Suppression of Bean Yellow Mosaic Virus by Plant Extracts in Faba Bean Plants
D S Khachatryan*
The Federal State Unitary Enterprise, Russia
Submission: October 14, 2021; Published: November 11, 2021
*Corresponding author: D S Khachatryan, The Federal State Unitary Enterprise, Institute of Chemical Reagents and High Purity Chemical Substances of National Research Centre, Kurchatov Institute, Bogorodsky val str.3, Moscow, Russia
How to cite this article: D S Khachatryan*. Derivatives of 6-Aminouracyl as Enamines in Reactions of Heterocyclization. Syntheses and Properties of a Pyrimidine Containing Heterocycles. Organic & Medicinal Chem IJ. 2021; 11(2): 555810. DOI: 10.19080/OMCIJ.2021.11.555810
Abstract
Uracil derivatives containing in their composition a natural (pharmacophore) ureic (acyl urea) group, are valuable objects for the development of new pharmaceutical product. Pyrimidine derivatives occupy a central position among the viral biomolecules, which are the elementary chains of the DNA and RNA. They have great potential for developing new possible therapeutic drugs. We have developed preparative methods for the synthesis of various binuclear nitrogen-containing heterocycles containing, on the one hand, a pharmacophore uracylic nucleus, and on the other hand, on the pyrimidine ring “hot” easily transformable groups (OCNHCO; SH; COOH), which open the possibility of introducing a wide range of different substituents with desired properties into the main matrix.
Keywords: Potassium carbonate; Alkylation; Pyrimidines; Azaheterocycles
Introduction
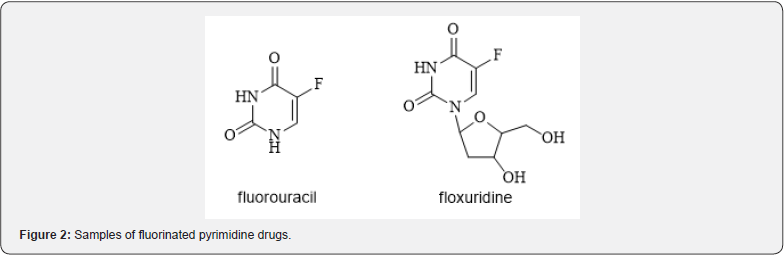
Pyrimidine derivatives occupy a central position among the viral biomolecules, which are the elementary chains of the DNA and RNA. They have great potential for developing new possible therapeutic drugs. The first drugs proposed as antiviral agents have been idoxuridine and cytarabine. They have limited clinical use due to low therapeutic index. Currently we still can find in use drugs such as amantadine, vidarabine, trifluridine, acyclovir, ribavirin, and zidovudine (Figure 1). A large number of fluorinated pyrimidine analogues have synthesized as potential anticancer drugs. The most extensively studied among them are fluorouracil and floxuridine. Wide application of anticancer uracil-based drugs in oncological practice contributes to continuous search for new, more efficient analogies [6] (Figure 2).
6-Aminouracyls as enamines, mechanisms
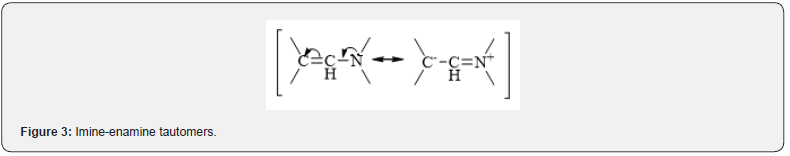
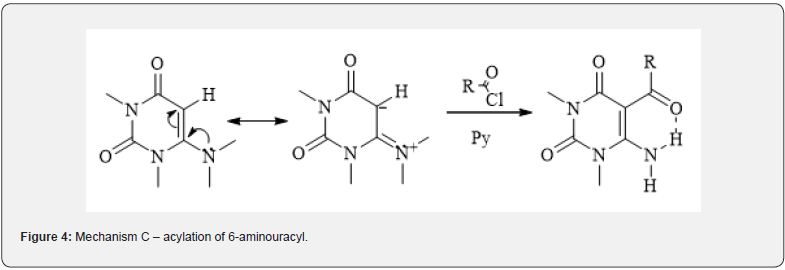
Pyrimidines, both unsubstituted 5th carbon atom and substituted by the amino group 6th carbon atom can be considered as cyclic enamines [7] and, thus, theoretically react with electrophiles. Structure of enamine can represented as resonance system containing two canonical forms (imine-enamine tautomer) (Figure 3). In this regard, electrophilic reagents can attack this structure either on nitrogen atom and giving ammonia salts or, what happens much more often-on β-carbon atom, giving iminium salts (Figure 4). In the case of 1,3-dimethyl-6-aminouracyls, it was identified that the carbon atom C-5 is region specifically attacked by electrophilic reagents [8], which is easily explained by the delocalization of the free electron pair of the exocyclic nitrogen atom, which increases the electron density in the 5th position, making the carbon atom C-5 more open to nucleophilic attack.
Discussion of Results
6-aminouracyls and heterocyclic systems based on them are analogues of natural compounds exhibiting antibacterial, antitumor, antimalarial, antihypertensive, anti-allergic, analgesic, anti-inflammatory, antipyretic and sedative effects. A wide range of pharmacological activity of these structures generates interest to their synthesis. This work is devoted to the synthesis of such derivatives of pyrimidines, which would be the building blocks for the further introduction of other pharmacophore groups.
Synthesis 6-aminuracyls
Synthesis of derivatives of 6-aminouracyls was proceeds by the reaction of substituted urea with cyan acetic ether in methanol under the action of sodium methoxide according to the following well-known scheme 1 [9]:
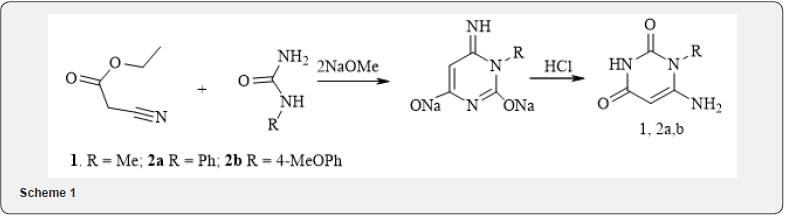
During the reaction, the disodium salt of amino uracil formed. During its neutralization by concentrated hydrochloric acid 6-aminouracils (1, 2 a, b) were formed with yields of 65-75%. The structures of the obtained compounds confirmed by 1H NMR spectroscopy and comparison of physical and chemical properties with known samples.
6-Aminouracyl reactions with maleic anhydride and maleimide. The synthesis of pyrrolidinouracyls
The dual reactivity of 6-aminouracil (direction of electrophilic attack on 5-C and/or 6-amino groups) is clearly manifested in the interaction of amine 2a with maleimide and maleic anhydride. Reaction with maleimide in acetic acid during boiling leads to mixtures of compounds 3 and 4, where compound 3, appears to be, forms as an initial Michael addition of the carbon atom C-5 to the double bond of maleimide (A’), followed by cyclization and acylation of 6-amino group through the disclosure maleimide cycle-transamination reaction type (2. path A). In the case of bicycle 4, the addition to the 6-amino group (B’) occurs first, followed by heterocyclization by electrophilic attack mechanism on the carbon atom C-5 with the opening of the maleimide cycle (path B).
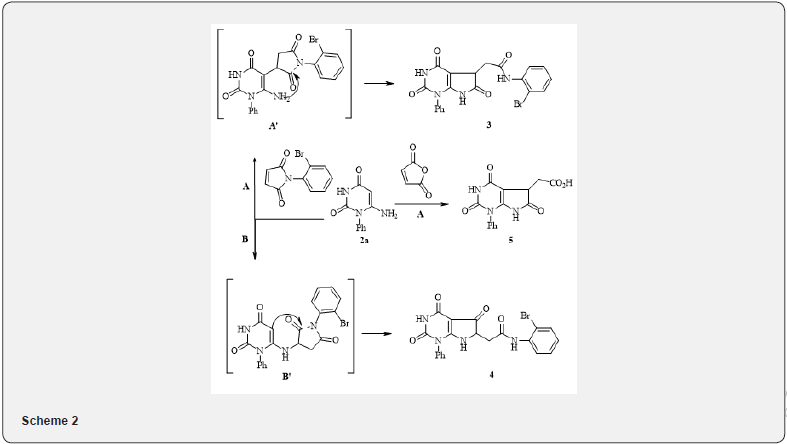
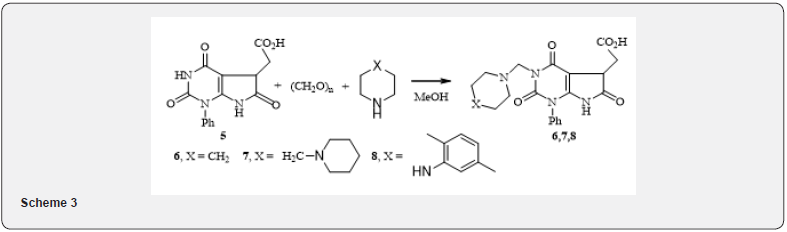
In contrast to maleimide, reaction of uracyl 2a with maleic anhydride under the same conditions proceeds regiospecifically on the way A with the formation of the bicyclic acid 5. We studied the transformation of the resulting acid 5 in the Munnich reaction, which proceeded smoothly enough during boiling the reaction mixture in methanol under the action of hydrochloric acid, that carry out in the following scheme 3. Under these conditions, the reaction went on the nitrogen atom in position 3 of pyrimidine ring of the bicycle 5. It is interesting to note that even with a twofold excess in paraldehyde and amine, nitrogen on position 7 not affected.
Other reactions with 6-aminouracyls
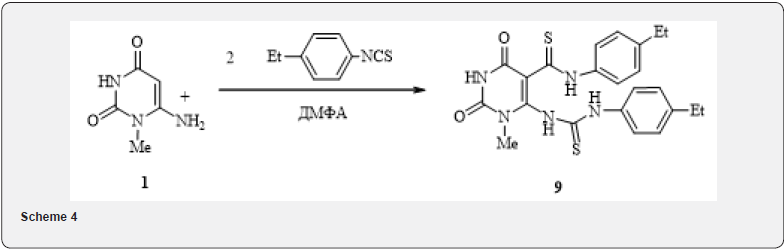
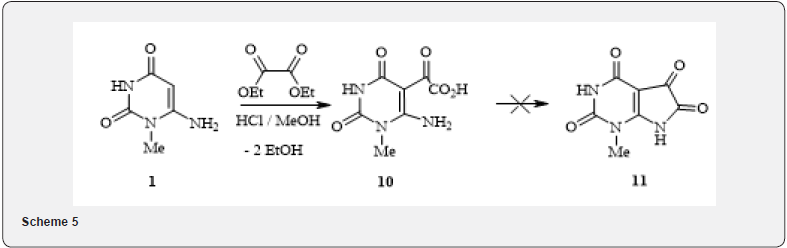
In literature [10] described the synthesis of analogues of purine by interaction of aminouracyls with phenylisothiocyanate in ethanol. Replacing ethanol with DMF and in the presence of catalytic amounts of triethylamine, we found out that under these conditions, the electrophilic attack of the isothiocyanate group is realized simultaneously in both possible directions (attack on 5-C and 6-amino positions of aminouracyls 1) with forming a linear byproduct 9 (4). An attempt to obtain Bicycle (11) by the interaction of 6-aminouracil 1 with oxalic ether during long boiling of the reaction mixture (TLC control after the disappearance of the 6-amnouracyl spot) in methanol in the presence of hydrochloric acid led to acylation at position 5 of the pyrimidine ring, followed by hydrolysis and producing acid 10 according to the following scheme 5.
Producing of 6-amino-5-bromine-1-phenyluracyl and its reactions
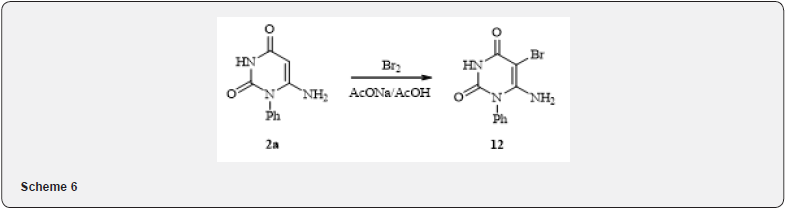
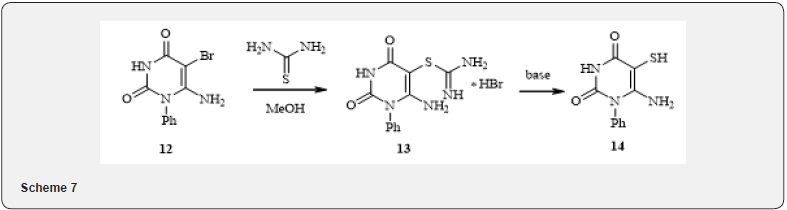
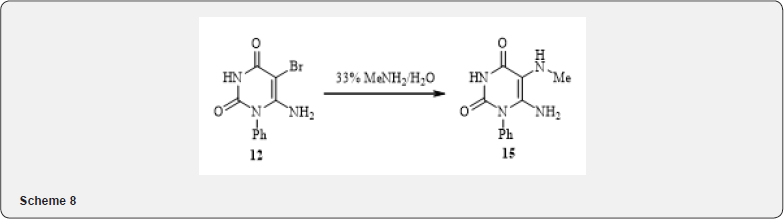
By bromine halogenation of 6-amino-1-phenyluracyl in acetic acid in the presence of sodium acetate 5-bromine-6-aminouracyl 12 was produced. Bromouracyl 12 involves in a classic reaction of formation of thiouronium salts when interacts with the thiourea in methanol with the formation of salt 13. The hydrolysis of salt 13 with 10% NaOH solution resulting to 1, 2-aminothiol 14 - compound that can be used in the synthesis of multinuclear heterocycles [11-13] (7). In the reaction of the substance 12 with 33% methylamine solution, bromine is replaced by an amino group and a derivative of 5, 6 - diaminouracyl 15 was produced (8).
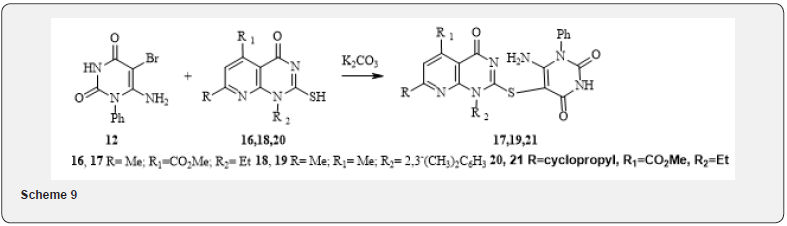
In reaction with bicyclic thiols* 16, 18, 20 when using compound 12 as an alkylating agent under the action of potassium carbonate according to the previously developed by us method of alkylation of heterocyclic thiols [14,15], the products of bromine substitution by the tio-group-compounds 17, 19, 21 are formed. Yields are satisfactory.
*Note: Description of the synthesis of pyridopyrimidine thiols 16, 18 and 20 will be given in subsequent publications.
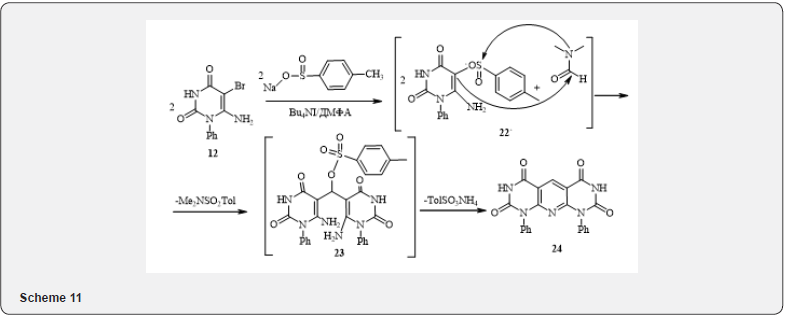
Further attempts to use uracyl 12 as an alkylating agent in reaction with CH-acids in the basic solutions (ethanol with K2CO3, KOH or sodium ethylate) led to the production of unbrominated initial aminouracyls and traces of brominated CH-acids (the presence of corresponding ions in the ESI MS spectra of reaction mixtures). Thus, 5-bromo-6-aminouracyl 12 in these conditions behaves like N-bromosuccinimide [16, 17] (10). Attempts to replace bromine with another - better leaving- toluene sulfate group by the classical method unexpectedly led to the production of condensed tricycle 24, which, apparently, can be explained by the further interaction of the resulting sulfinate 6-aminouracyl (22) with dimethylformamide (indirect evidence of this is the fact that the compound 24 does not obtained in the absence of sodium toluene sulfate) according to the scheme 11.
Experimental part
The completion of the reaction and purity of the obtained compounds monitored by TLC on Silica gel 60 F254 TLC plates using:
A. Chloroform: methanol-9: 1;
B. Chloroform: methanol – 5: 1
The visualization was performed by employing an UV lamp and iodine vapor. H1 NMR spectra were recorded on a Fourier NMR spectrometer with a superconducting magnet Bruker AVANCE III NanoBay 300 MHz (spectra were recorded in the deuterium stabilization mode, thermal stabilization 25℃, internal standard - TMS), and the elemental composition was determined on the CHNS analyzer Eurovector “EuroEA 3000”. Molecular masses were determined on a Finniga MAT INCOS50 mass spectrometer with an electron impact with an ionization energy of 70eV. Melting temperatures of the obtained compounds are determined on a standard melting-point apparatus (MPA), p. No. 26649 with certified thermometers. “ACROS” company’s solvents and reagents were used without further purification.
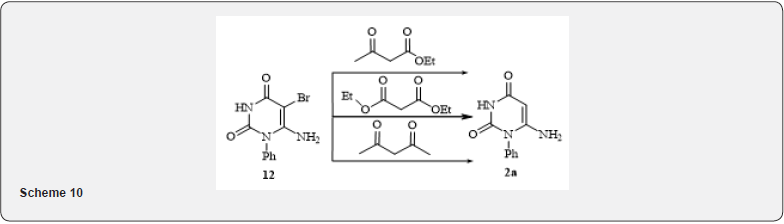
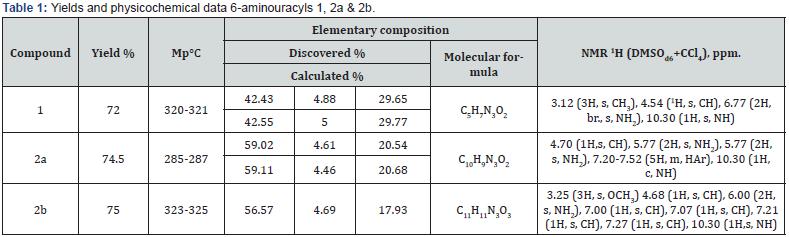
Producing 1-substituted 6-aminouracyls (1, 2a, 2b). To the cooled sodium methylate solution in methanol, produced from 6.8g (0.297mol) of sodium in 94ml of methanol, 0.147mole of the corresponding urea and 24.9g (0.221mol) of cyan acetic ether were added and boiled for 10hours. The reaction mass was cooled and solvents evaporated in a vacuum, the residue was dissolved in 150ml of water and neutralized with hydrochloric acid. The precipitate filtered, washed with distilled water and dried in air. Yields and physical & chemical data of produced 6-aminouracyls are shown in table 1.
Producing of bicyclic derivatives and their alkylation reaction. N-(2-bromophenyl)-2-(2,4,6-trioxo-1-phenyl-2,3,4,5,6,7- hexahydro-1H-pyrrolo[2,3-d]pyrimidine-5-yl)-acetamide (3) and N-(2-bromophenyl)-2-(2,4,5-trioxo-1-phenyl-2,3,4,5,6,7- hexahydro-1H-pyrrolo[2,3-d]pyrimidine-6-yl)-acetamide (4). In acetic acid under heating dissolved 0.50g (0.0025mol) of 6-amino-1-phenyluracyl, then added 0.76g (0.003mol) of 1-(2-bromophenyl)pyrrole-2,5-dione and boiled it for 7h. The reaction mass was cooled and filtered, washed with a small amount of acetic acid and water. The resulting mixture of products 3: 4=1:1 with a mass of 0.62g divided on a chromatographic column, obtaining solid products (see table 2).
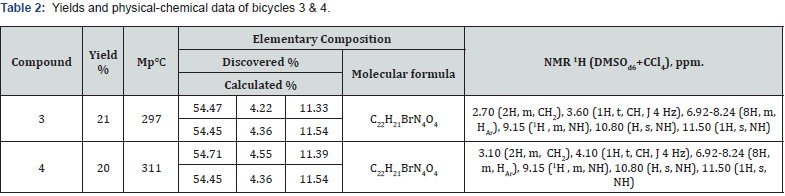
( 2 , 4 , 6 - t r i oxo - 1 - p h e nyl - 2 , 3 , 4 , 5 , 6 , 7 - h exa hyd ro - 1 H - pyrrolo[2,3-d]pyrimidine-5-yl) acetic acid (5). A solution of 3.00g (0.015mol) 6-amino-1-phenyluracyl (2a) in 60ml acetic acid was boiled until the compound 2a was completely dissolved, then slightly cooled and added 1.76g (0.018mole) maleic anhydride. Boiled 3.5h, cooled to room temperature and filtered white crystalline substance, which washed with a small amount of acetic acid and water, yields 3.59g (76%). Mp 325°C. NMR spectrum 1H (DMSOd6+CCl4), δ, ppm: 2.86 (2H, m, CH2), 3.54 (1H, t, CH, J 4 Hz), 7.22-7.58 (5H, m, HAr), 10.65 (1H, s, NH), 10.90 (1H, s, NH). Found,%: C 58.14; H 5.25; N 12.58. C16H17N3O5. Calculated, %: C, 58.00; H, 5.17; N, 12.68.
The general technique of the Munnich reaction
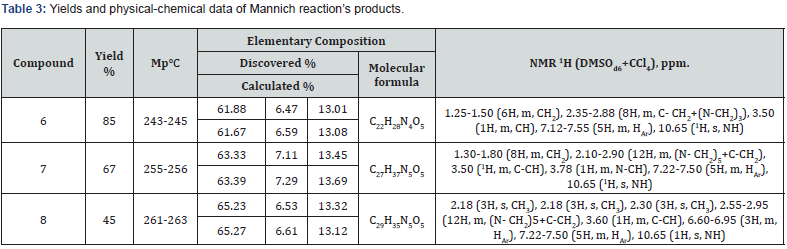
(2,4,6-trioxo-1-phenyl-3-amino-1-methyl-2,3,4,5,6,7- hexahydro-1H-pyrrolo[2,3-d]pyrimidine-5-yl)acetic acid (6, 7, 8). A mixture of 1.40mmol of the corresponding amine, 0.04g (1.4mmol) paraform, 0.20g (0.63mmol) of acid 5 with a drop of concentrated hydrochloric acid in 10ml of methanol was boiled for 3hours, while boiling rich white precipitate falls. The reaction mass was cooled, the precipitate was filtered and washed with a small amount of methanol. The yields and physical & chemical data of the obtained compounds 6, 7 and 8 are given in table 3.
Other reactions of aminouracyls
4-Ethylphenylamide 6-[3-(4-ethylphenyl) thioureido]-1- methyl-2,4-dioxo-1,2,3,4-Tetrahydropyrimidine-5-thiocarboxylic acid (9). 0.50g (3.5mmol) 6-amino-1-methyluracil was dissolved in 5ml of DMF, a trace of catalyst – triethylamine and 0.58g (3.5mmol) 4-ethyl-phenylisothiocyanate were added after. The reaction mixture was being stirred at 120-130°C for 10h, cooled to room temperature, filtering a small precipitate (original aminouracyl 1), and the mother liquor was diluted with water, formed yellow precipitate was filtered out and washed with distilled water. The yield of the substance 9 was 0.22g (27%). Mp - 270°C. NMR spectrum 1H (DMSOd6+CCl4), δ, ppm: 1.25 (6H, m, CH3), 2.60 (4H, m, CH2), 3.35 (3H, s, CH3), 7.00-7.50 (8H, m, HAr), 8.30 (1H, s, NH), 10.80 (1H, s, NH), 13.10 (1H, s, NH), 14.25 (1H, s, NH). Discovered, %: C 57.32; H, 4.92; N, 15.79; 14.68. C21H21N5O2S2. Calculated, %: C 57.38, H 4.82, N 15.93, S 14.59.
(6-Amino-1-methyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine- 5-yl)oxoacetic acid (10). Reaction mass was cooled after dissolving 2.00g (0.014mol) of compound 1 in 80ml of boiling methanol. Then 3.50ml (0.080mol) of concentrated hydrochloric acid, 8.18 g (0.056mol) of diethyl oxalate were added and boiled for 20hours. After the solvent was distilled, 30ml of water was added to the residue and NaHCO3 was added to form neutral pH. Formed precipitate was filtered, obtaining 1.22g of dimethyl oxalate (Melting point = 50-52°C). The mother liquor was evaporated in vacuum preliminarily adding 30ml of acetonitrile in it. Formed residue was boiled in 20ml of absolute ethanol, and the hot solution was filtered from precipitated NaCl. The mother liquor was evaporated to 1/3 of the volume, placed in a fridge for 2hours, and a precipitate was filtered and washed with cold ethanol, obtained 1.13g (37.9%) of the substance 10. Mp-130°C. 1H NMR Spectrum (DMSOd6+CCl4), δ, ppm: 3.05 (3H, s, CH3), 7.40 (2H, s, NH2), 11.25 (1H, s, NH). Discovered, %: C, 39.64; H, 3.47; N, 19.56. C7H7N3O5. Calculated, %: C, 39.45; H 3.31; N, 19.71.
6-Amino-5-bromine-1-phenyl-1H-pyrimidine-2,4-dione (12). To the 15.00g (0.0739mol) 6-amino-1-phenyluracil (2) boiling solution in 230ml of acetic acid 7.89g (0.096mol) of sodium acetate was added. The mixture was cooled to 65-70°C. Then, with vigorous stirring for 10minutes, 24.60g (0.153mol~7.92ml) bromine solution added by drops in 40 ml of acetic acid. The reaction mixture left for 15h at room temperature; a precipitate filtered out, washed with acetic acid and water. The reaction product was dried and recrystallized from 25% ethanol. The yield of 12 is 12.70g (61%). Mp 270°C. NMR Spectrum 1H (DMSOd6+CCl4), δ, ppm: 5.75 (2H, s, NH2), 7.35-7.55 (5H, m, HAr), 10.90 (1H, s, NH). Discovered, %: 42.63; H 2.99; Br 28.28.54; N 15.00. C10H8BrN3O2. Calculated, %: C, 42.58; H, 2.86; Br, 28.32; N, 14.90.
Hydrobromide 2-(6-amino-2,4-dioxo-1-phenyl-1,2,3,4- tetrahydro-pyrimidine-5-yl)-isothiourea (13). A solution of 9.00g (0.0319mole) of 6-amino-5-bromo-1-vinyluracyl (12) and 2.55g (0,0335mole) of thiourea in 180 ml of methanol was boiled for 6hours, the solvent was distilled, the precipitate washed with water and filtered, obtaining 6.44g (56%) of thiouronium salt 13 in the form of a white crystalline substance. Mp 255°C. NMR spectrum 1H (DMSOd6+CCl4), δ, ppm: 6.90 (2H, s, NH2), 7.35-7.80 (5H, m, HAr), 8.75-8.83 (4H, m, NH2), 11.05 (1H, s, NH). Discovered, %: 37.12; H, 3.31; Br, 22.26; N, 19.49; S, 9.17 C11H11BrN5O2S. Calculated, %: C, 36.99; H, 3.10; Br, 22.37; N, 19.61; S 8.98.
6-Amino-5-mercapto-1-phenyl-1H-pyrimidine-2,4-dione (14). 0.50g (1.4mmol) of compound 13 dissolved in 10 ml of 10% NaOH and stirred at room temperature for 24hours. The solution then acidified to pH~3 and the precipitate was filtered obtaining 0.32g (97%) of the yellow crystalline substance 14. Mp 345°C. NMR Spectrum 1H (DMSOd6+CCl4), δ, ppm: 2.90-3.20 ( + 3 Í O, SHexchange), 6.28 (2H, s, NH2), 7.30-7.60 (5H, m, HAr), 10.60 (1H, s, NH). Discovered, %: C 51.28; H, 4.03; N, 17.75; S, 13.88. C10H9N3O2S. Calculated, %: C, 51.05; H, 3.86; N, 17.86; S, 13.63. Mass spectrum, m/z: 234 [M]+, 203, 160, 132, 119, 93, 77, 64, 60, 55, 51, 43.
6-Amino-5-methylamine-1-phenyl-1H-pyrimidine-2,4-dione (15). A solution of 0.20g (0.71mmol) of the substance 12 was stirred for 20h in 4ml of 33% methylamine solution at room temperature. After evaporation of the solution by half, the precipitate filtered out, washed with cold acetone, obtained 0.10g (47%) diamine 15. Mp 174-175°C. NMR Spectrum 1H (DMSOd6+CCl4), δ, ppm: 2.45 (3H, m, CH3), 5.30 (2H, s, NH2), 6.86 (1H, m, NH), 7.25-7.55 (5H, m, HAr), 10.50 (1H, s, NH). Discovered, %: C, 57.13; H, 5.44; N, 23.87. C11H12N4O2. Calculated, %: C, 56.89; H, 5.21; N 24.12.
General technique of alkylation of heterocyclic thiols 16, 18, 20. A mixture of 0.67mmol of the corresponding mercaptopyrimidine (16, 18, 20), 0.12g (1.42mmol) NaHCO3, 0.14g (1.42mmol) triethylamine and 0.20g (0.71mmol) of the substance 12 in 3ml DMF was stirred at room temperature 3-4days. Then it was dissolved in 2% KOH solution and acidified with concentrated hydrochloric acid to pH~8. The resulting precipitate was filtered and washed with water. Yields and physical & chemical characteristics of the substances 17, 19 and 21 are shown in table 4.
1,8-diphenyl-1H,8H-1,3,6,8,9-pentahydroanthracene- 2,4,5,7-tetraen (24). A solution of a mixture of 0.50g (1.8mmol) of the compound 12, 0.36g (2.0mmol) 4-methylbenzenesodiumsulfinate, 0.07g (1.8mmol) Bu4NI was stirred in 5ml of DMF at 120-130°C for 2.5hours, then cooled, poured into 30ml of water, and filtered 0.34g (94.5%) of the substance 24. NMR spectrum 1H (DMSOd6+CCl4), δ, ppm: 6.60-7.20 (10H, m, HAr), 8.80 (1H, s, HAr), 11.75 (2H, s, NH). Discovered, %: C, 63.45; H 3.57; N, 17.39. C21H13N5O4. Calculated, %: C 63.16; H 3.28; N 17.54.
Conclusion
Thus, as a result of the research, possible pathways for the transformation of 6-aminouracils were found, methods for the preparation of various heterocycles were developed, and their chemical and physicochemical properties were studied. It was shown that in a two-phase system: potassium carbonate (solid phase) - solvent (liquid phase), the alkylation of pyridopyrimidines proceeds regioselectively at the nitrogen of the pyrimidine ring.
Acknowledgment
The work has been done carried out using the scientific equipment of the Center for Collective Use of the National Research Center” Kurchatov Institute “- IREA with the financial support of the project by the Russian Federation represented by the Ministry of Education and Science of Russia, Agreement No. 075-11-2021- 070 dated 19.08.2021.
References
- Preobrazhensky N A, Genkin E I (1953) Organic chemistry of medicinal substances. M Goskhimizdat pp 541.
- Papesch V, Elmer F S (1951) Synthesis of 1-Mono- and 1,3-Di-Substituted 6-Aminouracils. Diuretic Activity. J Org Chem 16: 1879-1890.
- S Senda, K Hirota (1974) Pyrimidine derivatives and related compounds. XXII. Synthesis and pharmacological properties of 7-deazaxanthine derivatives. Chem Pharm Bull 22(7): 1459-1467.
- R S Vardanyan (2004) Synthesis of essential drugs. MIA pp 844.
- Secrist III J A, Liu p S (1978) Reduction of aldehydes and ketones to alcohols and hydrocarbons through use of the organosilane-boron trifluoride system. J Org Chem 43(2): 374-375.
- Itoh T, Ishikawa I, Tomii Y, Mizuno Y, Ogura H, Kawahara N (1990) Syntheses of Pyrido[2,3-d]pyrimidine Nucleosides via 6-Allylaminouridines from 6,5’-Anhydroridine Derivative and Allyamines. Heterocycles 30(1): 231.
- Cobo J, Sánchez A, Nogueras M (1998) Reactions of 6-aminopyrimidin-4(3H)-ones with electron-deficient alkenyl derivatives. Easy preparation of heterocyclic analogues of Sangivamicine. Tetrahedron 54: 5753.
- Dudkin S, Iaroshenko V O, Sosnovskikh V Y, Tolmachev A A, Villinger A, et al. (2013) Synthesis and reactivity of 5-polyfluoroalkyl-5-deazaalloxazines. Org Biomol Chem 11(32): 5351.
- Tietze L. F., Eicher T (2001) Reaktionen und Synthesen im organisch-chemischen Praktikum und Forschungs laboratorium. Weinheim, Deutschland: Wiley-VCH Verlag 636.
- Prasad A S, Sandhu J S, Baruah J N J (1984) Pyrimidine analogues. Reaction of 1,3-dimethyl-6-hydroxylaminopyrimidine-2,4-dione with nitrophenyl isothiocyanates. Heterocycl Chem 21: 267.
- Chun S, Yang S, Chung Y K (2017) Synthesis of benzothiazoles from 2-aminobenzenethiols in the presence of a reusable polythiazolium precatalyst under atmospheric pressure of carbon dioxide. Tetrahedron 73(25): 3438.
- Nale B D, Bhanage B M (2015) N-Substituted Formamides as C1-Sources for the Synthesis of Benzimidazole and Benzothiazole Derivatives by Using Zinc Catalysts. Synlett 26(20): 2835.
- Wade A R, Pawar H R, Biware M V, Chikate R C (2015) Synergism in semiconducting nanocomposites: visible light photocatalysis towards the formation of C–S and C–N bonds. Green Chemistry 17(7): 3879.
- Khachatryan D S, Vardapetyan A A, Panosyan G A, Mirzoyan R G, Morlyan N M (1990) Potassium carbonate as a base for generation of carbanions from CH-acids in organic synthesis. Russ J Org Chem 26: 2092.
- Khachatryan D S, Razinov A L, A V Kolotaev, Belus S K, Matevosyan K R (2015) Alkylation of NH-, OH-, and SH-acids in the presence of potassium carbonate. Russ Chem Bull 64: 395.
- H M Meshram, P N Reddy, P Vishnu, K Sadashiv, Yadav J S (2006) A green approach for efficient α-halogenation of β-dicarbonyl compounds and cyclic ketones using N-halosuccinimides in ionic liquids. Tetrahedron Lett 47(6): 991.
- I Pravst, M Zupan, S Stavber (2008) Halogenation of ketones with N-halosuccinimides under solvent-free reaction conditions Tetrahedron 64(22): 5191.






























