The Impacts of Using Inorganic Chemical Fertilizers on the Environment and Human Health
Chali Abate Jote*
Department of Chemistry, Stream of Natural Sciences, Nekemte College of Teacher Education, Ethiopia
Submission: October 16, 2023;Published: November 27, 2023
*Corresponding author: Chali Abate Jote, Department of Chemistry, Stream of Natural Sciences, Nekemte College of Teacher Education, Post Box No. 88, Nekemte, Ethiopia
How to cite this article: Chali Abate J. The Impacts of Using Inorganic Chemical Fertilizers on the Environment and Human Health. Organic & Medicinal Chem IJ. 2023; 13(3): 555864. DOI: 10.19080/OMCIJ.2023.13.555864
Abstract
To meet the growing need for food, agricultural land per unit area is required to achieve maximum efficiency and the highest quality product. Plant nutrition is one of the most important factors to control agricultural productivity and quality. Rates of nutrients in the soil affect the quality of yield. In the permanent agricultural land, the soil will be very poor in nutrients, and as a result, inefficient. Therefore, fertilize the soil, combat pests, irrigation and process of agricultural activities to make more efficient to soil. Fertilization among these activities always remains a priority. However, excessive use of fertilizers is the need for additional land outside the public and environmental health effects. Excessive fertilization causes serious environmental problems (loss of biodiversity, heavy metal accumulation, water eutrophication, toxicity to different beneficial micro-organisms) and accumulation of nitrate, gases containing nitrogen and sulfur; giving and can lead to problems such as the greenhouse effect or global warming. In this review, aims to reveal environmental and health problems caused by improper fertilization and provides recommendation toward solving these problems.
Keywords:Chemical Fertilizers; Agriculture; Impact; Environment; Health problems
Introduction
The industrial revolution followed by the green revolution which fulfilled the food demands of the growing population caused an increase in yield per unit area in crop production, but they also increased the use of synthetic fertilizers in agriculture. Less soil fertility is one of the most vital constraints in improving agricultural production [1]. But the intensive use of inorganic fertilizer in agriculture worldwide for ensuring world food security caused so many health problems and unrecoverable environmental pollution.
In 1998/1999, total world consumption of nitrogen, phosphorus and potassium were 81, 14, and 18 tera-gram (1012g) per year, respectively [2]. 55% of the nutrients were used for cereal production, 12% for oilseed crops, 11% for grassland, 11% for commodities (e.g., cotton, sugar, and coffee), 6% for root crops, and only 5% for fruit and vegetable production. In 1950, fertilizers comprised only a small percentage of the nutrients needed for grain production, most of the supply being provided by the “natural fertility” of the soil and added manure [3]. By 2020, more than 70% of the grain yield will have to depend on fertilizers. The demand for plant nutrients is expected to increase continuously with population growth [4]. According to Keeney [4], the world population is expected to increase by about 2.3 billion by 2020 and double by the year 2050. If meat and food consumption in developed countries are matched by the rest of the world by the mid-21st century, then grain and nutrient demand are expected to triple [5]. Keeping in mind that the amount of land used for food production changed very slightly over the past few decades [3] and may even have decreased in parts of the world due to urbanization [5], the nutrient load per unit area is steadily increasing. All this implies that food production will have to be much more intensive and efficient than ever before.
Thus, to reduce and eliminate the adverse effects of synthetic fertilizers on human health and environment, nowadays a new agricultural practice has been developed called organic agriculture, sustainable agriculture or ecological agriculture [6]. Organic fertilizers are primarily cost-effective, easily available from locality products than chemical fertilizers [7]. Organic matter is the basis of soil fertility [8]. Microbial fertilizers are distinctly environment- friendly, non-bulk, cost-effective which plays a significant role in plant nutrition [9]. On the other hand, inorganic fertilizers are known for their high cost and their negative environmental effects if managed poorly [10]. All these give rise to reduced crop yields as a result of soil degradation and nutrients imbalance [11]. Some other technologies and management practices such as integrated nutrient management, using slow-release fertilizer or Nano-fertilizers, conservation tillage, cover cropping etc. can be adapted to supply balanced nutrients to plants. Fertilizers are very important for the crop growth, yield, quality parameters, even for soil health only when applied in optimum recommended dose or when used judiciously. Fertilizer improves the nutrient status and quality of soil by enriching it with nutrients which it lacks [12]. Crop plants require nitrogen, phosphorous and potassium to maintain the normal physiological function of the cell. In a similar way according to [13] lack of nitrogen results in poor growth and slow growth, but the excess use of nitrogen results in delayed maturity and low quality of leaf [14]. However intensive fertilizer application causes serious environmental problems, (for e.g., eutrophication of waters, loss of biodiversity, global warming and stratospheric ozone depletion), soil and plant health problems as some fertilizers also contains heavy metals, excess use of which leads fertilizer to enter the food chain via absorption from soil. Thus, fertilization leads to water, soil and air pollution [15].
Sources of Fertilizers
Fertilizer is any material of natural or synthetic origin (other than liming materials) that is applied to soils or to plant tissues to supply one or more plant nutrients essential to the growth of plants or to overcome the plant nutrient deficiency. Many sources of fertilizer exist, both natural and industrially produced. Any natural or manufactured material that contains at least 5% of one or more of the three primary nutrients (N, P, K) can be considered a fertilizer [16]. Industrially manufactured fertilizers are sometimes referred to as “mineral” fertilizers [17]. Fertilizers contain varying proportions of plant essential major (N, P, K) and minor (Zn, Mn, Fe, etc.) elements, as well as impurities and other non-essential elements. This definition includes both inorganic (mineral) and organic fertilizers and soil conditioners, such as lime and gypsum, which may promote plant growth by increasing the availability of nutrients that are already in the soil or by changing the soil’s physical structure. Fertilizers typically provide nutrients in varying proportions to plant growth and may be divided into three main categories. These are the primary macronutrients (N, P, K), the secondary macronutrients (Ca, Mg, S) and micronutrients (Cu, Fe, Mn, Mo, Zn, B, Si, Co, Cl) [16,18]. The importance of these elements to crop plants is described in Table 1.
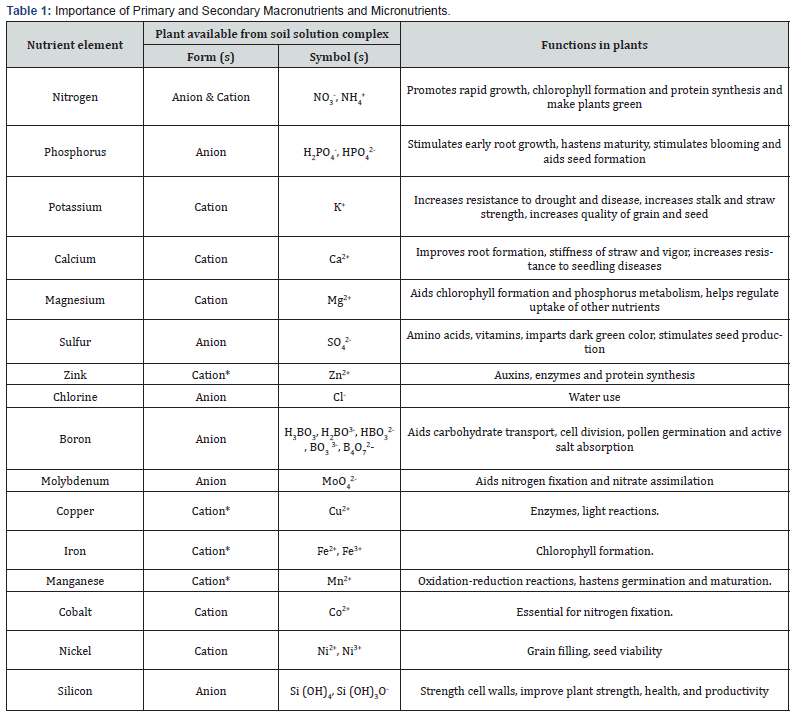
*Also available to plants in chelate form (a nutrient form having the essential nutrient linked to an organic compound so that it stays available for plant use within certain ranges of soil pH).
Classification of Fertilizers
Increased crop production largely depends on the type of fertilizers used for supplementing the essential plant nutrients [19]. The nature and the function of nutrient released from inorganic, organic and bio-fertilizers are different, also each type of fertilizer has its own advantages and limitations about crop growth and soil fertility [18]. Thus, sound fertilizer management must be done to ensure both an enhanced and safeguarded environment; therefore, a balanced fertilization strategy that combines the use of chemical, organic or bio-fertilizers must be developed and evaluated [20]. Fertilizers can be classified in numerous ways, like based on their nature [21]. Fertilizers based on their nature are classified as: Inorganic, Organic and Bio-fertilizers.
Inorganic Fertilizers: These include industrially synthesized fertilizers. Modern synthetic fertilizers are composed mainly of nitrogen, phosphorus, and potassium and they can be classified as Nitrogenous fertilizers (Urea, DAP, NH4NO3 & etc), Phosphorus fertilizers (super phosphate, triple phosphate, (NH4)3PO4, Nitro phosphate & etc.) and Potassium fertilizers (KCl, K2SO4, KNO3 & etc.) [17].
Organic Fertilizers: Fertilizers derived from living or formerly living materials or biological in origin. They are also known as organic manures, which are classified as farmyard manure, compost, and green manures. These organic fertilizers may be obtained from animal wastes (dung, urine & kitchen wastes), plant wastes (weeds, straw, sugarcane reuse, rotten vegetables & crop stubble, groundnut & rice husk), treated sewage sludge and leguminous plant [22].
Bio-Fertilizers are products that contain living microorganisms, which exert direct or indirect beneficial effects on plant growth and crop yield through different mechanisms. Biofertilizers containing biological nitrogen fixing organisms are of utmost importance in agriculture. Advantages of these fertilizers are help in the establishment and growth of crop plants and trees, enhance biomass production and grain yields by 10-20 %, useful in sustainable agriculture and suitable in organic farming. Some of the Bio-fertilizers are rhizobium, azotobacter, azospirillum, blue green algae, azolla, mycorrhize, & etc. [10].
Impacts of Chemical Fertilizers on the Environment
The world agricultural systems is using a large number of chemicals such a fertilizers, pesticides, herbicides to achieve more production per unit area but using more doses than optimum or recommended of these chemicals and fertilizers leads to several problems like environment pollution (soil, water, air pollution), reduced input efficiency, decreased food quality, resistance development in different weeds, diseases, insects, soil degradation, micronutrient deficiency in soil, toxicity to different beneficial living organism present above and below the soil surface, less income from the production, etc. [12]. Despite these many problems, there is also a challenge to meet the food demands of the world’s growing population. Therefore, there is a need to produce nutrition rich and chemicals free agricultural produce for the human and animal consumption without deteriorating are natural resources that is why emphasis should be laid on the production of food rich in quality as well as quantity [20]. Fertilizer use is no doubt beneficial to plant in providing deficient nutrients; also, they have several other conveniences such as the cheaper source of nutrient, higher nutrient content and its solubility hence immediate availability, then it’s required in less amount, which makes it more acceptable than organic fertilizer. There is abundance of evidence that inorganic fertilizers can improve the yield of crops significantly [16]. Fertilizers raise soil fertility so that the yield of crops is independent and no longer limited by the deficient amounts of plant nutrients [23]. Despite these benefits, fertilizer has several negative effects on the environment because of its growing consumption and lowering nutrient use efficiency. Therefore, the major challenge in intensive agricultural production systems is to combine intensive cultivation with high nutrient use efficiency [24].
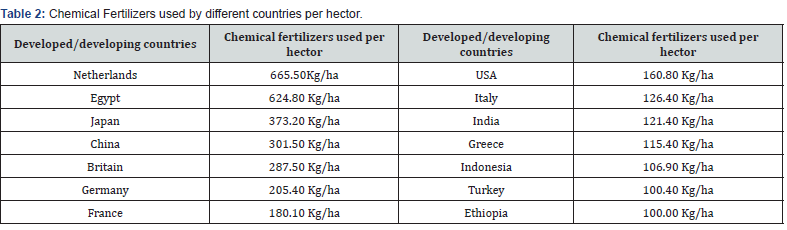
The use of chemical fertilizers in developed and developing countries per hectare were different due to their soil fertility. These were shown in Table 2 [25]. Soil nutrient level gets decreased over time when crop plants get harvested, and these nutrients get replenished either through natural decomposition process or by adding fertilizers. Hence fertilizer is an essential component of modern agriculture [12]. But though chemical fertilizers are the major cause of sufficient crop production for the world population their overuse is bringing serious challenges to the present and future generations like polluted air, water, and soil, the degraded lands, depleted soils and increased emissions of greenhouse gases. These synthetic fertilizers are not only becoming hazardous for our environment but also to humans, animals and to the microbial life forms too [8]. It’s high time that everyone understands the ill effects of using excess chemical fertilizers and take initiatives for reducing the use of chemical fertilizer and pesticides substituting it with other organic amendments like organic manures which not only provides essential nutrients to the plants but also maintains the soil health for the subsequent crops [22].
Effects on Soil
According to research and studies the effects of chemical fertilizers on the soil are not immediately obvious. Because soils have strong buffering power due to their components. Over time, it states that emerged from the pollution, deterioration of soil fertility, soil degradation reactions occurring in the soil leads to deterioration of the balance of the current element [26]. In addition, toxic substances accumulate within the vegetables and cause negative effects in humans and animals are fed [27]. Soil structures in agricultural productivity are very important and it is regarded as an indicator. Unconsciously, the fertilizing of soil, just as in the deterioration of the structure, is caused by industrial emissions. Especially NaNO3, NH4NO3, KCI, K2SO4, NH4Cl demolish the structure, such as fertilizers, soil, soil structure, deterioration is difficult to obtain high-quality and efficient product [26]. Particularly high level of sodium and potassium containing fertilizers, make a negative impact on soil, pH, soil structure deterioration and the increasing feature of acid irrigation or other agricultural operations or from the benefits derived from it is not possible or very scarce [28]. The overuse of chemical fertilizers can lead to soil acidification and soil crust thereby reducing organic matter content, humus content, beneficial organisms, stunting plant growth, can change the soil pH, increase pests, and even contribute to the release of greenhouse gases. The soil acidity diminishes phosphate intake by crops, increases the toxic ion concentration in the soil, and inhibits crop growth [12], [29]. The depletion of humus in the soil reduces its ability to store nutrients.
Nitrogen applied to fields in large amounts destroys the balance between the three macronutrients (N, P, K) over time which would result in lack of micronutrients; it also damages topsoil, resulting in reduced crop yields. In addition, limit the activities of nitrifying bacteria [26]. Sandy soils are much more prone to soil acidification than clay soils. Clay soil can buffer the effects of excess chemical fertilization. Repeated applications of chemical fertilizer may result in a toxic buildup of heavy metals such as arsenic, cadmium, and uranium in the soil. These toxic heavy metals not only pollute the soil but also get accumulated in food grains, fruits and vegetables. For example, Fertilizers like Triple superphosphate has trace elements like cadmium and arsenic that accumulate in plant and through food chains reach to human that may cause health problems. The effects of chemical fertilizers on soil are great and irreversible [15]. Given large amounts of potassium fertilizers in the soil of Ca and Fe with Zn disrupt the balance of nutrients by the plants and prevent the receipt. However, the negative effects on organisms, given the variety of worms and soil mite has been devastating and lethal effect [28]. Fertilizer application without the using soil testing recommendation can lead to implications such as soil degradation, nutrient imbalance, destruction of soil structure, increasing bulk density [30]. Fertilizers more than the recommended amounts cause formation, accumulation and concentration of mineral salts of fertilizers which leads to compaction layer and soil degradation in the long-term [26].
Effects on Water
Nitrogen in agricultural areas reaches the water environment in three ways: Drainage, leaching and flow. Nitrate leaching is particularly linked to agricultural practices such as fertilizing and cultivation. Irrigated agricultural land in some of the arid and semiarid regions, increased amounts of nitrate accumulation in the soil used and along with the evaporation of water. According to the conditions, nitrate accumulated leached in varying amounts. It reaches the depth of soil. In the soil, fertilizers are converted to nitrate through nitrification by microorganisms. Due to negatively charged nitrate can reach ground water. Even in ideal conditions, Plants use 50% of nitrogenous fertilizers applied to soil, 2-20% lost evaporation, 15-25% react organic compounds in the clay soil and the remaining 2-10% interfere surface and ground water [15, 31].
Most nitrogenous fertilizers aren’t absorbed products, and they interfere with both underground and surface water. Groundwater nitrate problem should be considered in a global context. 22% of cultivated areas in Europe for the internationally recommended drinking water nitrate concentration in groundwater concentration (≥ 11.3 mg/L) above. In European Countries, NO3- N concentration value is 23 mg/L and in the USA, it is 45 mg/L. NO3- and NH4+ concentration, Nottingham, United Kingdom exceeds the stated limits. The city of Nottingham is underlain by the unconfined Sherwood aquifer, which is vulnerable to contamination from various sources arising from urban and industrial activities of the region. According to that study, samples of aquifer recharge, both artificial and natural, and of shallow and deep groundwater were collected to determine the sources and level of contamination from nitrogen species. Deep groundwater contains low concentrations of ammonium (less than 0.3 N mg /L) throughout, however much higher nitrate concentrations (< 1.0 mg /L N to 28.0 mg/L N). Most remaining groundwater samples have a nitrogen fertilizer source, possibly derived from an influent river draining a rural catchment [32,33]. Similarly high concentrations of NO3- and NH4+ have also been reported in the USA. One of the most important parameters of the pollution of water is nitrate which is the basic component of fertilizer. Both the nitrate concentration of groundwater and surface water is increased by agricultural activities. Nitrate is the most common form of dissolved nitrogen present in groundwater or other water bodies. However, it can be found in the form of nitrite (NO2-), nitrogen (N2), nitrogen oxide (N2O) and organic nitrogen [34].
One of the most important negative effects of intensive fertilizer use is water eutrophication. The primary factor responsible for eutrophication is phosphate. Surface waters should contain ≤ 50 μg/L phosphorus. Nitrogen can also become a factor for eutrophication when increased biomass growth takes place [21]. Eutrophication results in increased growth of aquatic plants and algae in the water body covering the whole water body leading to the loss of other aquatic living species like fish due to the reduced oxygen supply. Eutrophication in the bottom layer, oxygen-free environment as a result, not suitable for drinking and water supply, reduction in the number of living species in the aquatic environment, proliferation of unwanted species and the media appear to be unsuitable for recreation due to bad odor, polluted water etc. [15, 35].
Effects on Air
High application rates of chemical fertilizer for enhancing crop production are generating numerous harmful greenhouse gases, depleting the protective ozone layer hence exposing humans to harmful ultraviolet rays [26]. Agriculture accounts for 60% of anthropogenic N2O emissions, and agricultural soils are the dominant source [34]. Greenhouse gases like CO2, CH4 and N2O are produced during the manufacture of nitrogenous fertilizer. The effects can be combined into an equivalent amount of CO2. Nitrogen fertilizer can be converted by soil bacteria into nitrous oxide, a greenhouse gas. Nitrogen fertilizer whose excess use results in an emission of nitrogen oxides (NO, N2O, NO2) is responsible for severe air pollution [29]. Other gases also responsible for the ozone depletion are H2O vapor, CO2, CH4, H2S and CFC’s [18]. Nitrous oxide (N2O) has become the third most important greenhouse gas after carbon dioxide and methane and increased from 0.2 to 0.3% each year. Its global warming potential is 310 times more than that of carbon dioxide. The main concern regarding the emission of nitrous oxides has to do with the effect of global warming and the role of nitrous oxides in ozone destruction that consequently leads to atmospheric “holes,” thus exposing humans and animals to excessive ultraviolet radiation [36].
Ammonia volatilized or emitted from fertilized lands, gets deposited in atmosphere and oxidized to become nitric acid, sulfuric acids, creating acid rain after the chemical transformations. Acid rain can damage vegetation and buildings; also, can damage organisms that live in both lakes and reservoirs [18]. Methane emissions from transplanted paddy fields are also a serious concern, as methane is a potent greenhouse gas, and its concentration is increased by the application of ammonium-based fertilizers. All these emissions contribute to global climate change [24].
Other Deleterious Effects
Excessive use of chemical fertilizer, especially nitrogen, can contribute to crop tip browning, lower leaf yellowing, wilting and crop lodging. When fertilizer scorches roots, the root may blacken and go limp. All these symptoms occur due to salt accumulation in the soil which would cause difficulty in water absorption by plants. Over application to plants also may cause the leaves to turn yellow or brown, damaging the plant and reducing the crop yield. Using higher doses of nitrogen fertilizers in malt barley may cause undesirable effect on quality of the beer [14]. The excessive accumulation of nitrate or nitrite in plant parts consumed by humans or animals is likely to cause the same detrimental effects associated with nitrate contamination of water sources [30]. Over fertilization effects also reduce the biodiversity resulting from ammonia deposition in forests and waters [27]. They reduce the mycorrhizal root colonization and inhibit symbiotic nitrogen fixation by rhizobia due to high nitrogen fertilization. Nutrients are easily lost from soils through fixation, leaching or gas emission and can lead to reduced fertilizer efficiency [20].
Effects on Human Health
Nitrates from drinking water of the body are absorbed in the intestinal tract 4-12hr and are excreted by the kidneys. The mechanism, as well as the salivary glands can concentrate nitrate.
As a result, the mouth is reduced to nitrite in the anaerobic
environment. It is possible to examine the toxicological effects
of nitrate in three stages. The primary toxic effect of nitrate
concentrations in drinking water exceeds of 50 mg /L NO3- the
value of the bowel in adults, digestive and urinary systems,
inflammation is seen. Seconder toxicity, high nitrate concentration
in drinking water caused disease methemoglobinemia in
infants and in ruminants, gastric cancer and other diseases
such as goiter, birth defects and heart disease [31]. Stomach
acid does not occur in infants younger than six months. In this
environment, nitrite reacts with hemoglobin in the blood and
is minimized methemoglobin consists of nitrite in the digestive
system. Meanwhile, iron contained in hemoglobin and blood
oxygen transport function lost. As a result, infants are found
strangled to death. Advancing age, it is eliminated because of the
increase in stomach acids. Toxicity in acid medium of secondary
and tertiary amines tertiary nitrites, alkyl ammonium bases and
react accordingly amides occurs as a result nitrosamines occurs,
because of this and nitrosamines, which consists of carcinogenic
substances [15,32]. The food produced using chemical fertilizers
also has very adverse effects on the health of humans as well as
animals [37].
i. Some other diseases like wheezing, nausea, lung
infections are also the result of deep inhaling and long-term
exposure.
ii. Due to the exposure with the chemical residues people
meet depression, insomnia, oral acetomatism, myoclonus and
hyper reflexia.
iii. Plants having excess quantity of nitrogen accumulation
causes infant diseases and methemoglobinemia, also the produced
amines from the N2 based fertilizers can cause cancer.
iv. Due to the higher aluminium exposure asthma is caused
together with Alzheimer’s and bone diseases.
v. Other Diseases like neurological toxicity, growth
retardation, cognitive delay, and damage to the nervous system
are caused due to the exposure to calcium.
vi. Lung damage occurred due to the extensive exposure to
cobalt and boron causing low sperm count, nose throat and eye
irritation.
Using Other Alternatives in Steady of Chemical Fertilizers
Excessive use of the chemical fertilizer for a long time on the same soil may lead to soil degradation, loss of beneficial soil microorganisms, and many other losses as discussed above [38]. Therefore, to ensure both the enhanced and sustainable agricultural production and to safeguard the environment integrated use of different types of nutrient suppliant such as organic manures, bio-fertilizers and other slow released or controlled released fertilizers should opt [22,39].
Organic Manures: the use of organic fertilizers together with chemical fertilizers, compared to the addition of organic fertilizers alone, had a higher positive effect on microbial biomass and hence soil health [19]. These are techniques that maintain and enrich the soil fertility and the soil humus content should be used like using compost, manure, agro-forestry, green manure, mulch manure etc.
Bio-Fertilizer: Bio-fertilizer is a substance which contains living micro-organisms and is known to help with the expansion of the root system and better seed germination. A healthy plant usually has a healthy rhizosphere which should be dominated by beneficial microbes. Bio-fertilizers differ from chemical and organic fertilizers in the sense that they do not directly supply any nutrients to crops and are cultures of special bacteria and fungi. The production technology for bio-fertilizers is relatively simple and installation cost is very low compared to chemical fertilizer plants [20].
Slow-Release Fertilizers: It involves the release of the
nutrient in a slower manner than common fertilizers. However,
the rate, pattern, and duration of release are not well controlled.
But the rate, pattern, and duration of release are well known in
controlled release fertilizers [40]. Different types of slow or
controlled release fertilizers are:
a. Organic-N low solubility compounds for e.g., Ureaformaldehyde
and Isobutyledene-diurea.
b. Fertilizers in which a Physical Barrier Controls the
Release for e.g., the coated fertilizers coated with organic polymer
coatings that are either thermoplastic or resins and fertilizers
coated with inorganic materials such as sulfur or mineral based
coatings etc.
c. Inorganic low solubility compounds: Fertilizers such as
metal ammonium phosphates and partially acidulated phosphates
rock.
Nano-Fertilizers are synthesized or modified forms of traditional fertilizers, fertilizers bulk materials or extracted from different vegetative or reproductive parts of the plant by different chemical, physical, mechanical or biological methods with the help of nanotechnology used to improve soil fertility, productivity and quality of agricultural produce. Nanoparticles can be made from fully bulk materials. For example, nano-TiO2 treated seed produced plant recorded more dry weight, higher photosynthetic rate, chlorophyll formation compared to the control [41].
Application Efficiency: application of any fertilizer should be done at an economic rate other than optimum rate. Also, application from right source, rate, placement and time will reduce the adverse effect on both the crop and the environment [30].
Conclusion
Today, use of fertilizers is seen as a necessary agricultural technology. Because soil restores nutrients and promotes crop growth and yield. But, to reduce the different kinds of hazards taking place due to excessive use of fertilizers, judicious and sustainable use of fertilizers should be made for that firstly soil testing and analysis should be done properly and then, fertilizer should be given to soil. Therefore, to ensure both the enhanced and sustainable agricultural production and to safeguard the environment, integrated use of different types of nutrient suppliant such as chemical fertilizer, organic manures, bio-fertilizers and other slow released or controlled released fertilizers should be adopted. To eliminate the pollution hazards due to chemical fertilizers, improved nutrient uses efficient fertilizers particularly nitrogen should be adopted by using organic manures, controlledrelease or slow-release fertilizers. Using different Nano-fertilizers which have the greater role in enhancing crop production will reduce the cost of fertilizer for crop production and minimize the pollution hazard.
References
- Ayoub AT (1999) Fertilizers and the Environment. Nutrient Cycling in Agro-ecosystems 55: 117-121.
- WFCSA (1999) World Fertilizer Consumption Annual Statistics.
- Kaarstad O (1950) Fertilizer’s significance for cereal production and cereal yield from.
- Keeney D (1990) What goes around comes around- the nitrogen issues cycle. In Third Int. Dahlia.
- Greidinger (1997) Sym. on Fertilization and the Environment. Technion, Haifa, Israel.
- Keeney DR, Follett RF (1991) Managing nitrogen for ground water quality and farm profitability: Overview and introduction. In Managing Nitrogen for Ground Water Quality and Farm Profitability.
- Aksoy U (2001) Ecological Farming. II. Ecological Farming Symposium in Turkey, Antalya, pp: 14-16.
- Solomon WGO, Ndana RW, Abdulrahim Y (2012) The Comparative study of the effect of organic manure cow dung and inorganic fertilizer NPK on the growth rate of maize (Zea mays L.). Int Res J Agri Sci Soil Sci 2(12): 516-519.
- Aboudrare A (2009) Agronomie Durable, Principes ET Pratiques. Rapport de Formation Continue. FAO.
- Mahajan Gupta RD, Sharma R (2008) Bio-fertilizers-Away to sustainable agriculture. Agrobios Newsletter 6: 36-37.
- Hijbeek R, Ten Berge H, Whitmore A, Barkusky D, Schroder JJ, et al. (2018) Nitrogen Fertilizer replacement values for organic amendments appear to increase with N application rates. Nutrient Cycling in Agroecosystems. 110(1): 105-115.
- Cooke G (1982) Fertilizing for maximum yield. Third Edition English Language Book society/Collins, pp. 465.
- Omidire Niyi S, Raymon Shange, Victor Khan, Russell Bean, Jewel Bean (2015) Assessing the impacts of Inorganic and organic fertilizer on crop performance under a micro-irrigation-plastic mulch regime. Professional Agri Work J 3(1): 1-9.
- Mani J (2002) Early events in environmental stresses in plants: Induction mechanisms of oxidative stress. In: Inzè D and Montague MV (eds.) Oxidative stress in plants. Taylor and Francis, New York 217-246.
- Sonmez Kaplan M, Sonmez S (2007) An investigation of seasonal changes in nitrate contents of soils and irrigation waters in greenhouses located in Antalya-Demre region. Asian J Chemist 19(7): 5639-5646.
- Scherer HW, Konrad M, Heinrich D, Manfred D, Ralf V, et al. (2005) Fertilizers. Ullmann's Encyclopedia of Industrial Chemistry.
- Helen NN (1991) Industrial Chemistry. University of Nairobi, Kenya, Nairobi.
- Sharma A, Chetani R (2017) A Review of the Effect of Organic and Chemical Fertilizers on Plants. Int J Res Appli Sci Engineer Techn (IJRASET) 5(2): 677.
- Usman M, VU Madu, Alkali G (2015) The combined use of organic and inorganic fertilizers for improving maize crop productivity in Nigeria. International Journal of Scientific and Research Publication 5(10): 1-7.
- Trenkel Martin E (1997) Controlled release and stabilized fertilizers in agriculture. Paris: International Fertilizer Industry Association.
- Neue Heinz-Ulrich (1993) Methane emission from rice fields. Bioscience 43(7): 466-474.
- Pandiselvi T, Jeyajothiand R, Kandeshwari M (2017) Organic nutrient management a way to improve soil fertility and Sustainable Agriculture. A review Int J Adv Life Sci 10(2): 175-181.
- Ojeniyi SO (2000) Effect of Goat Manure on Soil Nutrients and Okra Yield in the Rain Forest Area of Nigeria. Applied Tropical Agriculture 5: 20-23.
- Chen & Jen-Hshuan (2006) The combined use of chemical and organic fertilizers and/or bio-fertilizer for crop growth and soil fertility. In International workshop on sustained management of the soil- rhizosphere system for efficient crop production and fertilizer use, pp. 1-11.
- FAO (2009) Resource STAT-Fertilizer. Food and Agriculture Organization of the United Nations.
- Savci Serpil (2012) An agricultural pollutant: chemical fertilizer. Int J Environ Sci Develop 3(1): 73.
- Nelson DW (1984) Effect of nitrogen excess on quality of food and fiber. Nitrogen in Crop Production (RD Hauck, Ed.), pp: 643-661.
- Manik CK, Biswapati M (2008) Assessment of potential hazards of nitrate contamination in surface and ground water in a heavily fertilized and intensively cultivated district of India, Environ Monit Assess 146(1-3): 183-189.
- Cooper J, Eleanor R, Stefan H, Thomas L, Anne-Kristin L, et al. (2017) Phosphorus availability on many organically managed farms in Europe. Nutrient Cycling in Agro-ecosystems 110: 227–239.
- Bhattacharyya R, Birendra N, Pradeep D, Prasanta K, Priyabrata S, et al. (2016) Soil conservation issues in India. Sustainability 8(6): 565.
- Feigin A, Halevy J (1989) Irrigation-fertilization cropping management for maximum economic return and minimum pollution of ground water. Research report, Inst. Soil Water, ARO, The Volcani Center, Bet Dagan.
- Gross M J, Barry D, Rudolph D (1998) Contamination in Ontario Farmstead Domestic Wells and Its Association with Agriculture. 1. Results from Drinking Water Wells. Journal of Contaminant Hydrology 32(3-4): 267-293.
- Karacaoglu F, Günay G (1997) Groundwater Nitrate Pollution in An Alluvium Aquifer, Eskisehir Urban Area and Its Vicinity, Turkey. Environmental Geology 31: 178-184.
- Shoji S, Delgado J, Mosier A, Miura Y (2001) Use of controlled release fertilizers and nitrification inhibitors to increase nitrogen use efficiency and to conserve air and water quality. Communications in Soil Science and Plant Analysis 32(7-8): 1051-1070.
- Rivers CN, Barrett MH, Hiscock KM, Dennis PP, Feast NA, et al. (1996) Use of Nitrogen Isotopes to Identify Nitrogen Contamination of The Sherwood Sandstone Aquifer Beneath the City of Nottingham, UK. Hydrogeology J 4: 90-102.
- Rütting T, Aronsson H, Delin S (2018) Efficient use of nitrogen in agriculture. Nutrient Cycling in Agro-ecosystems 110: 1-5.
- Wimalawansa SA, Wimalawansa SJ (2014) Impact of changing agricultural practices on human health: chronic kidney disease of multi-factorial origin in Sri Lanka. Wudpecker J Agricult Res 3: 110-124.
- Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, et al. (1997) Human Alterations of the Global Nitrogen Cycle: Sources and Consequences: Ecological Applications 7(3): 737-750.
- Rai N, Ashiya P, Singh RD (2014) Comparative Study of the Effect of Chemical Fertilizers and Organic Fertilizers on Eiseniafoetida. Int J Innovat Res Sci Engineer Tech 3(5): 12991-12998.
- Shaviv A (2000) Advances in Controlled Release Fertilizers, Advances in Agronomy.
- Singh MD, Chirag G, Prakash PO, Mohan MH, Prakasha Vishawajith G (2017) Nano-Fertilizers is a New Way to Increas Nutrients Use Efficiency in Crop Production. Int J Agricult Sci 9(7): 3831-3833.






























