Suppression of Bean Yellow Mosaic Virus by Plant Extracts in Faba Bean Plants
Elsharkawy Mohsen Mohamed1̽*, Sidaros Samir Armia1, Elkewey Shawky Abd El Raouf1, ElAidy Nadia Abd Elsalam2 and Elkhiat Gamalat Morshedy2
1Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University, 33516 Kafr El-sheikh, Egypt
2Seed Technology Department, Field Crops Res. Inst., Agric. Res. Center (ARC), Giza, Egypt
Submission: July 11, 2021; Published: August 09, 2021
*Corresponding author: M M Elsharkawy, Agricultural Botany Department, Faculty of Agriculture, Kafrelsheikh University, 33516 Kafr El-sheikh, Egypt
How to cite this article: Elsharkawy Mohsen Mohamed1*, Sidaros Samir Armia, Elkewey Shawky Abd El Raouf, ElAidy Nadia Abd Elsalam and Elkhiat Gamalat Morshedy. Suppression of Bean Yellow Mosaic Virus by Plant Extracts in Faba Bean Plants. Organic & Medicinal Chem IJ. 2021; 11(1): 555805. DOI: 10.19080/OMCIJ.2021.11.555805
Abstract
The virus was obtained from faba bean plants that were naturally infected. The studied virus was identified as Bean yellow mosaic virus (BYMV) according to symptomology, host range, virus stability, modes of transmission, ELISA, and RT-PCR. ELISA test confirmed the presence of BYMV in all the collected leaf samples at different times after inoculation. A fragment of coat protein gene (352 bp) was amplified using PCR from infected faba bean plants. Growth and seed characters were significantly lower in infected plants compared with healthy faba bean plants. Plant extracts promoted systemic resistance to BYMV. Disease severity and BYMV titre were substantially decreased in faba bean plants treated with extracts of Cinnamomum zeylanicum bark (CZ), Syzygium aromaticum, Matericaria chamomilla L., Foeniculum vulgare, Zingiber officinale before or after BYMV inoculation. The lowest disease severity and virus concentration were achieved using cinnamon extracts treated at 1 day before or after virus inoculation. In faba bean plants treated with plant extracts, the expression of pathogenesis-related genes (PR1 and PR2) was substantially higher than in control plants. These results explain the role of PR1 and PR2 genes in enhanced resistance against BYMV by plant extracts.
Keywords: Faba bean; Bean yellow mosaic virus; Induced resistance; ELISA; Real time PCR
Introduction
In Egypt, faba bean (Vicia faba L., Fabaceae) is a main food legume crop. It represents an important source of protein, carbohydrate, vitamins, essential amino acids, and mineral salts [1]. It is used as a human food in developing countries and as animal feed in industrialized ones, mostly for pigs, horses, poultry, and pigeons. It may be eaten raw or cooked, fresh, or preserved. After infecting the susceptible host plant, viruses cause a sequence of physiological changes that may lead to disease symptoms such as systemic and local symptoms [2,3]. BYMV (Bean Yellow Mosaic Potyvirus) is a widespread disease of beans and other hosts that may be found all over the globe [4]. It is a severe disease wherever susceptible crops grown because it is transmitted through seeds and Aphids [5]. Recently, Skelton et al. [6] isolated and identified BYMV from Dactylorhiza foliosa (a hardy orchid species, native to the Island of Madeira) that showed symptoms of chlorotic mottle and streaking. Finally, the virus was mechanically inoculated into two indicator species. Chenopodium quinoa (chlorotic local lesions) and Nicotiana benthamiana (distortion and mosaic) developed leaf symptoms. The presence of BYMV was verified by ELISA testing of plants exhibiting symptoms.
Several investigators found that faba bean was the most susceptible host to BYMV causing the considered losses in the grain yield [7]. This virus was isolated, either alone or mixed with other viruses, from faba bean. Systemic symptoms produced by BYMV infection may not kill faba bean plants, but they can spread quicker and deeper throughout the crop, causing a larger total yield loss, despite inducing milder symptoms [8,9]. A long-known virus inhibitor from carnation leaves has now been shown to be an inducer of systemic resistance to virus as well [10]. Thus, it is possible that many of the other well-known virus inhibitors of plant origin may in fact be proven to be inducers of systemic resistance. The earliest evidence that plants possess inhibitory compounds came from virus-infected plants, and then viral inhibitory substances were discovered in a variety of healthy plants from several Angiosperm families, including Amaranthaceae, Caryophyllaceae, Chenopodiaceae, Solanaceae, and Verbenaceae [11].
While working on resistance induced by leaf extracts from carnation plants, they observed that a very short period was required to the induced antiviral state in host tissue [10]. Pyrethrin (Pyrethrum) is a source of synthetic pyrethroid insecticides that is generated in the flowers of Chrysanthemum cinerariaefolium. Pyrethrin is approved for the use against a wide range of pests. One formulation of pyrethrin was shown to be moderately to very efficient (61-100 % control) against the fruit pests, such as grape leafhoppers, potato leafhoppers, leaf curl plum aphids, blueberry flea beetles, blueberry thrips, and blueberry sawfly. It also works against the cranberry fruit worm. It decomposes rapidly in the environment and may be used up to harvest day [12].
Ribosome-inactivating proteins (RIPs), which have antiviral effects, are found in many plant species [13]. A RIP from pokeweed (Phytolacca americana) was shown to provide broad-spectrum viral resistance in tobacco and potato. All the tested RIPs exhibited strong antiviral action [14]. Purified antiviral proteins from roots, shoots, leaves, fruits, and seeds of Mirabilis jalapa were successful in protecting economically significant crops (tobacco, maize, and potatoes) against a wide range of plant viruses such as Tobacco mosaic virus and Tomato spotted wilt virus [15,16]. This study aims to isolate of the most common faba bean virus found in collected samples and to evaluate the induced systemic resistance activities of some medicinal plant extracts against Bean yellow mosaic virus.
Materials and Methods
Isolation and identification of the isolated virus
One type of naturally infected faba bean samples showing virus-like symptoms was collected from faba bean fields, Legume Department, Sakha Agricultural Research Station, Kafr EL-Sheikh Governorate. According to Elsharkawy et al. [17], mechanical inoculation was employed to inoculate the test plants (faba bean). The viral isolate was biologically purified using Chenopodium amaranticolor L. and the local lesion method was used [18]. The Sakha Agricultural Research Station in the Kafr El-Sheikh Governorate, Egypt, provided sixty plant species and cultivars from ten distinct families that were mechanically inoculated by the isolated virus to study the host range.
Isolation and identification of the isolated virus
One type of naturally infected faba bean samples showing virus-like symptoms was collected from faba bean fields, Legume Department, Sakha Agricultural Research Station, Kafr EL-Sheikh Governorate. According to Elsharkawy et al. [17], mechanical inoculation was employed to inoculate the test plants (faba bean). The viral isolate was biologically purified using Chenopodium amaranticolor L. and the local lesion method was used [18]. The Sakha Agricultural Research Station in the Kafr El-Sheikh Governorate, Egypt, provided sixty plant species and cultivars from ten distinct families that were mechanically inoculated by the isolated virus to study the host range.
Serological diagnosis of the isolated virus using indirect ELISA
Faba bean samples showing symptoms suggested to be due to Bean yellow mosaic virus (BYMV) was tested with their specific antiserum by indirect ELISA technique. This method was described by Elsharkawy et al. [19]. Leaves of faba bean were ground in a mortar with 0.02 M phosphate buffer (1:10) and mix the sap 1:1 with 2x concentrated coating buffer, pH 9.6, fill the wells with 200μ1 aliquots of test samples. The plates were covered and incubated at 30- 37 °C for 2 hrs. Washing with PBS-Tween was repeated 3 times. Cross-absorbed antiserum (200 μ1) was added to each well then Incubated for 1-1.5h at 30-37°C followed by washing with PBS-Tween. Goat anti-rabbit antibody conjugated to alkaline phosphatase (200 μ1) was added and incubated for one hour at 37°C followed by washing with PBS-Tween. P-nitrophenyle phosphate (200 μ1) was added and the absorbance values was measured at 405nm using Vniskan ELISA reader.
Extraction of total nucleic acid from plant tissues infected with BYMV
The Spin/ vacuum SV total RNA isolation system (Promega Corporation, Maison, WI) has been used successfully to isolate RNA from leaves of healthy or BYMV infected faba bean plants. Thirty mg of infected and uninfected faba bean leaves were powdered in liquid nitrogen. One hundred seventy-five μl of RNA lysis buffer (4M Guanidine thiocyanate, 0.01 Tris-HCl, pH 7.5, 0.97% β- mercaptoethanol) was added and the tube was mixed by inversion. The dilution buffer was added, and the tube was mixed by inversion followed by centrifugation at 15000rpm in a microcentrifuge for 15minutes. RNA precipitation and DNase treatment were carried out as described by Elsharkawy et al. [17]. RT-PCR primers used in this study were designed to detect the coat protein gene of BYMV as described by Shooman [20].
The selection of the primers was performed according to the primer analysis software Oligo 4.1 (National Bioscience Inc., Plymounth MN, USA). The primer bought from integrated DNA Technologies, Inc. The primer pair used for the detection of BYMV (NIF-5’-GAGCGCATCGTTTCAATTCT-3’ and NIR-5’- AGCATGGGGCTATCCAACT-3’) was designed based on GenBank accession AM884180. The PCR primers utilized for cDNA synthesis and amplification of the BYMV coat protein gene were based on the conserved sequence of other BYMV isolates from all over the world. The purified total RNAs extracted from infected and healthy faba bean plants by using SV total RNA isolation system were used as starting material in the Reverse transcriptase polymerase chain reaction (RT-PCR) process as described by Shooman [20] and Hataya et al. [21]. the PCR products were examined on a 1% agarose gel electrophoresis and the size of the full length BYMV DNA fragment was determined in accordance with the DNA molecular weight markers.
Induction of systemic resistance against BYMV by plant extracts
Extracted oils from five medicinal plants (Cinnamomum zeylanicum withbark (Cinnamon), Syzygium aromaticum (Clove), Matericaria chamomilla L. (Chamomile), Foeniculum vulgare (Fennel), Zingiber officinale (Ginger) were kindly provided by Horticulture Department, Faculty of Agriculture, Kafrelsheikh University. Plant extracts (oils) were stored in the refrigerator at 4°C until use. The isolated virus inoculum was prepared by triturating 1 g of young viral symptomatic faba leaves cv. “Giza 843” in a sterilized mortar by adding 20ml (1:20 w/v) of sterile distilled water. The extract was filtered through muslin cloth then centrifuged for 20min at 3000rpm. The supernatant was used as inoculum.
Plastic pots (25cm) were filled with suitable amount of sandclay- loamy sterilized soil mixture. Ten seeds per pot were planted and subsequently trimmed to three seedlings after 15 days from planting. When needed, pots were irrigated with tap water in equal quantities. NPK fertilizers were used (0.6g of urea/pot, 0.75g of Ca-super-phosphate/pot, and 0.25g of K-sulphate/pot). Phosphorus was supplied before sowing during soil preparation. N and K were supplied in two equal dosages at thinning and two weeks afterwards.
Faba bean plants were divided into eleven groups (15 plants for each in 5 pots), five of them sprayed 24 hours pretreatment (virus-inoculation) with medicinal plants. Five groups were sprayed 24 hours post BYMV-inoculation with medicinal plants. The last group (15 plants) served as control, pretreated with water, and subsequently inoculated with the sap from virus-infected plants. Crude extracts of selected medicinal plants adjusted as 1:5 w/v and sprayed using pressure sprayer (2L). After two weeks from virus inoculation, virus concentration was determine using indirect ELISA as described previously and also disease severity of BYMV was measured based on the following scale: 0=no symptoms; 2=mild mosaic of the youngest two leaves; 4 = pronounced leaf deformation and mosaic of the youngest two leaves; 6=pronounced leaf deformation and mosaic with progression of symptoms into sequentially older leaves; 8=pronounced leaf deformation and mosaic, with all leaves expressing some forms of BYMV induced symptoms and 10=similar symptoms as described for a rating of 8, with plants also being stunted in growth.
Molecular investigation of pathogenesis-related genes expression
The kit (Thermo Scientific, Fermentas, #K0731) was used for RNA extraction. Pure RNA was reverse transcribed using the reverse transcription kits (Thermo Scientific, Fermentas, #EP0451). RNA was quantified using Nanodrop. The Q5000 (Uv- Vis’s spectrophotometer Q5000/USA) automatically performs all necessary measurements and calculations. For pure RNA, the OD260/OD280 ratio is less than 2. Protein contamination (which has a maximum absorbance of 280 nm) or phenol contamination will result in a ratio that is considerably lower than these values. The expression of target genes (PR1 and PR2) was assessed using real-time PCR with SYBR Green, with elongation factor 1 alpha (EF1α) as an internal reference following the manufacturer protocol (Thermo scientific, USA, # K0221) and gene specific primers. Table 1 lists the primers used in the amplification. The housekeeping gene (EF1α) is utilized to determine the relative gene expression or fold change in the target gene. Therefore, the 2-ΔΔCt technique was used to normalize the amounts of the target gene’s critical threshold (Ct) with the quantities of the housekeeping gene (EF1α) [22].
Statistical Analysis
The data were all presented as means (±S.E.). Comparisons were obtained using Duncan’s multiple range test (DMRT) and statistical significance was determined using one-way analysis of variance (ANOVA) by SPSS 18.0 software, 2011. When p<0.05, values were determined statistically significant.
Results
Isolation and identification of virus isolate
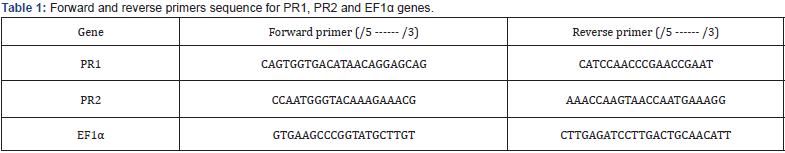
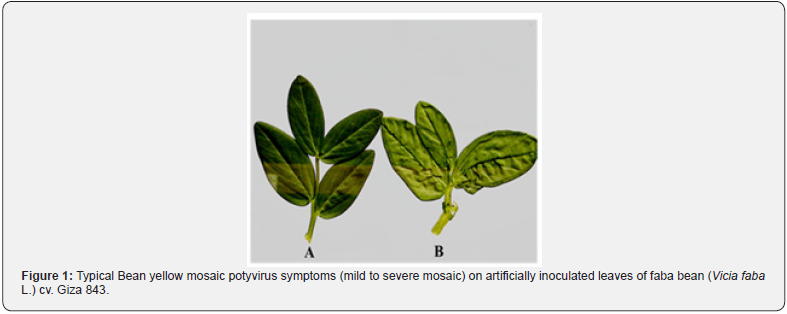
BYMV has a narrow host rang. Symptoms induced by the virus isolate in experimentally inoculated faba bean plants were like those expressed by field plants. BYMV induced severe and mild mosaic on all Vicia faba L. cultivars. The host range of the isolated virus was restricted to family leguminosae (Cicer arietinum, Lupinus termis, Phaseolus vulgaris, Trifolium alexandrinum and Vicia faba) and some members of Chenopodiaceae (Chenopodium album, C. amaranticolor and C. quinoa) (Figures 1&2). Stability of the isolated virus in sap indicated that, the thermal inactivation point was 60 to 65°C for BYMV, dilution end point was at 10-4 -10-5 and the longevity in vitro was 2 to 3 days. The isolated virus suggestive Bean yellow mosaic virus was mechanical transmissible readily (90–95%) with the infectious sap. Both green peach aphid (Myzus persicae Sulz.) and faba aphid (Aphis fabae) were found to be vectors of BYMV in a non-persistent manner with the average rate 80% and 40%, respectively. Seeds were harvested from mechanically inoculated faba bean (Vicia faba L.) plants and showed obvious viral infections with the isolated virus. The percentage of seed transmission was 10-13 % for BYMV in faba bean cultivar 843. BYMV was identified by ELISA. The experimental results confirmed the presence of BYMV in the infected samples. Positive reaction was obtained using BYMV-specific antiserum.
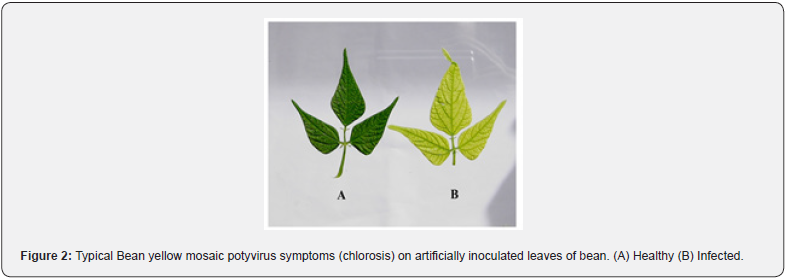
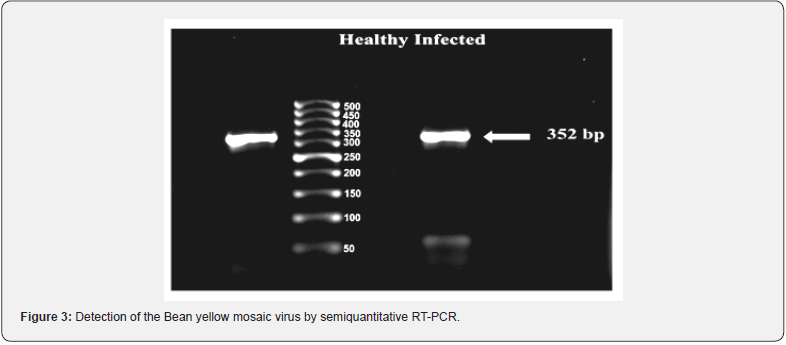
Detection of BYMV-E in infected faba bean plants was accomplished through the amplification of the viral RNA by RTPCR (Fig. 3). Isolation of RNA from plant tissues was done by SV total-RNA extraction method. The total RNA yield obtained was 137.7μg/100μl elution volume at 260nm, indicating high yield of RNA and the purity of total RNA also exhibited an A260/A280 absorbance ratio of 2.37 indicating high purity of total RNA isolated by the method described previously. Following PCR, the amplification products were analyzed by gel electrophoresis (Figure 3). The fragment was amplified from infected faba bean leaf tissues by using primer set. It appears from these data that the isolated virus is Bean yellow mosaic virus.
Effect of Bean yellow mosaic virus on faba bean growth
Chlorophyll a, b and total chlorophyll were significantly lower in infected plants (7.96, 8.37 and 15.36, respectively) compared with healthy plants (6.31, 7.13 and 13.58, respectively) at 60 days from sowing. Shoot and root length were significantly lower in infected plants compared to healthy plants. Similarly, seedling dry weight was significantly reduced due to BYMV infection in faba bean plants at 8 days after planting (Data not shown). Weight and volume of 100 seeds from infected faba bean were significantly lower compared with seeds from healthy plants. Moreover, relative density of seeds and germination percentage were also reduced in infected plants in comparison with healthy plants (Data not shown). Crude protein percentage in infected seeds was higher in infected faba bean plants (38.5) compared with the healthy seeds (33.5).
Effect of plant extracts application on BYMV disease severity
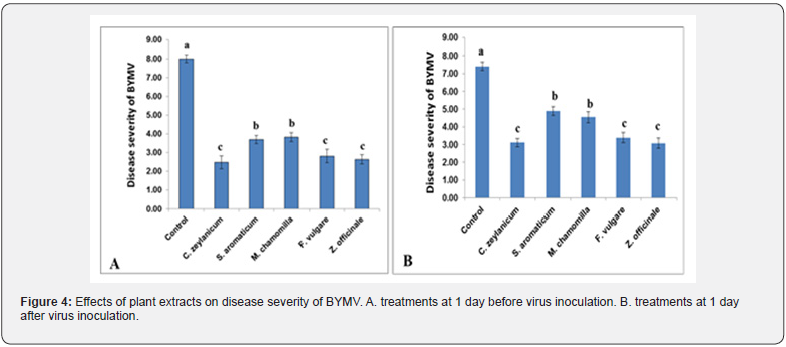
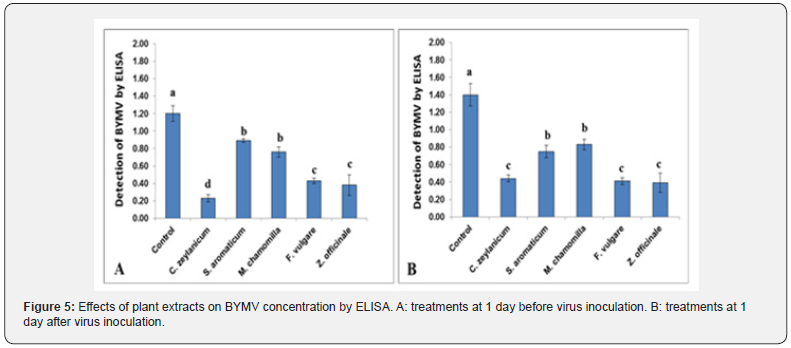
The lowest disease severity was achieved using cinnamon extracts under pot experiments. Ginger and fennel extracts (2.64, 2.81, respectively) were more effective than the extracts of clove and chamomile (3.69, 3.83, respectively). The protective activity of oil extracts was significantly effective compared with the control (8.00) (Figure 4A). On the other hand, the curative treatments of plant extracts after BYMV inoculation were also significant compared with the control (Figure 4B). Treatment with ginger extract achieved the best results (3.08) compared to the control (7.40). The lowest virus concentration was recorded in cinnamon (Cinnamomum zeylanicum) treatment (0.23) compared to control treatment (1.2). Similarly, BYMV concentration in plants treated with extracts before virus inoculation was also lower than the control (Figure 5A). Faba bean leaves sprayed with ginger extract showed the best results in this respect (0.39), compared to control treatment being 1.4 (Figure 5B).
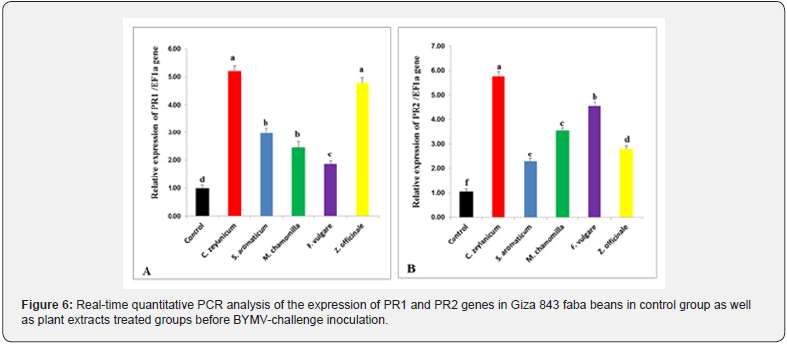
Effect of plant extracts treatments before BYMV inoculation on the relative expression of defense genes
Our results revealed a significant (P≤0.05) increase of PR1 gene expression level in plant extract treated faba bean plants before BYMV inoculation as compared to the control (Figure 6A). Plants treated with cinnamon extracts showed the highest expression values (5.21), as compared to non-treated plants (1.01). As compared to control treatment, PR2 gene expression level was significantly increased in plant extract treated faba bean plants before BYMV inoculation (Figure 6B). In addition, plants treated with cinnamon extracts resulted in the highest upregulation of PR2 gene expression followed by fennel, chamomile, ginger, and clove (5.78, 4.56, 3.56, 2.81 and 2.28, respectively).

Effect of plant extracts treatments after BYMV inoculation on the relative expression of defense genes
Our results revealed a significant (P≤0.05) increase in PR1 gene expression level in plants treated with plant extracts as compared to the control (Figure 7A). However, no significant difference in gene expression was found between clove, chamomile, and fennel treatments (1.71, 1.69 and 1.47, respectively). When comparing all treatments, treatment with cinnamon (3.43) showed the highest expression of PR1 gene than other treatments. PR2 gene expression level was significantly increased in plants treated with plant extracts as compared to control (Figure 7B). In all groups, cinnamon and fennel treated plants (2.89 and 2.28, respectively) showed a significant higher expression than other treatments. Moreover, treatment with cinnamon resulted in higher upregulation of PR2 gene expression than fennel sprayed plants (Figure 7B).
Discussion
Recently, faba bean crop in Egypt suffered great losses due to infection with mosaic diseases. Bean yellow mosaic potyvirus (BYMV) was reported to be one of the most prevalent viruses affecting leguminous crops in Egypt. Approximately 75% of aphidvectored potyviruses are transmitted in a non-persistent (noncirculative) manner [23]. The results confirmed the presence of BYMV in the infected samples of faba bean using indirect ELISA. ELISA proved to be reliable and sensitive method for detecting and identifying BYMV in infected faba bean extracts [6,24]. Bean yellow mosaic virus (BYMV) is composed of a protein molecular weight of 30-35kd. In this study, the detection and quantification of BYMV in infected plant tissues was based on semi-quantification reverse transcription polymerase chain reaction (RT-PCR). RT-PCR was used to detect low concentration of BYMV in total RNA extracts of infected gladiolus leaves [25].
In the present work, the decrease in growth, pigment contents and seed characters and viability probably explained based on an extensive production of excitation energy that occurred under virus stress, leading to photo-inhibitory damage in the reaction centers within chloroplasts [26]. Moreover, the lower number of chloroplasts is thought to be directly responsible for the decrease of chlorophyll content detected in BYMV-infected leaves. In compatibility with the previous results, a chloroplast is the most sensitive plant cell organelle to viral infection [27]. The application of antiviral principles derived from higher plants as biological virus control agents seems to be very promising. Antiviral proteins (AVPs) are most of these compounds, which are proteinaceous in origin.
However, deletion of the C-terminal of Pokeweed antiviral protein (PAP) mutants reduces viral infection but do not depurinate host ribosomes, indicating that PAP’s antiviral action may be separated from its ribosome inactivating proteins (RIPs) activity [28]. In the current study, faba bean plants were sprayed with plant extracts of Cinnamomum zeylanicum bark (Cinnamon), Syzygium aromaticum (Clove), Matericaria chamomilla L. (Chamomile), Foeniculum vulgare (Fennel), Zingiber officinale (Ginger) at 1 day before or after BYMV inoculation, to control BYMV in faba bean plants. Cinnamon extracts achieved the best results in reduction of disease severity, BYMV titre and activation of PR1 and PR2 gene expressions in treated plants compared with the control. Clerodendrum inerme contains basic protein which is resistant to proteases. Induced systemic resistance by antiviral agents has been introduced against many plant viruses [17,19,29,30]. A minimum concentration of 400Rg/ml of MAP (Mirabilis jalapa antiviral protein) was sufficient to inhibit TSWV [16].
In addition, Mirabilis antiviral protein (MAP) was isolated from roots and leaves of Mirabilis jalapa L. which possess repellent properties against aphids and white flies. MAP showed antiviral activity against mechanically transmitted viruses but not against aphid transmitted viruses [15]. Barakat et al. [14] reported that, Endogenous proteins have been discovered as ribosomeinactivating proteins (RIPs) such as enzymes that operate on ribosomes in a very particular manner in a variety of plant species. Six RIPs were isolated from leaves and/or seeds of several plant species, including types I and 2. Tobacco necrosis virus (TNV) on Phaseolus vulgaris plants, Tobacco mosaic virus (TMV) on Chenopodium amaranticolor plants, and Bean yellow mosaic virus (BYMV) on its systemic host (Vicia faba), all tested RIPs exhibited strong antiviral action.
Conclusion
In conclusion, the isolated virus was identified as Bean yellow mosaic virus based on host range, virus stability in crude sap, transmission, ELISA, and RT-PCR. Plant extracts was successfully used to control BYMV. The expression of pathogenesis-related genes (PR1 and PR2) was substantially higher in faba bean plants treated with plant extracts before or after BYMV inoculation which confirmed that the induced resistance was involved in disease control mechanisms.
References
- Guerrero F, Yáñez K, Vidal V, Cereceda Balic F (2019) Effects of wood moisture on emission factors for PM2.5, particle numbers and particulate-phase PAHs from Eucalyptus globulus combustion using a controlled combustion chamber for emissions. Sci. Total Environ 648: 737-744.
- Cereceda Balic F, Toledo M, Vidal V, Guerrero F, Diaz Robles L A, et al. Emission factors for PM5, CO, CO2, NOx, SO2 and particle size distributions from the combustion of wood species using a new controlled combustion chamber 3CE. Sci Total Environ 584-585: 901-910.
- Ravindra K, Sokhi R, Van Grieken R (2008) Atmospheric polycyclic aromatic hydrocarbons: Source attribution, emission factors and regulation. Atmos Environ 42: 2895-2921.
- Yan D, Wu S, Zhou S, Tong G, Li F, et al. (2019) Characteristics, sources, and health risk assessment of airborne particulate PAHs in Chinese cities: A review. Environ Pollut 248: 804-814.
- Kim K H, Jahan S A, Kabir E, Brown R J C (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60: 71-80.
- Guerrero F, Hernández C, Toledo M, Espinoza L, Carrasco Y, et al. (2021) Leaf Thermal and Chemical Properties as Natural Drivers of Plant Flammability of Native and Exotic Tree Species of the Valparaíso Region, Chile. Int J Environ Res Public Health 18: 7191.
- Perez P, Menares C, Ramírez C (2020) PM2.5 forecasting in Coyhaique, the most polluted city in the Americas. Urban Clim 32: 100608.






























