DDX3 Inhibitors: A Review of their Potential as an Anticancer and Antiviral Agents
Manikandan Palrasu1, Mohd Imran Ansari1, Aishwarya Sharma1, Scotty Branch1,2,3, Shea Harrelson1,2,3 and Saeed Khan1,3*
1KOR Life Sciences, USA
2KOR Medical, USA
3Vikor Scientific, Charleston, USA
Submission: June 16, 2022;Published: July 19, 2022
*Corresponding author: Saeed Khan, Department of Drug Research and Development, KOR Life Sciences/VikorScientific, 22 WestEdge Street, Charleston, SC, 29403, USA
How to cite this article: DDX3 Inhibitors: A Review of their Potential as an Anticancer and Antiviral Agents. Organic & Medicinal Chem IJ. 2022; 11(5): 555822. DOI: 10.19080/OMCIJ.2022.11.555822
Abstract
DDX3 (DEAD-box RNA helicase 3) is a multifunctional protein that has ATPase and RNA helicase activity. DDX3 plays a major role in RNA metabolism and several other several indispensable cellular processes. Over the past several decades, many studies demonstrated the role of DDX3 in the progression of several diseases including viral infection, and cancer. DDX3’s role in cancer is contradictory and ambiguous. DDX3 enhances viral replication by increasing the export of viral RNA from nucleus to cytoplasm and viral RNA translation. Oncogenic DDX3 is highly expressed in many cancers. Because of its oncogenic, anti-apoptotic and virus replicative potential, DDX3 has emerged as a promising target for the development of anticancer and anti-viral agents. A plethora of small molecular inhibitors for DDX3 have been discovered and RK-33 is the most investigated DDX3 inhibitor against cancer. This article discusses the complex relationships between DDX3 and its oncogenic signaling mechanisms, as well as the possibility of targeting DDX3 for the treatment of cancer and viral infection.
Keywords: DDX3 inhibitor; RNA; Anti-viral agents; Anticancer; Drugs
Introduction
DEAD-box RNA helicase 3 (DDX3), a highly conserved family member of DEAD-box proteins plays a crucial role in numerous aspects of energy-dependent RNA metabolism including RNA splicing, RNA export, transcription and translation initiation in all eukaryotes [1,2]. DDX3 is a multifunctional protein involved in a variety of physiological functions, including stress response, cell death, cell cycle control, and virus infection. Dysregulation of DDX3 has been linked to diverse disease entities including cancer and viral infection [1,2]. The role of DDX3 in cancer progression and viral replication has recently ignited a fervent exploration on its potential anti-cancer and anti-viral properties. Hundreds of small molecules have been developed to target the ATPase and helicase activity of DDX3 proteins. In this mini review, we summarize and discuss the roles of DDX3 in cancer and viral infection, and we discuss the potential antiviral and anticancer effects of drugs targeting DDX3.
The Role of DDX3 in Viral Infection
DDX3 is involved in the nucleocytoplasmic export of viral RNA (vRNA) as well as vRNA translation in the cytoplasm during viral infections, promoting viral replication [3-5]. There is overwhelming evidence to indicate that the DDX3 helicase is involved in the replication of numerous viruses including HCV, Dengue virus, HIV, hepatitis B virus (HBV) and several others [3-6]. DDX3, on the other hand, participates in the antiviral innate immune signaling system via type I IFN induction mediated by TBK1/IKK. In turn, the viruses try to outsmart the host’s defensive system by targeting DDX3 [7]. DDX3 detects abortive HIV-1 RNA transcripts in dendritic cells and triggers type I interferon immune responses via the mitochondrial antiviral signaling protein [8]. As a result of its critical role in modulating innate immunity and viral replication, it was quickly exploited as an antiviral target.
The Role of DDX3 in Cancer
DDX3’s role in cancer is contradictory and ambiguous. DDX3 demonstrates both oncogenic and tumor suppressor properties depending on cytoplasmic expression levels and its involvement in signaling pathways that involve the proliferation of different cancers. It has been reported that DDX3 expression was localized to the cytoplasm in various cancers including skin and liver cancer, however, nuclear localization was found in paired normal skin and liver tissue [1,2,9,10]. Accumulating studies have demonstrated the oncogenic function of DDX3 in the development of a variety of cancers [1,2]. One of the most important oncogenic functions of DDX3 is its involvement in the Wnt/β-catenin signaling pathway, a signal transduction cascade involved in developmental processes and various cancers [11-13]. A growing body of evidence suggests that breast cancer, glioblastoma, medulloblastoma, prostate, head and neck, and liver cancer have all been associated to a dysregulated or mutated Wnt/β-catenin signaling pathway that promotes cancer cell proliferation and growth [13,14]. When Wnt/β-catenin signaling is activated, β-catenin is localized in the nucleus, and many target genes involved in cell proliferation, cell adhesion, cell invasion, metastasis, and angiogenesis, such as Snail1, MMP7, PAI1, RAS, and Fibronectin, are dysregulated. It has been reported that mutation in DDX3 activates β-catenin signaling pathways in medulloblastoma by binding to the TCF/LEF promotor [13]. DDX3 also enhances cell proliferation, adhesion, motility and metastasis through Rac1- β-catenin signaling pathways [11]. In addition, DDX3 enhances KRAS-mediated β-catenin/ ZEB1 signaling to promote KRAS-mediated tumor invasion [15]. DDX3 promotes the epithelial-mesenchymal-like transformation (EMT) process in various cancers through several mechanisms. The mechanism includes 1. Activation of β-catenin signaling pathways 2. HIF-1α-transcriptionally increases the expression of DDX3, which decreases E-cadherin levels either directly or increasing Snail expression. 3. DDX3 increases cell aggressiveness via a DDX3/KRAS/ROS/HIF-1α/DDX3 cascade feedback loop or the KRAS/HIF-1α/YAP1/SIX2 cascade (Figure 1) [10-12,16-18]. Figure 1 depicts the DDX3 mediate oncogenic signaling pathways. These findings collectively demonstrate that DDX3 performs an oncogenic role in cancer cells, enhancing the EMT process.
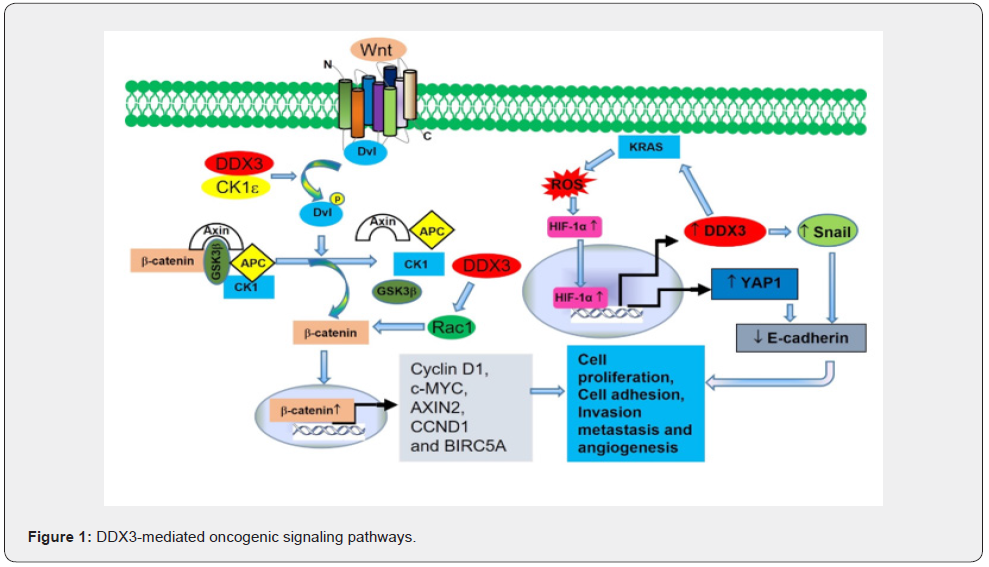
DDX3 was discovered to be involved in apoptosis evasion, a key hallmark of cancer cells. Li et al found that the tumor cell resistance to apoptosis against TRA-8, an anti-TRAILR2 antibody was due to blockade of TRAIL-R2 (a novel apoptosis-mediating receptor for TRAIL) by DDX3 [19]. DDX3 suppresses apoptosis by reducing caspase 3 activation in cells expressing mutant or non-functional p53 [20]. DDX3 inhibits apoptosis by forming a death antagonizing signaling complex with GSK3 and cIAP-1 at each of the four major death receptors (Fas, TNF-R1, TRAIL-R1, and TRAIL-R2) [21]. Inhibition of DDX3 caused G1 cell cycle arrest and induced apoptosis suggesting its oncogenic role in cancer progression [22,23]. However, the role of DDX3 remains controversial, as few studies suggested that loss of DDX3 promotes tumor growth and proliferation. DDX3 exerts its tumor suppressive roles on the cell cycle and apoptosis by p53-DDX3 mediated transcriptional activation of p21 [9]. Furthermore, DDX3 functions as a tumor suppressor by increasing the tumor suppressor E-cadherin and impeding the EMT progression through sp1-MDM2 mediated suppression of Slug, which negatively regulates the expression of E-cadherin [24,25]. Given its diametrically opposed functions, more research is needed to figure out what the mechanisms are behind DDX3’s dual role in cancer biogenesis.
DDX3 Inhibitors as Potential Antiviral and Anticancer Drugs
Because of its oncogenic function in cancer proliferation, invasion, and metastasis, DDX3 could be a promising target for cancer treatment. Hundreds of small compounds targeting the ATPase/helicase of the DDX3 helicase have been developed over the last two decades. FE-15 was the first small molecule developed to suppress the HIV-1 replication by inhibiting the ATPase activity of human DDX3. Even though these small compounds have been shown to reduce viral replication and cancer cell growth by inhibiting DDX3, they have low activity and selectivity for DDX3 [26,27]. Yedavalli et al developed a series of ring expanded nucleoside (REN) analogues that demonstrated to inhibit human immunodeficiency virus type 1 (HIV-1) replication via inhibiting RNA-Helicase activity of DDX3 [28]. Subsequently, secondgeneration inhibitors of the cellular ATPase DDX3 including FE87, FE98, FE109, triazine, and pyrazolo-analogs were developed with improved anti-viral activity [27]. Notably, Radi et al. discovered a series of diarylurea analogs utilizing homology model of hDDX3 crystalized with AMP that block the helicase activity of DDX3 and hence the replication of HIV-1 [29]. Using this homology model, a series of diarylurea derivatives were docked within the hDDX3 pre-RNA binding pocket and found that NH groups of urea functionality of the molecules acted as a hydrogen bond donor for the backbone carbonyl oxygen of Pro274 and Gln360 [29]. Brai and coworkers developed a series of triazole containing diarylurea derivatives that inhibits broad range of viral replication in infected cells. Interestingly, the lead compound had complete effectiveness against drug-resistant HIV-1 strains while causing less cellular damage, implying that DDX3 inhibitors could be a promising antiviral treatment for treating individuals with drugresistant viruses [30]. Following that, the same research group developed diarylurea-containing trazole compounds, with BA-103 being the most active with an IC50 of 0.4 micromolar, targeting DDX3X helicase activity [31,32]. FHP01 (BA103) also suppresses the growth of various breast and glioblastoma cells and in vivo breast cancer and glioblastoma xenograft and orthotopic models via inhibiting DDX3- β-catenin signaling pathways [31,32].
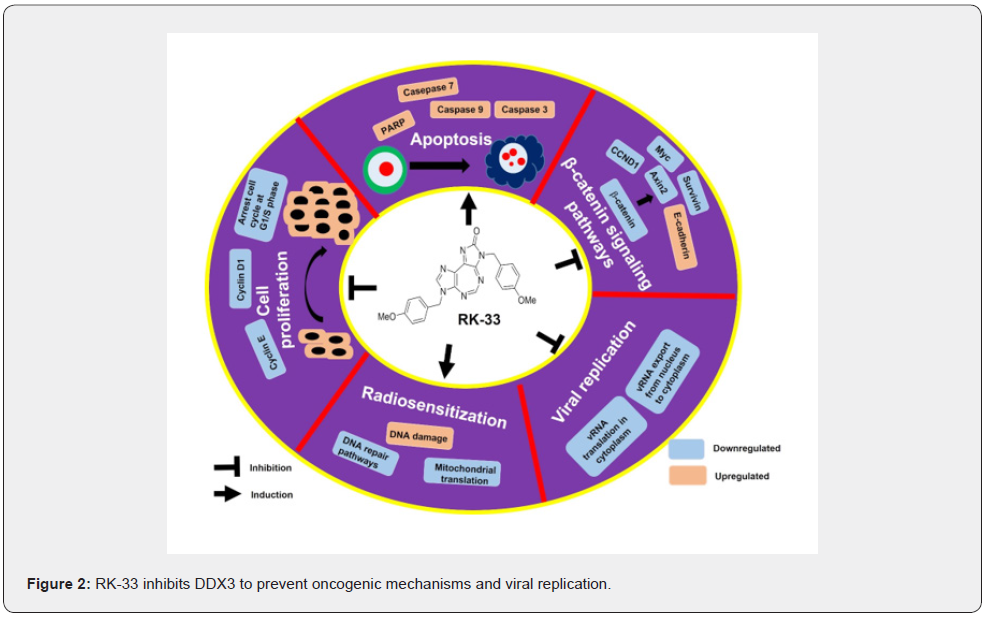
Although REN analogs were investigated for their antiviral properties, NZ51, the most active of the REN analogs and one of the first molecules tested against cancer, did not cause DDX3 to degrade, but it did significantly reduce its functionality, inhibiting breast cancer cell motility and viability. However, this compound had no effect on primary tumor growth in mouse model suggesting a lack of effective drug delivery in the animals [33]. Following that, REN analogues were chemically altered, and a series of compounds were synthesized, with RK-33 being the most active and well-studied DDX3 helicase activity inhibitor against various cancers and viruses. In vitro and in vivo studies have shown that RK-33 inhibits the growth of many cancers including Ewing sarcoma, breast cancer, medulloblastoma, colorectal, prostate, and lung cancer [22,23,34-38]. RK-33 inhibition of DDX3 inhibited the cell cycle in the G1 phase and caused apoptosis in cancer cells. Inhibition of DDX3 functions by RK-33 disrupt the DDX3-β-catenin complex and modulates various β-catenin target genes involved in cell differentiation, cell proliferation, apoptosis, inhibiting tumor growth [22,23]. RK-33 shows significant in vivo radiosensitizing effect against various cancers including lung, human xenograft model of prostate and breast cancer by inhibiting double strand break repair (non-homologous end joining) pathways and mitochondrial translation [22,34,38]. RK-33 increases the susceptibility of BRCA1-positive breast cancer cells to PARP inhibitors [35]. The mechanism of anticancer effects of RK33 is represented in Figure 2. RK-33 had no adverse effects on mice, indicating that more preclinical study is needed to enhance drug formulation, dose, and delivery. In effort to improve its delivery and to overcome chemical hydrophobicity of RK-33, the poly (lacticco- glycolic acid) (PLGA) encapsulated RK-33 nanoparticles were developed with potent cytotoxicity to human breast carcinoma MCF-7 cells in vitro [39]. Despite its anti-cancer effects, RK-33 inhibits various viral replication including Dengue, West Nile, Zika, Respiratory Syncytial, human Parainfluenza Type-3, HIV and SARS-CoV-2 viral infections via targeting DDX3 [6,40]. However, further clinical trials in human are needed to evaluate the safety and efficacy of RK-33 against various cancers and viruses.
Ketorolac salt, a newly discovered bioactive compound against the ATPase activity of DDX3 has been demonstrated to inhibit the growth of oral squamous cell carcinoma (OSCC) cell lines by increasing tumor suppressor E-cadherin. Ketorolac salt also reduces tongue lesions in in-vivo mice models of oral cancer [41]. Since Ketorolac salt is less studied in the cancer field, further in vivo evaluation of this bioactive compound is needed. Doxorubicin, an FDA approved antitumor drug, has inhibited the growth of OSCC cells by reducing cellular DDX3 ATPase activity [42]. However, doxorubicin caused cardiotoxicity, which was a primary cause of death. Thus, although the advent of potential small molecule inhibitors of DDX3 have proven to be successful, there are still many potential analogs or small molecules yet to be discovered in the field of cancer treatment.
Conclusion
DDX3 plays a crucial role in various physiological functions including RNA metabolism and stress responses. Dysregulation of DDX3 results in the development of cancer and viral infection. The crystal structure of human DDX3 will help in the development of novel DDX3 inhibitors, as well as the understanding of DDX3’s molecular mechanism in cancer. Several small molecule inhibitors that target ATPase and helicase activity of DDX3 have showed promising therapeutic effects in several cancer cell lines and animal tumor models. Currently, the most studied DDX3 inhibitor RK-33 is expected to arrive in upcoming clinical trials. Notably, further research and evaluation of some unexplored DDX3 inhibitors such as Ketorolac salts are warranted in preclinical models of aggressive cancer types.
Funding Sources
This work was supported by Department of Biomedical Sciences and Drug Discovery, KOR Life Sciences, Biotechnology. The funding was provided by KOR life sciences.
References
- Bol GM, Xie M, Raman V (2015) DDX3, a potential target for cancer treatment. Molecular Cancer 14(1):188.
- Mo J, Liang H, Su C, Li P, Chen J, et al. (2021) DDX3X: structure, physiologic functions and cancer. Molecular Cancer 20(1): 38.
- Hernández Díaz T, Valiente Echeverría F, Soto Rifo R (2021) RNA Helicase DDX3: A Double-Edged Sword for Viral Replication and Immune Signaling. Microorganisms 9(6): 1206.
- Valiente Echeverría F, Hermoso MA, Soto Rifo R (2015) RNA helicase DDX3: at the crossroad of viral replication and antiviral immunity. Rev Med Virol 25(5): 286-299.
- Yedavalli VSRK, Neuveut C, Chi YH, Kleiman L, Jeang KT (2004) Requirement of DDX3 DEAD box RNA helicase for HIV-1 Rev-RRE export function. Cell 119(3): 381-392.
- Rao S, Lungu C, Crespo R, Steijaert TH, Gorska A, et al. (2021) Selective cell death in HIV-1-infected cells by DDX3 inhibitors leads to depletion of the inducible reservoir. Nature Communications12(1): 2475.
- Soulat D, Bürckstümmer T, Westermayer S, Goncalves A, Bauch A, et al. (2008) The DEAD-box helicase DDX3X is a critical component of the TANK-binding kinase 1-dependent innate immune response. EMBO J 27(15): 2135-2146.
- Gringhuis SI, Hertoghs N, Kaptein TM, Zijlstra Willems EM, Sarrami Fooroshani R, et al. (2017) Erratum: HIV-1 blocks the signaling adaptor MAVS to evade antiviral host defense after sensing of abortive HIV-1 RNA by the host helicase DDX3. Nat Immunol 18(4): 474.
- Chao CH, Chen CM, Cheng PL, Shih JW, Tsou AP, et al. (2006) DDX3, a DEAD box RNA helicase with tumor growth-suppressive property and transcriptional regulation activity of the p21waf1/cip1 promoter, is a candidate tumor suppressor. Cancer Res 66(13): 6579-6588.
- He Y, Zhang D, Yang Y, Wang X, Zhao X, Zhang P, et al. (2018) A double-edged function of DDX3, as an oncogene or tumor suppressor, in cancer progression (Review). Oncol Rep 39(3): 883-892.
- Chen HH, Yu HI, Cho WC, Tarn WY (2015) DDX3 modulates cell adhesion and motility and cancer cell metastasis via Rac1-mediated signaling pathway. Oncogene 34(21): 2790-800.
- He TY, Wu DW, Lin PL, Wang L, Huang CC, et al. (2016) DDX3 promotes tumor invasion in colorectal cancer via the CK1ε/Dvl2 axis. Sci Rep 6: 21483.
- Pugh TJ, Weeraratne SD, Archer TC, Pomeranz Krummel DA, Auclair D, et al. (2012) Medulloblastoma exome sequencing uncovers subtype-specific somatic mutations. Nature 488(7409): 106-110.
- Zhan T, Rindtorff N, Boutros M (2017) Wnt signaling in cancer. Oncogene 36(11): 1461-1473.
- Wu DW, Lin PL, Cheng YW, Huang CC, Wang L, et al. (2016) DDX3 enhances oncogenic KRAS‑induced tumor invasion in colorectal cancer via the β‑catenin/ZEB1 axis. Oncotarget 7(16): 22687-22699.
- Sun M, Song L, Zhou T, Gillespie GY, Jope RS (2011) The role of DDX3 in regulating Snail. Biochim Biophys Acta 1813(3): 438-447.
- Botlagunta M, Krishnamachary B, Vesuna F, Winnard PTJ, Bol GM, et al. (2011) Expression of DDX3 is directly modulated by hypoxia inducible factor-1 alpha in breast epithelial cells. PLoS One 6(3): e17563.
- Wu DW, Lin PL, Wang L, Huang CC, Lee H (2017) The YAP1/SIX2 axis is required for DDX3-mediated tumor aggressiveness and cetuximab resistance in KRAS-wild-type colorectal cancer. Theranostics 7(5): 1114-1132.
- Li Y, Wang H, Wang Z, Makhija S, Buchsbaum D, et al. (2006) Inducible resistance of tumor cells to tumor necrosis factor-related apoptosis-inducing ligand receptor 2-mediated apoptosis by generation of a blockade at the death domain function. Cancer Res 66(17): 8520-8528.
- Sun M, Zhou T, Jonasch E, Jope RS (2013) DDX3 regulates DNA damage-induced apoptosis and p53 stabilization. Biochim Biophys Acta 1833(6): 1489-1497.
- Sun M, Song L, Li Y, Zhou T, Jope RS (2008) Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ 15(12): 1887-1900.
- Bol GM, Vesuna F, Xie M, Zeng J, Aziz K, et al. (2015) Targeting DDX3 with a small molecule inhibitor for lung cancer therapy. EMBO Mol Med 7(5): 648-669.
- Tantravedi S, Vesuna F, Winnard PTJ, Martin A, Lim M, et al. (2019) Targeting DDX3 in Medulloblastoma Using the Small Molecule Inhibitor RK-33. Transl Oncol 12(1): 96-105.
- Wu DW, Lee MC, Wang J, Chen CY, Cheng YW, et al. (2014) DDX3 loss by p53 inactivation promotes tumor malignancy via the MDM2/Slug/E-cadherin pathway and poor patient outcome in non-small-cell lung cancer. Oncogene 33(12): 1515-1526.
- Wang SP, Wang WL, Chang YL, Wu CT, Chao YC, et al. (2009) p53 controls cancer cell invasion by inducing the MDM2-mediated degradation of Slug. Nat Cell Biol 11(6): 694-704.
- Maga G, Falchi F, Garbelli A, Belfiore A, Witvrouw M, et al. (2008) Pharmacophore modeling and molecular docking led to the discovery of inhibitors of human immunodeficiency virus-1 replication targeting the human cellular aspartic acid-glutamic acid-alanine-aspartic acid box polypeptide 3. J Med Chem 51(21): 6635-6638.
- Maga G, Falchi F, Radi M, Botta L, Casaluce G, et al. (2011) Toward the discovery of novel anti-HIV drugs. Second-generation inhibitors of the cellular ATPase DDX3 with improved anti-HIV activity: synthesis, structure-activity relationship analysis, cytotoxicity studies, and target validation. ChemMedChem 6(8): 1371-1389.
- Yedavalli VSRK, Zhang N, Cai H, Zhang P, Starost MF, et al. (2008) Ring expanded nucleoside analogues inhibit RNA helicase and intracellular human immunodeficiency virus type 1 replication. J Med Chem 51(16): 5043-5051.
- Radi M, Falchi F, Garbelli A, Samuele A, Bernardo V, et al. (2012) Discovery of the first small molecule inhibitor of human DDX3 specifically designed to target the RNA binding site: towards the next generation HIV-1 inhibitors. Bioorg Med Chem Lett 22(5): 2094-2098.
- Brai A, Fazi R, Tintori C, Zamperini C, Bugli F, et al. (2016) Human DDX3 protein is a valuable target to develop broad spectrum antiviral agents. Proc Natl Acad Sci U S A 113(19): 5388-5893.
- Brai A, Riva V, Clementi L, Falsitta L, Zamperini C, et al. (2021) Targeting DDX3X Helicase Activity with BA103 Shows Promising Therapeutic Effects in Preclinical Glioblastoma Models. Cancers (Basel) 13(21): 5569.
- Gherardini L, Inzalaco G, Imperatore F, D’Aurizio R, Franci L, et al. (2021) The FHP01 DDX3X Helicase Inhibitor Exerts Potent Anti-Tumor Activity In Vivo in Breast Cancer Pre-Clinical Models. Cancers (Basel) 13(19): 4830.
- Xie M, Vesuna F, Botlagunta M, Bol GM, Irving A, et al. (2015) NZ51, a ring-expanded nucleoside analog, inhibits motility and viability of breast cancer cells by targeting the RNA helicase DDX3. Oncotarget 6(30): 29901-29913.
- Heerma van Voss MR, Vesuna F, Bol GM, Afzal J, Tantravedi S, Bergman Y, et al. (2018) Targeting mitochondrial translation by inhibiting DDX3: a novel radiosensitization strategy for cancer treatment. Oncogene 37(1): 63-74.
- Heerma van Voss MR, Brilliant JD, Vesuna F, Bol GM, van der Wall E, et al. (2017) Combination treatment using DDX3 and PARP inhibitors induces synthetic lethality in BRCA1-proficient breast cancer. Med Oncol 34(3): 33.
- Wilky BA, Kim C, McCarty G, Montgomery EA, Kammers K, et al. (2016) RNA helicase DDX3: a novel therapeutic target in Ewing sarcoma. Oncogene 35(20): 2574-2583.
- Heerma van Voss MR, Vesuna F, Trumpi K, Brilliant J, Berlinicke C, et al. (2015) Identification of the DEAD box RNA helicase DDX3 as a therapeutic target in colorectal cancer. Oncotarget 6(29): 28312-28326.
- Xie M, Vesuna F, Tantravedi S, Bol GM, Heerma van Voss MR, et al. (2016) RK-33 Radiosensitizes Prostate Cancer Cells by Blocking the RNA Helicase DDX3. Cancer Res 76(21): 6340-6350.
- Bol GM, Khan R, Heerma van Voss MR, Tantravedi S, Korz D, et al. (2015) PLGA nanoparticle formulation of RK-33: an RNA helicase inhibitor against DDX3. Cancer Chemother Pharmacol 76(4): 821-827.
- Yang SNY, Atkinson SC, Audsley MD, Heaton SM, Jans DA, et al. (2020) RK-33 Is a Broad-Spectrum Antiviral Agent That Targets DEAD-Box RNA Helicase DDX3X. Cells 9(1): 170.
- Samal SK, Routray S, Veeramachaneni GK, Dash R, Botlagunta M (2015) Ketorolac salt is a newly discovered DDX3 inhibitor to treat oral cancer. Sci Rep 5: 9982.
- Botlagunta M, Kollapalli B, Kakarla L, Gajarla SP, Gade SP, et al. (2016) In vitro anti-cancer activity of doxorubicin against human RNA helicase, DDX3. Bioinformation 12(7): 347-353.






























