NSF-Funded Green Drug Discovery Research Program Based on a 90MHz NMR Spectrometer
Joseph Kipronoh Rugutt*
Department of Chemistry, Missouri State University-West, USA
Submission: November 15, 2020;Published: December 04, 2020
*Corresponding author: Joseph Kipronoh Rugutt, Department of Chemistry, Missouri State University-West, USA
How to cite this article: Joseph K R. NSF-Funded Green Drug Discovery Research Program Based on a 90MHz NMR Spectrometer. Organic & Medicinal Chem IJ. 2020; 10(3): 555786. DOI: 10.19080/OMCIJ.2020.09.555786.
Keywords:Green Drug; Spectrometer; Nanosynthesis; Bioactive chalcones; Medicinal plants; Silver nanoparticle; Chromatography-grade silica gel; Cycloaddition; Acetylated chalcone; Labile diene; Thai ginger; Boesenbergia pandurate; Water; Ionic liquids; Methyl acrylate
Mini Review
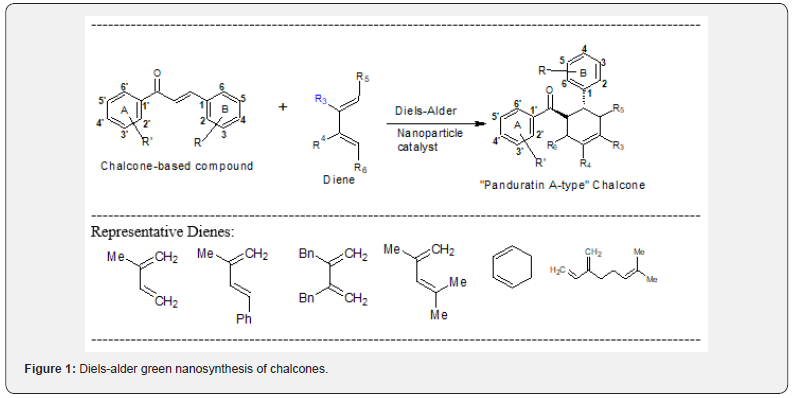
My ongoing research is focused on green Diels-Alder nanosynthesis of bioactive chalcones [1] derived from medicinal plants endemic to Kenya [2-5]. The versatile Diels-Alder [4+2] cycloaddition [6] is an enviable asset in the organic chemist’s toolbox because it triggers the simultaneous setting of asymmetric centers, ring(s), and functional groups. Cong and co-workers [7] were the first to report the use of silver nanoparticle (AgNP) catalysts in the Diels-Alder total synthesis of the cytotoxic chalcone-based natural product panduratin A. The poorly reactive but electron-rich trans-2’-hydroxychalcone was used as a dienophile. Their initial studies revealed that trans-2’-hydroxychalcone and 1-phenyl-3-methylbutadiene in the presence of a mixture of 30 mol % AgBF4 and 10 mol % Bu4NBH4 gave the desired [4+2] cycloadduct in 98% yield as a single Regio isomer. Conversions (“yields”) and the endo/exo ratios were calculated based on 1H-NMR integration. Catalytically active AgNPs were fixed onto chromatography-grade silica gel in simple steps: A 3:1 AgBF4/ Bu4NBH4 supernatant in CH2Cl2 was stirred with silica gel for three hours at 25oC. The resulting light brown silica-supported AgNPs catalyzed the cycloaddition of acetylated chalcone and the labile diene (trans-β-ocimene) affording the desired endo cycloadduct (panduratin A) in 98% yield. Panduratin A and its regioisomer (1′R,2′S,6′R)-2-hydroxyisopanduratin A) have been isolated [8] from the rhizomes of Thai ginger, Boesenbergia pandurata. Both compounds showed significant preferential cytotoxicity against human pancreatic PANC-1 cancer cells in nutrient-deprived medium.
In my research group, we have implemented successful nanosynthetic protocols [6-9] in the preparation of new [4+2] cycloadducts based on chalcones and dienes (Figure 1). The polar organic solvents used in the cycloaddition reaction [7] have been replaced with environmentally benign water, poly (ethylene glycol) (PEG), or ionic liquids (ILs). ILs were synthesized as described in the literature [10]. Breslow & Zhu [11] discovered that Diels-Alder reactions proceed faster in water (as high as 700-fold) and with a higher endo/exo selectivity than in organic solvents. The first study using ILs in Diels-Alder cycloaddition was the reaction of cyclopentadiene with two dienophiles (i.e., methyl acrylate and methylvinyl ketone) in ethylammonium nitrate ([EtNH3] [NO3]) [12]. These cycloadditions lead to a mixture of exo and endo products with a preference for the endo product. We have synthesized various chalcones through green synthetic methods [13]. Additional chalcones were purchased from common chemical suppliers (e.g., Sigma-Aldrich) and used as dienophiles.
Hundreds of chalcone-containing natural products continue to be discovered each year [8]. Retro-Diels-Alder (rDA) [14] of bioactive chalcone-based synthetic and natural products containing cyclohexenyl nucleus has guided us in selecting the additional dienes [15]. We are currently evaluating how cycloadditions of chalcones and a range of mono-, di-, tri-, and tetrasubstituted dienes (Figure 1) affect the yields of reactions and bioactivities. Our potentially transformative Diels-Alder reactions are clearly bioinspired because the cycloadducts (Figure 1) are analogues of the bioactive prenylated chalcones natural products,(-)-panduratin A and nicolaioidesin C [16]. A library of biologically relevant chalcone scaffolds generated from Diels-Alder reactions will be bio-tested against several biological targets (human cancer cell lines, Caenorhabditis elegans, mosquitoes, Drosophila melanogaster, Mycobacterium smegmatis, etc.) to decipher the structure-activity relationships (SAR).
This NSF-funded chalcone project is a minefield for generating interesting research data. Undergraduate and graduate students are already synthesizing the beautiful and inspiring “Pina’s-type” [17] crystalline compounds of the anthocyanin family. They are learning important research skills including green nanosynthesis, chromatographic purification, and characterization of compounds using NMR spectroscopy. In our research, [18,19] we have used Anasazi’s Fourier Transform (FT) [20] 90MHz NMR spectrometer [21] as a “workhorse” instrument for both research and teaching purposes. Despite the inherent drawbacks of low sensitivity and low resolution, the permanent magnet spectrometer can be used for a robust drug discovery project like ours. It is important to emphasize that the compound(s) of interest must be synthesized in gram quantities to reduce data acquisition time. Advantages of the permanent magnet are numerous including durability, low power consumption, user-friendly, ability to do various 1D/2D NMR experiments, and low maintenance (does not require cryogens, an NMR technician, etc.).
Acknowledgement
The author (Professor Joseph Kipronoh Rugutt) gratefully acknowledges the National Science Foundation for a grant (# 1920137) that supports an ongoing (2019-2022) project entitled “Undergraduate Student Achievers in Research (USTAR) Program” at Missouri State University-West Plains (MSU-WP) and collaborating institutions in Kenya.
References
- Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, et al. (2017) Chalcone: A Privileged Structure in Medicinal Chemistry. Chem Rev 117(12): 7762-7810.
- Rugutt J K, Henry C W, Franzblau S G, Warner I M (1999) NMR and molecular mechanics study of pyrethrins I and II. J Agric Food Chem 47(8): 3402-3410.
- Rugutt J K, Rugutt K J, Berner D K (2001) Limonoids from Nigerian Harrisonia abyssinica and their stimulatory activity against Striga hermonthica seeds. J Nat Prod 64(11): 1434-1438.
- Rugutt J K, Rugutt K J (2012) Antimycobacterial activity of steroids, long-chain alcohols and lytic peptides. Nat Prod Res 26(11): 1004-1011.
- Thornburg C C, Britt J R, Evans J R, Akee R K, Whitt J A, et al. (2018) NCI Program for Natural Product Discovery: A Publicly Accessible Library of Natural Product Fractions for High-Throughput Screening. ACS Chem Biol 13(9): 2484-2497.
- Ashburn B O, Carter R G (2008) Diels-Alder approach to biaryls (DAB): importance of the ortho-nitro moiety in the [4 + 2] cycloaddition. Org Biomol Chem 6(2): 255-257.
- Cong H, Becker C F, Elliott S J, Grinstaff M W, Porco J A (2010) Silver nanoparticle-catalyzed Diels-Alder cycloadditions of 2'-hydroxychalcones. J Am Chem Soc 132(21): 7514-7518.
- Win N N, Awale S, Esumi H, Tezuka Y, Kadota S (2007) Bioactive secondary metabolites from Boesenbergiapandurata of Myanmar and their preferential cytotoxicity against human pancreatic cancer PANC-1 cell line in nutrient-deprived medium. J Nat Prod 70(10): 1582-1587.
- Hobbs J M, Patel N N, Kim D W, Rugutt J K, Wanekaya A K (2013) Glucose Determination in Beverages Using Carbon Nanotube Modified Biosensor: An Experiment for the Undergraduate Laboratory. J Chem Educ 90(9): 1222-1226.
- Goossens K, Lava K, Bielawski C W, Binnemans K (2016) Ionic Liquid Crystals: Versatile Materials. Chem Rev 116(8): 4643-4807.
- Breslow R, Halfon S (1992) Quantitative effects of antihydrophobic agents on binding constants and solubilities in water. Proc Natl Acad Sci U S A 89(15): 6916-6918.
- Soldaini G, Cardona F, Goti A (2005) Catalytic oxidation-phosphorylation of glycals: rate acceleration and enhancement of selectivity with added nitrogen ligands in common organic solvents. Org Lett 7(4): 725-728.
- Siddiqui Z N, Musthafa T N, Ahmad A, Khan A U (2011) Thermal solvent-free synthesis of novel pyrazolyl chalcones and pyrazolines as potential antimicrobial agents. Bioorg Med Chem Lett 21(10): 2860-2865.
- Liu Y, Lu K, Dai M, Wang K, Wu W, et al. (2007) An Efficient One-Pot Asymmetric Synthesis of Biaryl Compounds via Diels−Alder/Retro-Diels−Alder Cascade Reactions. Org. Lett 9(5): 805-808.
- Cragg G M, Grothaus P G, Newman D J (2009) Impact of Natural Products on Developing New Anti-Cancer Agents. ChemRev 109(7): 3012-3043.
- Tidgewell K J (2016) Review of Natural Products Chemistry: Sources, Separations, and Structures. J Nat Prod 79(10): 2762-2762.
- Pina F (2014) Chemical Applications of Anthocyanins and Related Compounds. A Source of Bioinspiration. Journal of Agricultural and Food Chemistry 62(29): 6885-6897.
- Rugutt J K, Travis L Pohl, Benton G Grills, Taewan Park, ChulapolThanomsing, et al. (2013) Infusing the Undergraduate Curriculum with Medicinal Chemistry Techniques Using Real-World Examples Involving Bioassay-Directed Synthesis of Chalcone-based Citrus Fruit Bioregulators. Chem. Educator 18: 295-301.
- Rugutt J K, Alexandra E Graham, ChulapolThanomsing, Katie R Wilson, Keil B Harris, et al. (2013) Adding Value to Organic Chemistry Curriculum by Infusing Guided Inquiry Nanoscience Experiments Chem. Educator 18: 116-120.
- Bell H M (1993) Fourier transform NMR simulations. J Chem Educ 70(12): 996.
- Manzano Maria R, Colnago L A, Aparecida Forato L, Bouchard D (2010) Fast and Simple Nuclear Magnetic Resonance Method to Measure Conjugated Linoleic Acid in Beef. J Agric Food Chem 58(11): 6562-6564.






























