four diastereomers
1H NMR (400 MHz, CDCl3) [δ= 1.29 ppm (d, J=6.4 Hz, 0.30H, CH3 IV); 1.36 ppm (d, J=6.4 Hz, 2.28H, CH3 I&II); 1.37 ppm (d, J=6.4 Hz, 0.42H, CH3III); 2.45 (d, J=2.4Hz, 0.10H, H-C≡C IV); 2.52 (d, J=2.4Hz, 0.36H, H-C≡C II); 2.58 (d, J=1.6Hz, 0.14H, H-C≡C III); 2.61 (d, J=2.4Hz, 0.40H, H-C≡C I); 2.99 (at, J=6.0Hz, 0.10H, CH-CHCH3 IV); 3.05 (dd, J=6.4, 3.6 Hz, 0.36H, CH-CHCH3 II); 3.18 (dd, J=6.8, 3.8Hz, 0.14H, CH-CHCH3 III); 3.32 (at, J=6.4Hz, 0.40H, CH-CHCH3 I); 4.22 (dd, J=3.8, 2.4Hz, 0.36H CH-C≡C II); 4.28 (dd, J=6.6, 1.6Hz, 0.10H CH-C≡C IV); 4.77 (dd, J=6.4, 2.4Hz, 0.40H CH-C≡C I); 4.85 (m, 1.14H, CH-CH3 I-IV & CH-C≡C III); 5.95 (s, 0.40H, HC-Ar I); 5.98 (s, 0.14H, HC-Ar III); 6.02 (s, 0.10H, HC-Ar IV); 6.04 (s, 0.36H, HC-Ar II); 7.44 (m, 5H, Ar-H).] 13C NMR (100 MHz, CDCl3): [δ= 21.1 ppm (CH3 I), 21.1 (CH3 IV), 21.4 (CH3 III), 21.5 (CH3 II), 56.1 (CH-Ar II), 56.2 (CH-Ar IV), 56.8 (CH-Ar III), 56.9 (CH-Ar I), 59.7 CH-CH-CH3 I), 59.9, 60.0 60.2 (CH-CH-CH3 II-V, CH-C≡C II&III), 61.8 (CH-C≡C IV), 62.2 (CH-C≡C I), 75.2 (HC≡C II), 75.4 (HC≡C III), 75.5 (HC≡C IV), 75.9 (HC≡C I), 80.2 (CH-CH3 II), 80.6, 80.7, 80.9 (C≡CH II-IV), 81.0 (C≡CH I), 81.1 (CH-CH3 III), 81.2 (CH-CH3 I), 81.3 (CH-CH3 IV), 127.3, 127.7, 127.8, 128.0, 128.3, 128.4, 128.6 128.7, 128.9, 129.0, 129.2 (Ar CH I-V), 136.0, 136.2, 136.5 (Ar C I-V), 155.4 (C=N II), 155.9 (C=N III), 156.3 (C=N IV), 156.8 (C=N I)].
4.6.2. Synthesis of (±)-5-[1-bromo-1-phenylmethyl]-4-[1-hydroxyprop-2-yn-1-yl]- 3-methyl-3,4-dihydroisoxazole (9): 0.138 g (0.607 mmol) of 4-ethynyl-3-methyl-6-phenyl-3, 3a, 4, 6-tetrahydrofuro[3, 4-c]isoxazole was placed in a 5 mL round bottom and dissolved it in 2 mL of dichloromethane and purged with nitrogen. The reaction flask was then placed in a bath of acetone saturated with dry ice ( -78˚C). Next 1.2 mL of 1M (1.2 mmol) boron tribromide was added. The reaction mixture was allowed to stir for 45 min. It was then tested for completion by TLC (1:6 EtOAc:Hex) by disappearance of the furoisoxazolines (Rf 0.26/0.27). Diethyl ether (Et2O) was added and the mixture was poured into cold water. The organic layer was isolated after vigorous stirring and the aqueous layer was extracted with Et2O. The combined organic layers were then dried over MgSO4 and placed under reduced pressure until the solvent was no longer present. The resulting mixture was then placed on a strong vacuum to further remove any residual volatiles. The result was a dark green sappy solid with a crude yield of 0.138 g (78.0%). The compounds were found to decompose with attempts to purify by column chromatography. Rf values could not be obtained. The products decomposed even upon storage under nitrogen in freezer overnight. A reaction was run with just the major isomer of 6, to help assign all the diastereomers.
FT-IR: 3287 cm-1 ≡C-H & OH v, 3063 cm-1 Ar-H v, 2974, 2926 cm-1 sp3C-H v, 2116 cm-1 C≡C v, 1602 cm-1 C=C Ar v, 1455 cm-1 C=N-O v, 1377 cm-1 CH3 ip d, 1262 cm-1 N-O v, 1193 cm-1 C-C v, 1080 cm-1 C-O v, 770 cm-1 Ar d, 698 cm-1 C-Br v.
Major Diastereomer of furoisoxazoline reacted to give a 2:1 ratio of I:II Br-isoxazoline.
1H NMR (400 MHz, CDCl3): δ= [1.28 ppm (d, J=6.4Hz, 1H, CH3 II), 1.40 ppm (d, J=6.4Hz, 2H, CH3 I), 2.48 (d, J=2.4 Hz,0.33H, H-C≡C II), 2.61(d, J=2.0 Hz, 0.66H, H-C≡C I), 2.96 (at, J=6.4 Hz, 0.33H, CH-CH-ON II), 3.47 (at, J=6.4 Hz, 0.66H, CH-CH-ON I), 4.31 (dd, J=6.8,2.4 Hz, 0.33H, CH-C≡C II), 4.67 (dd, J=6.8,2.0 Hz, 0.66H, CH-C≡C I), 4.75 (ap, J=6.4 Hz, 0.66H, CH-CH3 I), 4.82 (ap, J=6.4 Hz, 0.33H, CH-CH3 II), 5.99 (s, 0.66H, CH-Ph I), 6.03 (s,0.33H, CH-Ph II), 7.44 (m, 5H, Ar-H)]. 13C NMR (100 MHz, CDCl3): δ= [21.0 ppm (CH3 I), 21.1(CH3 II), 44.6 (CH-Ph II), 46.9 (CH-Ph I), 60.1 (CH-C=N II), 60.2 (CH-C=N I), 61.9 (CH-C≡C II), 62.2 (CH-C≡C I), 75.5 (C≡C-H II), 75.9 (C≡C-H I), 81.1(C≡C-H II), 81.2 (C≡C-H I), 81.3 (CH-CH3 I), 81.7 (CH-CH3 II), 128.3, 128.4, 129.0, 129.1 (Ar CH I,II), 136.4 (Ar C I), 137.0 (Ar C II), 156.3 (C=N II), 157.5 (C=N I)].
Two diastereomers of furoisoxazoline gave 4 diastereomers: 0.4 I :0.27 II :0.2 III :0.13 IV.
1H NMR (400 MHz, CDCl3): δ= [1.28 ppm (d, J=6.4Hz, 0.6H, CH3 III), 1.34 ppm (d, J=6.4 Hz, 0.4H, CH3 IV), 1.40 ppm (d, J=6.4Hz, 1.2H, CH3 I), 1.42 ppm (d, J=6.4Hz, 0.8H, CH3 II), 2.48 (d, J=2.4Hz, 0.27H, H-C≡C II), 2.61(d, J=2.0 Hz, 0.4H, H-C≡C I), 2.96 (at, J=6.4 Hz, 0.2H, CH-CH-ON III), 3.01 (m, .13H, CH-CH-ON IV), 3.39 (dd, J=7.2,4.4 Hz, .27H, CH-CH-ON II) 3.47 (at, J=6.4 Hz, 0.4H, CH-CH-ON I), 4.26 (dd, J=4.2,2.2 Hz, 0.13H, CH-C≡C IV), 4.31 (dd, J=6.8,2.4 Hz, 0.27H, CH-C≡C II), 4.67 (dd, J=6.8,2.0 Hz, 0.4H, CH-C≡C I), 4.80 (m, 1.2H, CH-C≡C III & CH-CH3 I,II,IV), 4.88 (ap, J=6.4 Hz, 0.2H, CH-CH3 III), 5.97 (s, 0.27H, CH-Ph II), 5.99 (s, 0.4H, CH-Ph I), 6.03 (s, 0.33H, CH-Ph III&IV), 7.44 (m, 5H, Ar-H)]. C13 NMR (100 MHz, CDCl3): δ= [21.0 (CH3 I), 21.1 (CH3 III), 21.2 (CH3 II), 21.5 (CH3 IV), 44.6 (CH-Ph III), 44.7 (CH-Ph IV), 46.6 (CH-Ph II), 47.0 (CH-Ph I), 60.1 (CH-C=N III), 60.2 (CH-C=N I&II), 60.3 (CH-C=N IV), 61.9 (CH-C≡C III&V), 62.3 (CH-C≡C I&II), 75.4 (C≡C-H IV), 75.5 (C≡C-H III), 75.7 (C≡C-H II), 75.9 (C≡C-H I), 80.3 (CH-CH3 II), 80.7 (C≡C-H III), 81.1 (C≡C-H II), 81.2 (C≡C-H I), 81.3 (CH-CH3 I), 81.7 (CH-CH3 III), (80.2-81.7 CH-CH3 IV, C≡C-H IV), 128.2, 128.3, 128.4, 128.5, 128.9, 129.0, 129.1, 129.2 (Ar CH I-IV), 136.3 (Ar C II), 136.5 (Ar C I), 136.7 (Ar C IV), 137.1 (Ar C III), 155.5 (C=N IV), 156.3 (C=N III), 156.6 (C=N II), 157.5 (C=N I)].
Determination of Bacterial Growth Inhibition
The ability of the furoisoxazole compounds to inhibit bacteria growth was detected qualitatively using diffusion assays on agar plates and using growth curves made by measuring the growth rate of bacteria in LB medium [16]; as detected by an increase in absorbance at 600 nm on a Molecular Devices SpectraMaxM2 plate reader. Examination of various concentrations of DMSO with Luria-Bertani (LB) broth were examined over a several hour time frame to track the growth of Escherichia coli. It was determined that a 5% DMSO solution gave optimum results. Mixed solvents of ethanol, methanol, and acetone with LB broth were analyzed and DMSO gave the best growth results. The isoxazolines were dissolved in 5% DMSO and pipetted onto LB agar plates that had been spread with E. coli. for diffusion assay. Positive reactions were assessed based on a zone of clearing of bacterial growth after incubation at 37o C.
Results and Discussion
It was understood that the spectra of the halogenated alkynyl isoxazolines would be difficult to assign due to the presence of many diastereomers. This work will examine all the possible isooxazoline isomers. Duffy-Matzner’s group previously discovered that the cycloaddition (ISNC) to produce 6 yielded a mixture of two diastereomers [11]. Careful NOE studies showed that the structures of the two diastereomers as listed in Figure 1, have the major diastereomer in a “cis” conformation vs a “trans” minor in respects to the phenyl and triple bond substituents. Lewis Acid catalyzed cleavage of these ethers would result in the possibility of six different isomers (excluding enantiomers) as displayed in Scheme 2. Nucleophilic strikes at the benzylic methine would result in four diastereomers. Nucleophilic attacks of the alkenyl methine provides an additional two diastereomers. The stereocenter of the methines attached to the methyl are fixed arbitrarily in a S configuration for the six diastereomers of 7 and 8 as shown in Scheme 2(enantiomers are assumed). Additionally, the methine’s hydrogen at the fusion point for the ring must be behind the ring, due to the trans double bond of the starting nitroether. The configurations of the benzylic and propargylic methines are determined by the ring opening processes. A Lewis acid mediated SN2 mechanism would provide four isomers (71, 73, 81, 82), while a Lewis acid mediated SN1 process would yield all the possible six isomers. Regio control would provide four isomers for either the benzylic (top) or progargylic (bottom) reactive sites.
It was decided to pursue Spartan calculations to assist in understanding the diastereomers and regioisomers produced from the ring cleavage. Corrected enthalpies of formation (zero point energy {ZPE} and temperature {25 ˚C}) were calculated for each isomer. Chemical proton and carbon chemical shift predictions from both Spartan (Spartan 2024) and ChemDraw (Perkin Elmer,19.1.1.32) programs were used to determine the isomers formed. These results can be seen in Table 1. The corrected enthalpies of formation were found via semi-empirical (PM6) calculations for the six different isomers. Chemical shift predictions for proton and carbon NMR experiments were obtained via Hartree Fock (HF/3-21G & HF/6-31G*) and DFT (wB97X-D/6-31G*) calculations. The authors also included the results obtained from predictions of the six isomers from Chem Draw (Professional, 19.1.1.32). A quick comparison of enthalpy values indicated that there might be a preference between propargylic attacks (8) vs benzylic attacks (7) in that the former resulted in lower energy products on average. The H1 and H3 trans isomers were lower in energy than the corresponding cis isomers except for 71 & 72. The two Hartree Fock calculations gave similar results for the proton and carbon chemical shift estimations. In comparison, the DFT calculations gave higher estimations for both proton and carbon shifts. The three calculations showed similar trends: C2 (methyl methine) would have the highest carbon ppm. C1 (benzylic methine) shifts would be higher for the 81,2 vs 71,2,3,4. Also the carbon chemical shifts C3 (propargylic methine) were much lower for 81 & 82.
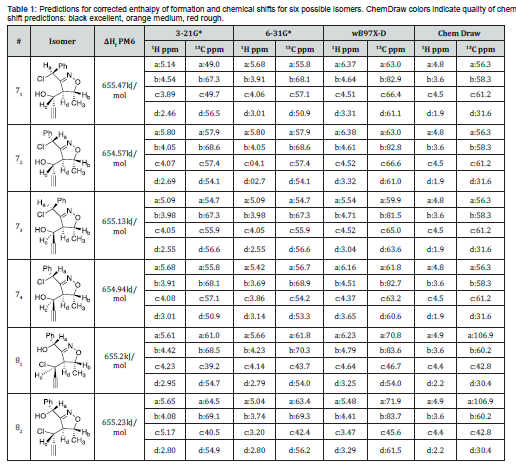
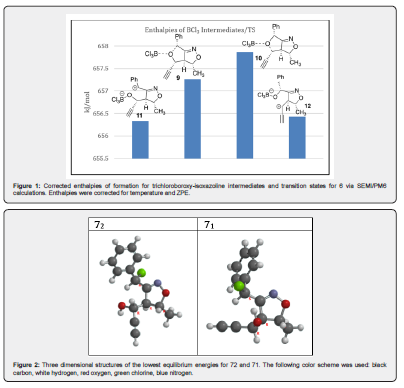
These investigations demonstrated several key findings: Calculations would be needed for the transition states of the six isomers to indicate which are formed since former publications on isoxazole systems indicated that only benzylic attacks were present [2,13]. Carbon NMR could be used to determine a preference between benzylic substitutions (71,2,3,4) and/or alkenyl attacks (81,2). ChemDraw predictions would not be helpful. The program could not differentiate between diastereomers in any of its calculations. The results listed in red or orange indicate a rough or medium estimation quality for the methine hydrogens, thus these predictions would not be useful. While the program indicated that the carbon predictions had good estimation qualities, it gave unrealistically low predictions for C3 and unrealistically high values for C1 for 81 & 82. The DFT calculations gave closer predictions for the 13C values as compared to the HF prediction as compared to the observed chemical shifts. The authors discovered that four inseparable diastereomers were apparent in the proton and carbon NMR spectrums from the chloride and bromide ring opening reactions of the two isomers of 6. Two inseparable diastereomers were present when the major isomer of 6 was treated with boron trichloride, which indicates regiospecificity. It was also clear from the integrations of the products’ proton NMRs that not only SN1 mechanisms were followed since the selectivity was not 50:50. The authors decided to thoroughly investigate the possible trichloroboroxy-isooxazoline intermediates with SEMI/PM6 calculations. It has been stated that the Semi-Empirical quantum chemistry method of James P. Stewart calculates heats of formation of a wide variety of molecules with higher accuracies than Hartree-Fock and better overall than Density Functional Theory. This accuracy is due to the addition of experimental parameters along with the quantum chemistry calculations [17,18]. These results are listed in Figure 2.
The transition state and the intermediate zwitterion are higher in energies for the alkenyl opening site (10 & 12) as compared to the benzylic ones (9 & 11) as expected. This means that very little of none of 8a1 and 8 a2 should be detected. The proton hydrogen chemical shifts of the halogenated isoxazolines were carefully assigned through COSY experiments and then the carbons through DEPT and HMQC experiments. The four peaks seen for the propargylic methine carbon diastereomers could be found at 60.2-62.1 ppm. The four diastereomeric carbon peaks for the benzylic methines were found at 56.1 – 56.9 ppm. This demonstrates that compounds 71-74 were selectively formed over compounds 81-2 as predicted by the computational results. The bromide reactions provided more diastereoselectivity control (~2:1) as compared to the chloride ring openings (~1.1:1) based on 1H NMR integration values. Therefore, the chloride ring openings primarily followed SN1 pathways. It is expected that the product amounts are in the following order: 72 > 71 >> 74 > 73. This was determined based on the 1H integration ratios of the two diastereomers of the furoisoxazoline (6) in comparison to the 1H integration ratios of the stereoisomers produced during ether cleavage. These ratios are also supported by the PM6 studies of the enthalpies of formation of these four diastereomers. This demonstrates that while the SN1 like mechanism dominates, there is some small stereocontrol that yields the lower energy isomers. Figure 3 depicts the three-dimensional structures for 71 and 72. The latter (7a2) places the hydroxy/chloro substituents and the phenyl/alkenyl moieties further away from each other. This could be explained by less steric strain between the Cl and OH groups for 72 and 74. The hydroxy and phenyl substituents with the larger A values [19] are not forced as far apart as possible in the conformers, which is somewhat surprising.
Examinations of the inhibition of bacterial growth of the halogenated propargylic isoxazolines proved problematic. The isomers of the bromo-propargylic isoxazolines (9) were not tested as they decomposed within 24 hours of synthesis. The chlorinated propargylic isoxazolines (7) were stable and soluble in DMSO, but precipitated when added to the agar plates. Diffusion assays demonstrated that the propargylic furoisoxazoline (6) dissolved in 5% DMSO inhibited bacterial growth, while 5% DMSO alone did not. A zone of clearing present indicated inhibition of E. coli by the furoisoxazoline. The doubling times of E. coli in 5% DMSO and furoisoxazole (6) dissolved in 5% DMSO to a final concentration of 50 mg/ml, were 33 min and 60 min, respectively. This demonstrates that 50 mg/ml furoisoxazoline inhibits growth of E. coli.
Conclusion
This work continues to examine propargylic dihydrofuroisoxazolines whose syntheses were recently published by the corresponding author [11]. The furoisoxazolines underwent boron trihalide mediated ring openings to provide novel halogenated propargylic isoxazolines. The stereoselectivity and regioselectivity of the Lewis acid opened compounds were analyzed via NMR predictions and computational modeling. 13C calculations were helpful in assigning the corresponding complex 13C spectra of the isomers. Careful 2D NMR experiments allowed the assignment of the possible stereoisomers/regioisomers that were formed. Both the BCl3 and BBr3 ether cleavages demonstrated excellent regioselective for the benzylic halogen products (7 & 9), this is supported by the PM6 calculations for the possible intermediates and similar studies. The boron trichloride ring opening showed that it primarily followed a SN1 Lewis acid mediated pathway while the bromide version demonstrated products with greater stereoselectivity. The propargylic furoisoxazoline (6) demonstrated moderate growth inhibition of E. coli. when dissolved in 5% DMSO in LB broth. Unfortunately, the instability of the bromo- and insolubility issues for the propargylic chloro-isoxazoline limited the anti-microbial studies of those compounds. It is anticipated that in-depth further studies will include other aromatic and electron withdrawing substituents to increase both the stability and the biological activity. Toxicity studies for all the compounds will also be carried out. Additional antimicrobial experiments to include antifungal activities from these initial explorations of halogenated propargylic isoxazolines will also be explored from these preliminary findings.
Acknowledgement
Research reported in this publication was supported in part by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health [P20GM103443]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would also like to acknowledge Cameron Jensen, Jared Mays, and Brandon Gustafson for their contributions. The corresponding author would like to thank Dr. Mark Kurth and Dr. Arlen Viste for their continuing friendship and guidance.
References
- Kumar A, Fernandes J, Kumar P (2014) Synthesis and Biological Evaluation of Some Novel Isoxazoline Derivatives of Carbostyril. World J Pharm Pharmacuetical Sci 3 (2): 1267-1277.
- Kim HJ, Hwang KJ, Lee JH (1994) Synthesis and Fungicidal Activities of 4 H ,6 H -Furo [3,4- c] Isoxazole Derivatives as Potential New Fungicides. Biosci. Biotechnol. Biochem 58(6): 1191-1192.
- Zhu J, Mo J, Lin H, Chen Y, Sun H (2018) The Recent Progress of Isoxazole in Medicinal Chemistry. Bioorg Med Chem 26(12): 3065-3075.
- Indorkar D, Chourasia OP, Limaye SN (2012) Synthesis, Characterization, Antimicrobial, Antifungal Activity of Some s-Triazine Derivatives of Isoxazoline, Pyrazoline and PC Model Computational Studies. Res J Pharm Sci 1(4): 10-16.
- Kaur K, Kumar V, Sharma AK, Gupta GK (2014) Isoxazoline Containing Natural Products as Anticancer Agents: A Review. Eur J Med Chem 77: 121-133.
- Zhou X, Hohman AE, Hsu WH (2022) Current Review of Isoxazoline Ectoparasiticides Used in Veterinary Medicine. J Vet Pharmacol Ther 45(1): 1-15.
- Rae Kim H, Jin Kim H, Duffy JL, Olmstead MM, Ruhlandt-Senge K, et al. (1991) Double Diastereoselectivity in the Intramolecular Nitrile Oxide-Olefin Cycloaddition (INOC) Reaction. Tetrahedron Lett 32(34): 4259-4262.
- Duffy Jetty L (1993) An Investigation into the Diastereoselectivity of the Intramolecular Nitrile Oxide Olefin Cycloaddition vs. the Intramolecular Silyl Nitronate Olefin Cycloaddition. Ph.D. Thesis, University of California, Davis, Davis, CA, U.S.A.
- Duffy JL, Kurth MJ (1994) A Novel Intramolecular Silyl Nitronate Cycloaddition Route to Dihydrofuraldehydes and Dihydropyranaldehydes. J OrgChem 59(14): 3783-3785.
- Grandbois ML, Betsch KJ, Buchanan WD, Duffy-Matzner JL (2009) Synthesis of Novel 2H,5H-Dihydrofuran-3-Yl Ketones via ISNC Reactions. Tetrahedron Lett 50(47): 6446-6449.
- Stevens K, Schull A, Kaufman E, Stevens J, Shik KL, et al. (2024) Double Diastereoselectivity and Chemoselectivity of the Intramolecular Silyl Nitronate Cycloaddition with Allylic/Propargylic Nitroethers. Molecules 29: 5816.
- Jiang D, Xiao J, Zhang Y, Liu K, Li J, et al. (2024) Lewis Acid-Initiated Ring-Opening Reactions of Five- and Six-Membered Cyclic Ethers Based on the Oxonium Ylide Intermediates. Organics 5(3): 219-236.
- Kim HJ, Lee JH, Olmstead MM, Kurth MJ (1992) A Facile Synthesis of Furo[3,4-c]Isoxazoles: Precursors to 3,4-Disubstituted Isoxazoles. J Org Chem 57(24): 6513-6519.
- Namboothiri INN, Hassner A, Gottlie HE (1997) A Highly Stereoselective One-Pot Tandem Consecutive 1,4-Addition-Intramolecular 1,3-Dipolar Cycloaddition Strategy for the Construction of Functionalized Five- and Six-Membered Carbocycles (,)( 1). J Org Chem 62(3): 485-492.
- Cheng Q, Oritani T, Horiguchi T, Shi Q (1999) High Stereoselectivity in One-Pot Intramolecular Cycloadditions of Olefinic Silyl Nitronates. Eur J Org Chem 10: 2689-2693.
- Johnson BP, Jensen BJ, Ransom EM, Heinemann KA, Vannatta KM, et al. (2009) Interspecies signaling between Veillonella atypical and Streptococcus gordonii requires the transcription factor CcpA. J Bacteriol 191(17): 5563-5565.
- Stewart JJP (2007) Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J Mol Model 13(12): 1173-1213.
- Stewart JJP (2013) Optimization of Parameters for Semiempirical Methods VI: More Modifications to the NDDO Approximations and Re-Optimization of Parameters. J Mol Model 19(1): 1-32.
- Corey EJ, Feiner NF (1980) Computer-Assisted Synthetic Analysis. A Rapid Computer Method for the Semiquantitative Assignment of Conformation of Six-Membered Ring Systems. 2. Assessment of Conformational Energies. J Org Chem 45(5): 765-780.






























