Solute-Solute and Solute-Solvent Interactions of l-Methionine, l-Threonine and l-Histidine in Aqueous-Carbohydrate Solutions using Kirkwood-Buff Theory: A Theoretical Study
Yogita Bisht1 and Anil Kumar Nain2*
1Department of Chemistry, St. Stephan’s College (University of Delhi), India
2Department of Chemistry, Dyal Singh College (University of Delhi), India
Submission: June 29, 2021; Published: August 23, 2021
*Corresponding author: Anil Kumar Nain, Department of Chemistry, Dyal Singh College (University of Delhi), Delhi 110 007, India
How to cite this article: Y Bisht, A K Nain. Solute-Solute and Solute-Solvent Interactions of l-Methionine, l-Threonine and l-Histidine in Aqueous- Carbohydrate Solutions using Kirkwood-Buff Theory: A Theoretical Study. Organic & Medicinal Chem IJ. 2021; 11(2): 555806. DOI: 10.19080/OMCIJ.2021.11.555806
Abstract
The Kirkwood-Buff (K-B) theory can be used to investigate the solute-solute, solute-solvent, solvent-solvent interactions in protein-aqueous carbohydrate system in terms of K-B integrals (G12, G11 and G22), which provide a direct estimation of these interactions. The K-B integrals of nonpolar amino acid (l-methionine), positively charged polar amino acid (l-histidine) and neutral polar amino acid (l-threonine) in aqueous-glucose/sucrose (10% and 20% of glucose/sucrose in water, w/w) solvents have been evaluated from the ultrasonic speed and density data at 298.15 K. The results obtained by theoretical calculations are found in good agreement with those of experimental findings. It has been observed that there exist strong solute-solvent interactions in these systems.
Keywords:Kirkwood-Buff integrals; l-histidine; l-threonine; l-methionine; Solute-solvent interaction
Introduction
Proteins are the building blocks of body tissue and play important role in almost all the biological processes. But being too complex in nature their direct study is arduous. Since proteins are the polymer chains of amino acids linked together by peptide chains, therefore amino acids are studied as model constituents of proteins. The physicochemical and thermodynamic properties of amino acids in aqueous solutions provide valuable information on solute-solvent, solute-solute, and solvent-solvent interactions [1-4]. The stability of different conformations of protein is affected by different non-covalent interactions including electrostatic, hydrophobic interaction and hydrogen bonding. Therefore, to understand the stability factors of protein, it is important to study above mentioned interactions of amino acid in aqueous-saccharides system [5-8]. Carbohydrates are the polyhydroxy compounds which help in stabilizing globular structure of proteins [9,10]. The studies on carbohydrate-protein interactions are very important for the field of immunology, biosynthesis, pharmacology, and medicine. Because of conformational flexibility, saccharides play significant roles in many biological processes [11]. In living organisms, interactions of saccharides with model molecules of proteins play a key role in understanding the thermodynamic behaviour of biochemical process in the body system [12,13].
Although a large amount of experimental data exists in the literature on the physicochemical properties of amino acids in aqueous and aqueous-carbohydrate solutions [14-21], but in contrast there are few reports on theoretical studies [22,23]. The Kirkwood and Buff [24] theory can be used for such kind of theoretical studies. K-B theory of solutions is a general statistical mechanical theory of solutions which is applicable to all types of intermolecular interactions and is valid both classically and quantum mechanically. This theory relates the radial distribution functions of various molecular species in a mixture to the derivatives of the thermodynamic properties of the species. It is one of the most accepted theories of solutions that directly correlate the thermodynamic quantities with the solution structure without any assumptions. In the present paper, K-B theory has been used to study solute-solvent, solute-solute and solvent-solvent interactions of l-methionine, l-threonine, and l-histidine in aqueous-glucose/sucrose solvents (10% and 20% of glucose/sucrose in water, w/w). Density and ultrasonic speed data used in the present study have been taken from our previous studies [25-30].
Theory
The Kirkwood-Buff (KB) theory [24] expresses thermodynamic properties of a solution in terms of K-B integrals (Gij). Mathematically it can be represented as
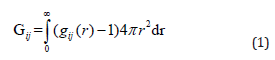
where subscripts i and j refer to the two components that may be the same or different and gij is the pair correlation function, denoting the probability of finding a molecule of species i in a volume element at the distance r from the centre of a molecule of species j. The K-B integral Gij represents the affinity of ith molecule towards jth molecule and vice-versa. This theory co relate compositional fluctuations to both integrals of the radial distribution functions of the different types of molecular pairs present in the solution as well as to the derivatives of the chemical potentials of different components.
Procedure to compute K-B integrals (Gij)
Subscripts 1 and 2 are used for solute (amino acid) and solvent (aqueous glucose/sucrose), respectively. KB integrals G12=G21, G11 and G22 represent solute-solvent, solute-solute, and solventsolvent interaction parameter respectively. Following equations were used to calculate these KB parameters [31,32]
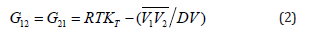
where, R is the gas constant, T is the absolute temperature,
V is the molar volume of the mixture, KT is the isothermal
compressibility. 
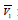 is the apparent molar volume of amino acid,
D is the derivative of chemical potential. The
is the apparent molar volume of amino acid,
D is the derivative of chemical potential. The 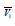 can be calculated using following relation
can be calculated using following relation
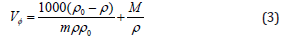
where M is the molar mass of the solute (amino acid), m is the molal concentration of the solute (amino acid), ρ and ρo are the densities of the solution and the solvent, respectively. KT can be calculated using following equation

where u is the ultrasonic speed in the solution. Here the KT values of solvents are being used as the isothermal compressibility of solutions contributes negligibly to the Gij values [33,34]. The parameter D in equation (2) is the derivative of chemical potential and can be solved using the equation
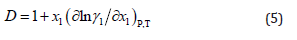
Here, x1 and γ1 are the mole fraction and activity coefficient
of solute, respectively. The 
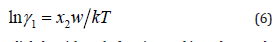
where w varies slightly with mole fraction and its value can be taken as 10-21, k is the Boltzmann constant, x2 is the mole fraction of solvent and T is the absolute temperature. Solute-solute and solvent-solvent interaction parameters, i.e., G11 and G22 can be calculated using solute-solvent interaction parameter G12
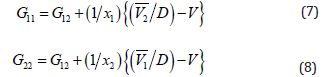
Results and Discussion
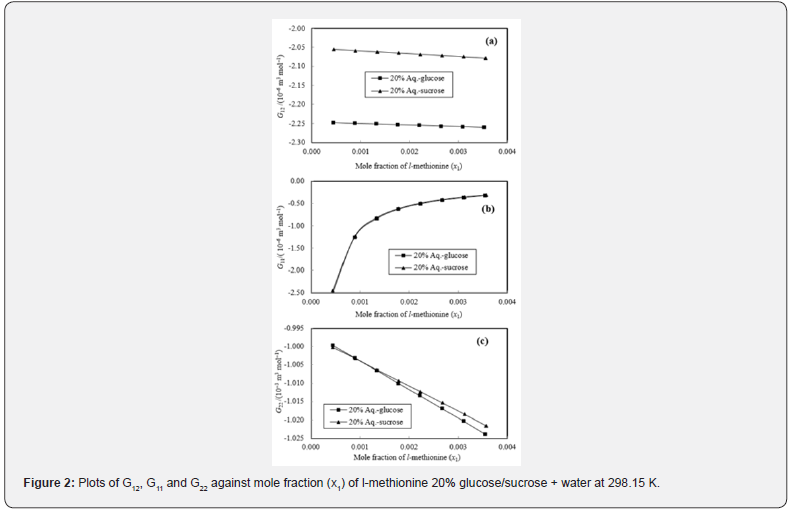
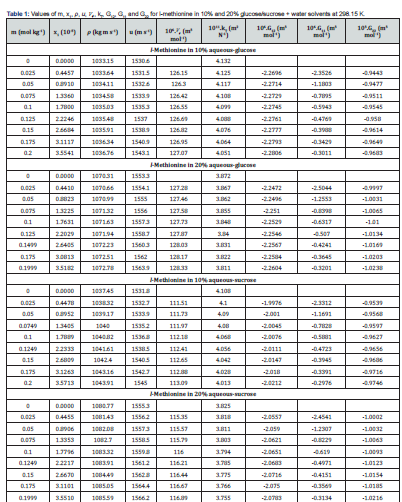
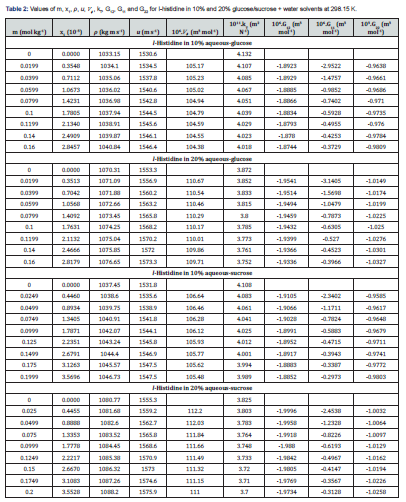
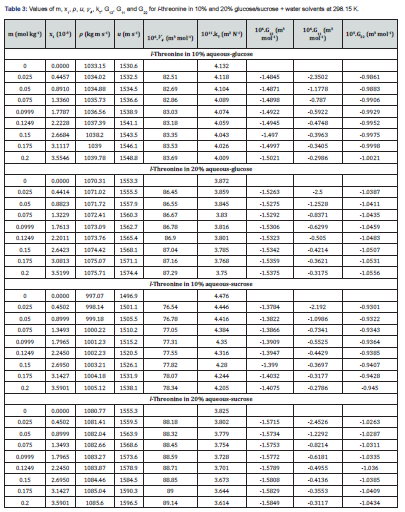
The KB integrals for nonpolar amino acid (l-methionine), positively charged polar amino acid (l-histidine) and neutral polar amino acid (l-threonine) in aqueous-glucose/sucrose system were calculated using the above-mentioned procedure. The calculated values of K-B integrals along with experimental data used in calculation are listed in Tables 1-3. The variations of different K-B interaction parameters, i.e., G12, G11 and G22 with x1 (mole fraction of amino acid) for 10% and 20% concentration of glucose/sucrose in water (w/w) individually for l-methionine, l-threonine and l-histidine are shown graphically in Figures 1-6.
Figures 1&2 shows the variation of K-B integrals for l-methionine in aqueous-glucose/sucrose solution for different mole fractions of l-methionine. Figures 1a & 2a indicates that G12 decreases with increase in l-methionine concentration in both aqueous-glucose and aqueous-sucrose systems. This suggests that solute-solvent interaction decrease with increase in l-methionine concentration, which in turn indicates the structure breaking ability of l-methionine in both these systems, which supports our earlier experimental studies [25,26] on these systems. In general, the types of interactions occurring between l-methionine and carbohydrate molecules can be classified as follows [36,37]
a. The hydrophilic-ionic interaction between OH groups of carbohydrate molecules and zwitterions of l-methionine molecule.
b. Hydrophilic-hydrophobic interaction between the OH groups of carbohydrate molecules and non-polar (–CH2) inside chain of l-methionine molecule.
c. Hydrophobic-hydrophobic group interactions between the non-polar groups of carbohydrate molecules and non-polar (– CH2) inside chain of l-methionine molecule.
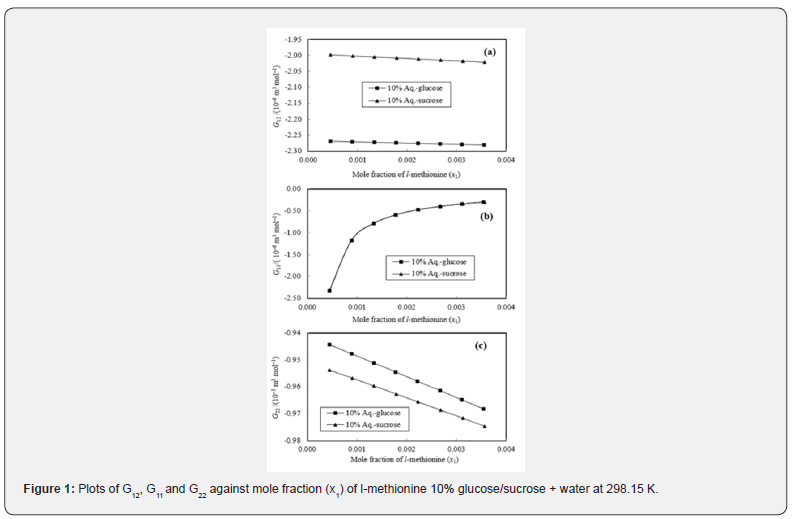
The magnitude of solute-solvent interaction in aqueoussucrose is larger than that of aqueous-glucose for same concentration of l-methionine. This can be explained based on greater hydrophilic-ionic group interactions in sucrose molecules due to presence of more hydroxyl groups as compared to glucose molecules. Figures 1b & 2b shows the variation of G11 representing solute-solute interaction of l-methionine in aqueous-glucose/ sucrose media. It has almost same magnitude in both glucose and sucrose media with sudden increase for 0.0005 mole fraction to 0.0015 mole fraction of l-methionine then gradual increase for rest of the compositions till 0.0040 mole fraction. Solventsolvent interactions, G22 show linear decrease with increase in l-methionine concentration (Figures 1c & 2c) in aqueous-glucose/ sucrose indicating that the solvent-solvent interaction decreases with increase in l-methionine concentration, which further reflects that the solute-solvent interaction dominates in these systems.
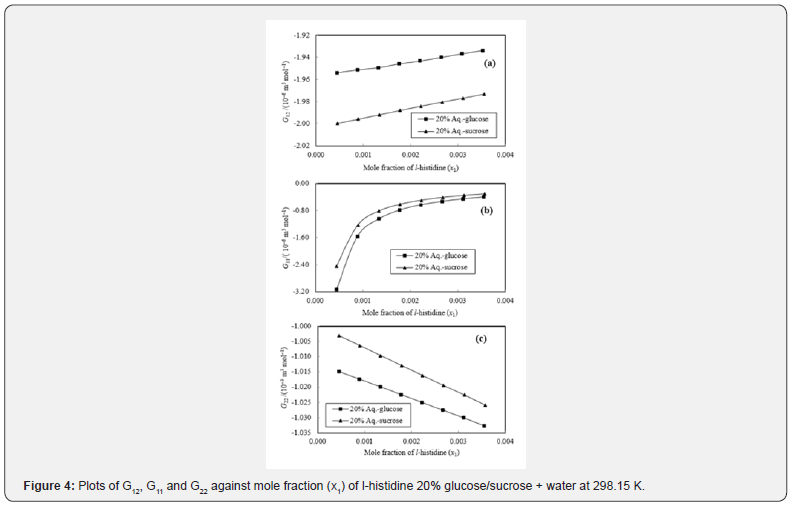
The K-B integrals of l-histidine in aqueous-glucose/sucrose system are shown in Figures 3 & 4. Figures 3a & 4a indicate that G12 the increase with increase in l-histidine concentration in both aqueous-glucose and aqueous-sucrose systems. This suggests that solute-solvent interaction increase with increase in l-histidine concentration, indicating the structure making ability of l-histidine in both these systems, which supports our earlier experimental studies [37,28] on these systems. The interactions occurring between l-histidine and carbohydrate molecules can be classified as follows [38,39]
i. The hydrophilic-ionic interaction between OH groups of carbohydrate molecules and zwitterions of l-histidine molecule.
ii. Hydrophilic-hydrophilic interaction between the OH groups of carbohydrate molecules and NH groups in the side chain of l-histidine mediated through hydrogen bonding.
iii. Hydrophilic-hydrophobic interaction between the OH groups of carbohydrate molecules and non-polar (–CH2) inside chain of l-histidine molecule.
iv. Hydrophobic–hydrophobic group interactions between the non-polar groups of carbohydrate molecules and non-polar (– CH2) inside chain of l-histidine molecule.
The variation of K-B parameter G11 representing solute-solute interaction among l-histidine molecules in aqueous-glucose/ sucrose media are shown in Figures 3b & 4b. Almost similar trend and magnitude was observed for l-histidine in both the media with sudden increase initially and almost constant value for higher l-histidine concentration. Figures 3c & 4c represents solventsolvent interaction parameter, G22, which decrease continuously with l-histidine concentration indicating that the solvent-solvent interaction decreases with increase in l-histidine concentration, which further reflects that the solute-solvent interaction dominates over solute-solute and solvent-solvent interactions in these systems.
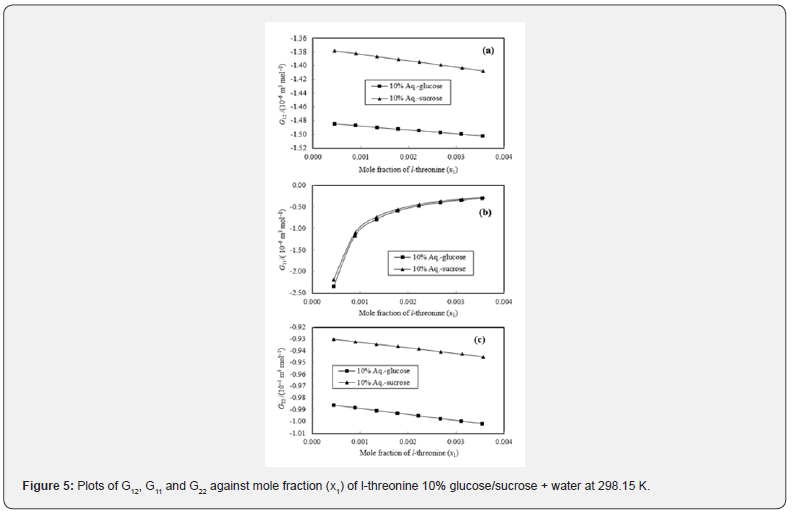
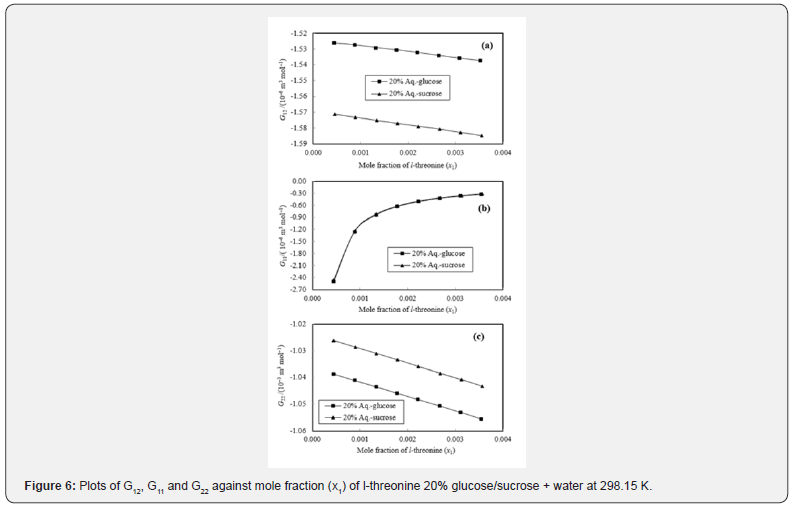
The K-B integrals of l-threonine in aqueous-glucose/sucrose solvents are shown in Figures 5 & 6. Figures 5a & 6a indicate that G12 the decrease with increase in l-threonine concentration in both aqueous-glucose and aqueous-sucrose systems. This suggests that solute-solvent interaction decrease with increase in l-threonine concentration, indicating the structure breaking ability of l-threonine in both these systems, which supported our earlier conclusions derived from experimental volumetric, ultrasonic, and viscometrical properties [29,30] of these systems. The interactions occurring between l-threonine and carbohydrate molecules following types of interactions are possible [38,39]
a) The hydrophilic-ionic interaction between OH groups of carbohydrate molecules and zwitterions of l-threonine.
b) Hydrophilic-hydrophilic interaction the OH groups of carbohydrate molecules and OH groups in the side chain of acid l-threonine mediated through hydrogen bonding.
c) Hydrophilic-hydrophobic interaction between the OH groups of carbohydrate molecules molecule and non-polar (–CH2) inside chain of l-threonine molecule.
d) Hydrophobic-hydrophobic group interactions between the non-polar groups of carbohydrate molecules and non-polar (– CH2) inside chain of l-threonine molecule.
Figures 5b & 6b variation of K-B parameter G11 representing solute-solute interaction among l-threonine molecules in aqueousglucose/ sucrose solvents, wherein sudden increase followed by very small increase in interaction with increase in l-threonine concentration is visible. The magnitude of all the three interaction parameters (G12, G11 and G22) is negative, but as compared to G12 and G11 has higher negative values. This confirms weaker solute-solute interaction and comparatively stronger solutesolvent interaction. A perusal of Figures 5c & 6c reveals that the solvent-solvent interaction parameter, G22 decreases continuously with increase in l-threonine concentration indicating that the solvent-solvent interaction decreases with increase in l-threonine concentration, which further reveals that the solute-solvent interaction dominates over solute-solute and solvent-solvent interactions in these systems.
Conclusion
The K-B integrals of nonpolar amino acid (l-methionine) positively charged polar amino acid (l-histidine) and neutral polar amino acid (l-threonine) in aqueous-glucose/sucrose (10% and 20% of glucose/sucrose in water, w/w) solvents have been evaluated from the ultrasonic speed and density data at 298.15K using the Kirkwood-Buff theory. The results indicated that solutesolvent interactions (hydrophilic-ionic interaction) between OH groups of carbohydrate molecules and zwitterions of these amino acid dominates over solute-solute and solvent-solvent interactions in these systems, and solute-solvent is found to increase with carbohydrate concentration. The variation of G12 with amino acid concentration revealed that l-histidine acts as structure maker while l-methionine and l-threonine act as structure breaker in these systems.
References
- JC Ahluwalia, C Ostiguy, G Perron, JE Desnoyers (1977) Volumes and heat capacities of some amino acids in water at 25 °C. Can J Chem 55: 3364-3367.
- M Sahin, Z Yesil, M Gunel, S Tahiroglu, E Ayranci (2011) Interactions of glycine with polyethylene glycol studied by measurements of density and ultrasound speed in aqueous solutions at various temperatures Fluid Phase Equilib 300: 155-161.
- MS Santosh, DK Bhat (2010) Excess molar volumes, viscosity deviations and isentropic compressibility changes in glycylglycine–NiCl2 aqueous ethanol mixtures Fluid Phase Equilib 298: 169-172.
- Riyazuddeen, S Afrin (2012) Interactions in (l-phenylalanine/l-histidine+ 001 mol· kg-1 aqueous β-cyclodextrin) systems at T=(29315, 29815, 30315 and 30815). K J Chem Thermodyn 54: 179-182.
- F Franks (2002) Protein stability: the value of ‘old literature’. Biophys Chem 96: 117-127.
- PH von Hippel, T Schleich (1969) Ion effects on the solution structure of biological macromolecules. Acc Chem Res 2: 257-265.
- A Taravati, M Shokrzadeh, AG Ebadi, P Valipour, ATM Hassan, et al. (2007) Various effects of sugar and polyols on the protein structure and function: role as osmolyte on protein stability. World Appl Sci J 2: 353-362.
- K Gekko (1981) Mechanism of polyol-induced protein stabilization: solubility of amino acids and diglycine in aqueous polyol solutions. J Biochem 90: 1633-1641.
- J F Back, D Oakenfull, MB Smith (1979) Increased thermal stability of proteins in the presence of sugars and polyols. Biochemistry 18: 5191-5196.
- JC Lee, SN Timasheff (1981) The stabilization of proteins by sucrose. J Biol Chem 256: 7193-7201.
- M Wusteman, S Boylan, DE Pegg (1996) The effect of cooling rate and temperature on the toxicity of ethylene glycol in the rabbit internal carotid artery. Cryobiology 33: 423-429.
- A Pal, N Chauhan (2009) Densities, speeds of sound and viscosities of L- alanine in aqueous fructose, maltose and lactose solutions at different temperatures. Indian J Chem A 48: 1069-1077.
- GA Kulikova, EV Parfenyuk (2008) Influence of side chain of L-α-amino acids on their interaction with D-glucose in dilute aqueous solutions. J Solut Chem 37: 835-840.
- SK Sharma, G Singh, R Kataria, H Kumar (2014) Volumetric and ultrasonic studies of solute–solute and solute–solvent interactions of N-acetyl glycine in aqueous sucrose solutions at different temperatures. Thermochim Acta 589: 23-130.
- I Banik, MN Roy (2012) Study of solute–solvent interaction of some bio-active solutes prevailing in aqueous ascorbic acid solution. J Mol Liq 169: 8-14.
- SK Sharma, G Singh, H Kumar, R Kataria (2015) Study of solute–solute and solute–solvent interactions of N-acetyl glycine in aqueous d-fructose solutions at different temperatures. Thermochim Acta 607: 1-8.
- AK Nain, M Lather (2013) Probing solute–solute and solute–solvent interactions in (l-arginine+ d-xylose/l-arabinose+ water) solutions at different temperatures by using volumetric and viscometric methods. J Chem Thermodyn 63: 67-73.
- AK Nain, R Pal (2014) Physicochemical study of solute–solute and solute–solvent interactions of l-phenylalanine in (water+ arabinose/glucose/sucrose) solutions at different temperatures. J Chem Thermodyn 68: 169-182.
- AK Nain, M Lather (2015) Study of solute–solute and solute–solvent interactions of l-serine in d-xylose/l-arabinose+ water solutions using volumetric, ultrasonic and viscometric methods at different temperatures. Phys Chem Liq 53: 599-618.
- AK Nain (2016) Physicochemical study of solute–solute and solute–solvent interactions of glycine, l-alanine, l-valine and l-isoleucine in aqueous-d-mannose solutions at temperatures from 29315 K to 31815 K. J Chem Thermodyn 98: 338-352.
- AK Nain, P Droliya, J Yadav, A Agarwal (2016) Physicochemical study of (solute+ solute) and (solute+ solvent) interactions of l-asparagine and l-glutamine in aqueous-d-mannose solutions at temperatures from (29315 to 31815). K J Chem Thermodyn 95: 202-215.
- Y Bisht, AK Nain (2019) Study of Kirkwood-Buff integrals of selected polar and nonpolar amino acids in aqueous-streptomycin sulphate solutions at 29815 K. Indian J Chem A 58: 281-287.
- AK Nain, P Droliya, J Gupta (2017) Theoretical study of molecular interactions of amino acids in aqueous carbohydrate solutions by scaled particle theory. Indian J Chem A 56: 939-944.
- JG Kirkwood, FP Buff (1951) The statistical mechanical theory of solutions. J Chem Phys 19: 774-777.
- AK Nain, M Lather, RK Sharma (2013) Study of solute–solute and solute–solvent interactions of l-methionine in aqueous-sucrose solutions at different temperatures. J Chem Thermodyn 58: 101-109.
- AK Nain, M Lather, RK Sharma (2011) Volumetric, ultrasonic and viscometric behavior of l-methionine in aqueous-glucose solutions at different temperatures. J Mol Liq 159: 180-188.
- AK Nain, R Pal, RK Sharma (2011) Volumetric, ultrasonic, and viscometric behaviour of l-histidine in aqueous-glucose solutions at different temperatures. J Chem Thermodyn 43: 603-612.
- AK Nain, R Pal, RK Sharma (2012) Physicochemical study of solute–solute and solute–solvent interactions of l-histidine in water+ sucrose solutions at different temperatures. J Mol Liq 165: 54-160.
- AK Nain, R Pal (2013) Study of solute–solute and solute–solvent interactions of l-threonine in aqueous-glucose solutions at different temperatures by using volumetric and viscometric methods. J Chem Thermodyn 60: 98-104.
- AK Nain, R Pal (2013) Volumetric, ultrasonic and viscometric studies of solute–solute and solute–solvent interactions of l-threonine in aqueous-sucrose solutions at different temperatures. J Chem Thermodyn 64: 172-181.
- A Ben Naim (1977) Inversion of the Kirkwood–Buff theory of solutions: application to the water–ethanol system. J Chem Phys 67: 4884-4890.
- E Matteoli, L Lepori (1984) Solute–solute interactions in water II An analysis through the Kirkwood–Buff integrals for 14 organic solutes. J Chem Phys 80: 2856-2863.
- KJ Patil (1981) Appliation of Kirkwood-Buff theory of liquid mixtures to water-butanol system. J Solut Chem 10: 315-320
- KJ Patil, DH Dagade (2004) Studies of molecular interactions in aqueous and CCl4 solutions involving 18-crown-6 by application of Kirkwood–Buff theory. J Chem Thermodyn 36: 677-682.
- JD Pandey, R Verma (2001) Inversion of the Kirkwood–Buff theory of solutions: application to binary systems. Chem Phys 270: 429-438.
- GR Hedwig, H Høiland (1993) Thermodynamic properties of peptide solutions 9 Partial molar isentropic pressure coefficients in aqueous solutions of sequence isomeric tripeptides with a single-CH3 sidechain. J Chem Thermodyn 25: 349-354.
- AK Mishra, JC Ahluwalia (1984) Apparent molal volumes of amino acids, N-acetylamino acids, and peptides in aqueous solutions. J Phys Chem 88: 86-92.
- R Bhat, N Kishore, JC Ahluwalia (1988) Thermodynamic studies of transfer of some amino acids and peptides from water to aqueous glucose and sucrose solutions at 29815 K. J Chem Soc Faraday Trans 84: 2651-2665.
- T Banerjee, N Kishore (2005) Interactions of some amino acids with aqueous tetraethylammonium bromide at 29815 K: A volumetric approach. J Solut Chem 34: 137-153.






























