N- and / or O- Alkylation of Quinazolinone Derivatives
Anton V Kolotaev1,2, Vasiliy N Osipov1,3, Karine R Matevosyan4, Eugene A Bondarenko1,4, Arthur R Mavlianberdiev1,4 and Derenik S Khachatryan1,2*
1The Federal State Unitary Enterprise, Institute of Chemical Reagents and High Purity Chemical Substances of National Research Centre, Kurchatov Institute, Russia
2National Research Centre, Kurchatov Institute, Russia
3N N Blokhin National Medical Research Center of Oncology, Russia
4Mendeleev University of Chemical Technology of Russia, Russia
Submission: October 12, 2020;Published: November 16, 2020
*Corresponding author: Derenik S Khachatryan, The Federal State Unitary Enterprise, Institute of Chemical Reagents and High Purity Chemical Substances of National Research Centre, Kurchatov Institute, Bogorodsky val 3, Moscow 107076, Russia and National Research Centre, Kurchatov Institute, Akademika Kurchatova pl. 1, Moscow 123182, Russia
How to cite this article: Anton V K, Vasiliy N O, Karine R M, Eugene A B, Arthur R M, et al. N- and / or O- Alkylation of Quinazolinone Derivatives. Organic & Medicinal Chem IJ. 2020; 10(2): 555781. DOI: 10.19080/OMCIJ.2020.10.555781.
Abstract
Here we report on a strategy based on the capabilities of 2D NMR spectroscopy, which focuses on determining the exact structures of promising HDAC / VEGF-2 inhibitors and intermediate N- or O-alkylated building blocks for their construction. Due to which, in contrast to the erroneous conclusions of other studies, it has been unequivocally established that quinazolin-4-ones with alkyl halides under the classical conditions of two-phase catalysis: the solid phase (alkali metal carbonates) and liquid (aprotic solvents) are subjected to alkylation in the 3-N- position.
Keywords:Analgesic; Anti-inflammatory; Antibacterial; Antifungal; Antiviral; Antihistamine; Antihypertensive; Anti-cancer; Antihyperglycemic; Anti-HIV
Introduction
The study of the regiochemistry of the alkylation process takes one of the key positions in the development of the organic synthesis methodology [1-8]. A classic synthetic problem that remains unresolved is a reaction that involves the control of N- and / or O-alkylation of ambident anions, in particular, the study of the process of N- and O-alkylation of quinazolinones. Uncertainty (inaccuracy) of whether the product(s) are N- and / or O-alkylated is common and can be an unpleasant (expensive) omission (especially in pharmacology) if the structure of the final compound is not adequately defined.
Currently, one of the promising strategies in creating new pharmaceuticals is the design and synthesis of hybrid compounds consisting of two or more different bioactive fragments and acting through the activation / blocking of several targets. The combination of two active groups in one molecule can lead to a more pronounced therapeutic effect, compared with the individual components [9-10]. Thereby, successful work appeared on the development of hybrid molecules based on quinazoline. Quinazolinone derivatives, which contain a natural uracil fragment, exhibit a wide range of biological activity: analgesic [11], anti-inflammatory [12], antibacterial [13,14], antifungal [15], antiviral [16], antihistamine [17], antihypertensive [18], anti-cancer [19], antihyperglycemic [20] and anti-HIV [21]. Combining the foregoing fragment with other pharmacophores, there were constructed polyfunctional inhibitors containing a quinazoline cycle and hydroxamic acid acting on HDAC and other targets: vascular endothelial growth factor (VEGF-2) [21-23], tyrosine kinases (EGFR, HER2) [24-27]. The most successful compound (CUDC-101) is undergoing clinical trials as a multi-target antitumor drug [28,29].
Results
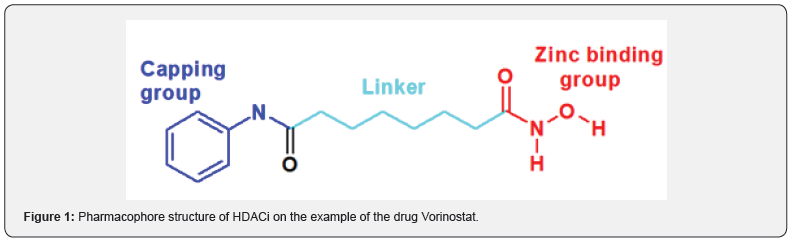
Based on the foregoing, in order to create more efficient hybrid molecules with bi-inhibitory activity of HDAC / VEGF-2, we decided to construct a system similar to Vorinostat (Figure 1) by functionalizing it with N- and/or O-alkylation based on quinazoline derivatives (analogs of VEGF-2 inhibitors) as a “capping” group (Scheme 1).
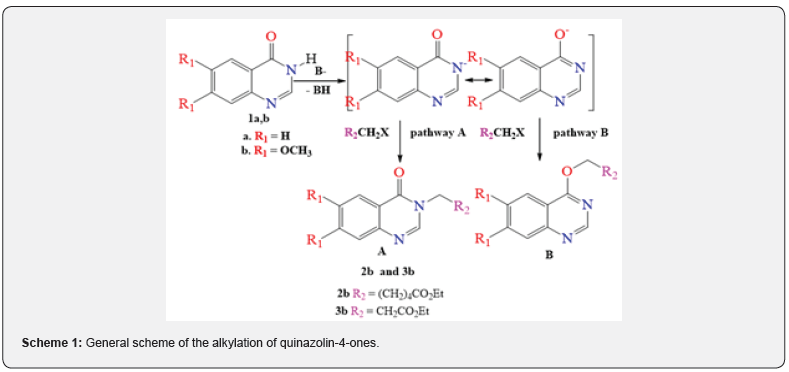
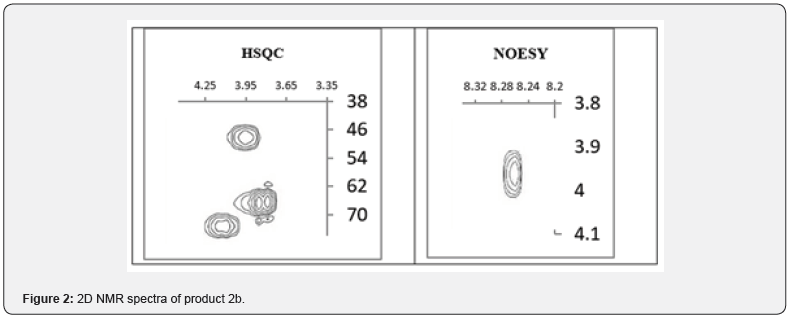
To see of literature sources afford that for the O-alkylation of quinazolin-4-ones, good results are obtained when carrying out processes in a two-phase system: solid phase (K2CO3 or Cs2CO3) – liquid phase (DMF, DMSO, acetone, etc. aprotic solvents) [30-31]. However, when we carried out the alkylation of quinazolin-4-one with ethyl 6-bromohexanoate in the described conditions, it was found out that, in contrast to the expected O–isomer B, the product of N-alkylation 2b (R1= OCH3, R2 = (CH2)5CO2CH2CH3) was formed with 85% yield (pathway A). The structure of product 2b was established based on 2D NMR spectroscopy (HSQC/ NOESY, HMBC and 13C)1e-f, which allow one to determine the regioselectivity of alkylation during optimization of synthetic routes. In particular, the formation of the N-alkylation product is confirmed by the correlation of the protons of the NCH2 group with the 2-H proton of the quinazolinone ring in the NOESY spectrum, as well as the chemical shift of the carbon of the NCH2 group equal to 45.57ppm (Figure 2).
This discrepancy led us to consider the process more carefully, given that studying the process of N- and O-alkylation of quinazolinones by changing the conditions of the alkylation and / or the nature of the substituents (donor-acceptor properties and steric features) in the used reagents opens up wide possibilities for obtaining from the same reagents of both 3-N-alkylquinazolinone and 4-alkoxyquinazoline. This circumstance has recently become an important tool in creating libraries of various scaffolds for SAR in the search for biologically active substances [32-34]. The urgent need was the repetition of these [30-31] studies, which contradicts the results of work [35], where instead of the O-alkylation product, the N-alkylation product was obtained in the course of reactions under almost the same conditions, which we proved using various methods of NMR spectroscopy of compounds obtained by the above methods. The alkylation of 6,7-dimethoxyquinazolin-4(3H)- one with ethyl chloroacetate under the above [30-31,35-38] conditions also led to the formation of the N-alkylation product 3b (R1=OCH3, R2=CH2CO2CH2CH3) with 82 % yield (pathway A), which was confirmed by 2D NMR spectroscopy. The formation of the N-alkylation product is confirmed by the correlation of the protons of the NCH2 group with the 2-H proton of the quinazolinone in the COSY spectrum, as well as the chemical shift of the carbon of the NCH2 group equal to 47.79ppm (Figure 3).
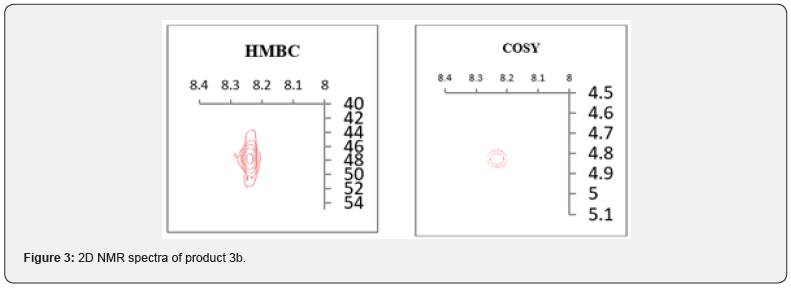
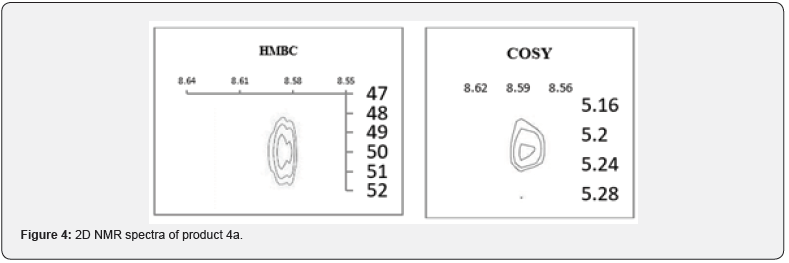
Alkylation of quinazolin-4(3H)-one (1a) with benzyl chloride in the presence of potassium carbonate in DMF upon heating for 3 hours at 100°C expectedly led to the product of N-alkylation 4a with a yield of 82 %. It turned out that the Regio chemistry of the process is also independent of the nature of the base (when using cesium carbonate the N-alkylation product yield is 81.0 %, and sodium hydride is 77.8 %). Here, the correlation of the 2-H proton of the quinazolinone and α-methylene protons of the NCH2R group is also observed in the COSY spectra, and the correlation of the 2-H proton of the quinazolinone and the carbon of the NCH2R group (49.32ppm) in the HMBC spectrum (Figure 3).
This is also confirmed by the fact that the chemical shift of the protons of the benzyl group is at 5.21ppm, which coincides with the 1H NMR data [39] of compound 4a obtained by counter synthesis using benzylamine (Scheme 2). The formation of the N-alkylation product is confirmed by the correlations of the NCHN proton of quinazolinone with the proton and carbon of the NCH2R group shown in the two-dimensional HMBC and COSY spectra, as well as the signal of the C=O group at 160.10ppm 13C NMR spectrum (Figure 4).
In the case of alkylation of 6,7-dimethoxyquinazolin-4-one with benzyl chloride, potassium carbonate was replaced by cesium carbonate and the process was carried out at 70°C, which also led to the product of N-alkylation 4b with a yield of 82%. The introduction of two methoxy groups into the quinazolinone molecule did not affect the chemical shift of methylene protons of the benzyl group-5.21ppm, which is consistent with the 1H NMR spectrum of compound 4b obtained by counter synthesis according to the condensation technique [40] of 2-amino- 4,5-dimethoxybenzoic acids with triethylorthoformate and benzylamine.

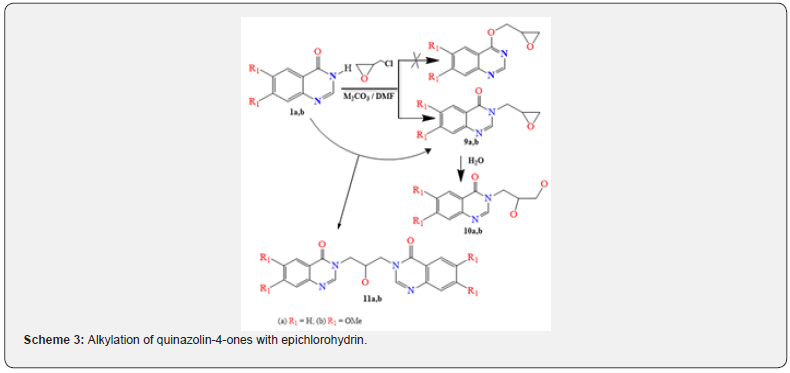
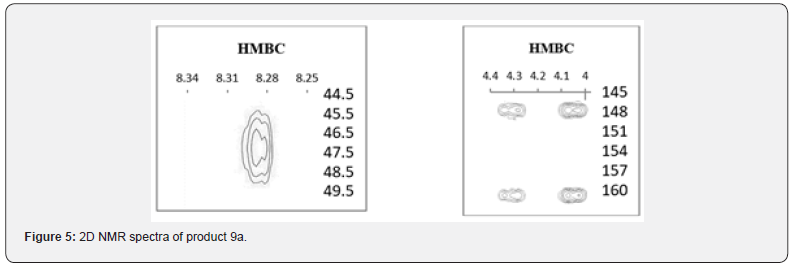
A similar situation that we observed during a repeat of work [31] when quinazolinone was alkylated with epichlorohydrin. Isolation of N-alkylation product 9a (LCMS, m/z = 203 [M + H]+, tR = 10.9 min) with a yield of 27%, contains 13% dimer (LCMS, m/z=349 [M + H]+, tR = 12.2 min). We suppose that this product was obtained by N- but not O-alkylation, and, moreover, does not contain an oxirane ring, which was hydrolyzed to give the corresponding diol, most likely when authors of work [31] isolated reaction products or used wet acetone as a solvent for alkylation process. Indeed, a comparison of the 1H NMR spectra showed the identity of the compound they obtained in the reaction of quinazolinone with epichlorohydrin and the product 10a obtained by hydrolysis of quinazolinone 9a.
The presence of the oxirane ring in the compound 9a we obtained is confirmed by the presence of two proton groups in the PMR spectrum: the CH group in the form of a multiplet at 3.37- 3.34ppm and CH2 groups in the form of a doublet of doublets at 2.58ppm with J values equal to 4.8 and 2.6 Hz. The protons of the NCH2 group, due to nonequivalence, give two groups of signals: at 4.32 and 4.08ppm in the form of a doublet of doublets with J values equal to 14.3 and 5.4 Hz (Figure 5). The formation of the N-alkylation product is confirmed with two-dimensional HMBC spectra by correlations of the NCHN proton of quinazolinone with carbon and both carbons of C-2 and C-4 quinazolinone with protons of the NCH2R group, as well as the C = O group signal at 160.34ppm of 13C NMR spectra.
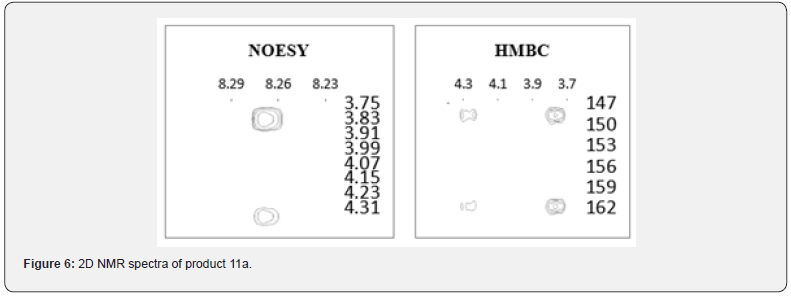
When carrying out the same reaction in the presence of cesium carbonate in DMF at room temperature, dimer 11a is formed (LCMS, m/z = 349 [M + H]+, tR = 12.2 min) with a yield of 46 % (Scheme 3). The formation of the N-alkylation product is confirmed by the presence of NCH2 group signals in the 1H NMR spectrum in the form of two groups of signals: at 4.29 and 3.83ppm in the form of a doublet of doublets with J values at 13.4 and 3.4 Hz, and at 49.73ppm in the 13C NMR spectrum. Also, in the 2D NOESY spectrum, a 2-H correlation of the quinazolinone proton with the protons of the NCH2R group is observed along with the signal of the C = O group at 160.50ppm in the 13C NMR spectrum. In the HMBC 2D spectrum, there are correlations of the 2-H quinazolinone proton with the carbon of the NCH2R group and both carbons of C-2 and C-4 quinazolinone with the protons of the NCH2R group (Figure 6).
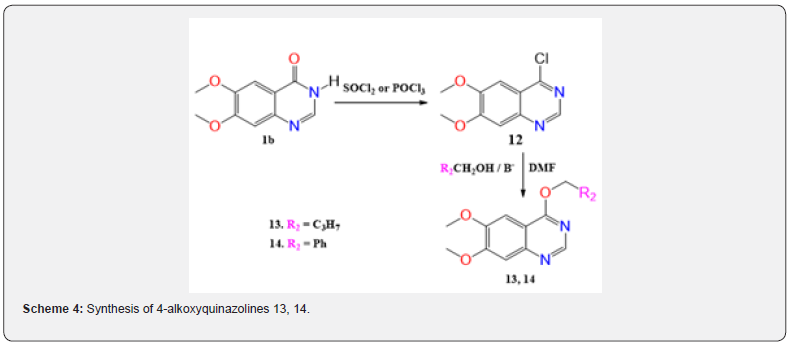
The results show that a different synthesis route must be used to obtain O-alkylation products. It turned out that in order to realize this, quinazolin-4-one derivatives must first be converted to the corresponding 4-chloroquinazolines by the action of thionyl chloride [41] or phosphorus oxychloride [42] followed by a stage of alcoholysis (Scheme 4). The 4-chloro-6,7-dimethoxyquinazoline (12) obtained in this way was subjected to alcoholysis with butanol and benzyl alcohol. In the case of n-butyl alcohol during the reaction in the presence of sodium hydride in DMF for 48 hours at room temperature and then heating for 3 hours at 70°C, compound 13 was obtained with a 56 % yield, and 4-O-ether 14 was formed with benzyl alcohol at reflux in dioxane at 100°C for 21 hours with 63% yield. The chemical shift of the protons of the benzyl group of the O-alkylation product 14 appears at 5.64ppm, while for the N-alkylation products 4a at 5.21ppm, 4b at 5.18ppm
Conclusion
Thus, the studies have shown that under classical two-phase conditions, quinazolin-4-ones to use the solid phase (alkali metal carbonates) - liquid (aprotic solvents) are regioselectively subjected to 3-N-alkylation. It is unequivocally proved by detailed analysis of two-dimensional (HSQC/NOESY, HMBC and 13C) NMR spectra of the reaction products, as well as by comparing the physicochemical properties of the products we obtained with those products obtained by counter synthesis. The above is very important for us when conducting our SAR in the search for effective HDAC/VEGFR-2 bi-inhibitors, since the authors of work [29,35] performed SAR to search for VEGFR-2 inhibitors on supposedly 4-O-alkylquinazolines, whereas in reality they had products of N-alkylation (Scheme 4). This led us to develop an alternative route [37,38] for the synthesis of 4-alkyloxyquinazolines.
Acknowledgement
The authors are grateful to the staff of the CCU of the NRC, Kurchatov Institute-IREA, for their help in taking the spectra and determining the physicochemical properties of the obtained compounds. We appreciate the Ministry of Education and Science of Russia (grant agreement № 075-11-2018-172 from 03.12.18. RFMEFI62418X0051) for financial support.
References
- La Plante S R, Bilodeau F, Aubry N, Gillard J R, O’Meara J, et al. (2013) N- versus O-alkylation: utilizing NMR methods to establish reliable primary structure determinations for drug discovery. BioorgMed Chem Lett 23(16): 4663-4668.
- Torhan, M C, Peet N P, Williams J D (2013)A comparison of N- versus O-alkylation of substituted 2-pyridones under Mitsunobu conditions. Tetr Lett 54(30): 3926-3928.
- Hopkins G C,Jonak J P,Minnemeyer H J,Tieckelmann H (1967)Alkylations of Heterocyclic Ambident Anions II. Alkylation of 2-Pyridone Salts. J OrgChem 32: 4040-4044.
- Kornblum N, Smiley R A, Blackwood R K,Iffland DC (1955)The Reaction of Silver Nitrite with Secondary and Tertiary Alkyl Halides1,2. J Am Chem Soc 77: 6269-6280.
- Zwahlen C, Legault P, Vincent S J F, Greenblatt J,Konrat R, et al. (1997) J Am ChemSoc 119: 6711.
- Boyer R D, Johnson R, Krishnamurthy K (2003) Compensation of refocusing inefficiency with synchronized inversion sweep (CRISIS) in multiplicity-edited HSQC. J MagnReson 165(2): 253-259.
- Khachatryan D S,Razinov A L,Kolotaev A V, Belus S K,Matevosyan K (2015)Alkylation of NH-, OH-, and SH-acids in the presence of potassium carbonate. R Russ Chem Bull64(2): 395-404.
- Khachatryan D S,Matevosyan K R (2016)Potassium carbonate as a base for generation of carbanions from CH-acids in organic synthesis. Russ Chem Bull 65(1): 14-28.
- Osipov V N, Khachatryan D S,Balaev A N (2020)Discovery of Novel, Highly Potent, and Selective Matrix Metalloproteinase (MMP)-13 Inhibitors with a 1,2,4-Triazol-3-yl Moiety as a Zinc Binding Group Using a Structure-Based Design Approach. Med Chem Res 29: 831-845.
- Kolotaev A V,Matevosyan K R,Osipov V N, Khachatryan D S (2019)Synthesis of new quinazoline-containing hydroxamic acids as potential HDAC/VEGFR inhibitors. Unusual rearrangements with pyrrolidone ring opening and dehydration of 3-N-hydroxyquinazoline fragment containing tetracycles. Tetr Lett 60(51): 151315.
- Hemalatha K,Girija K (2011)Synthesis of Some Novel 2, 3 Disubstituted Quinazolinone Derivatives As Analgesic And AntiInflammatory Agents. Int J Pharm Pharm Sci 3(2): 103-106.
- Alagarsamy V, Solomon V R, Meena R,Ramaseshu K V,Thirumurugan K, et al. (2007) Design and synthesis of 2-methylthio-3-substituted-5,6-dimethylthieno [2,3-d] pyrimidin-4(3H)-ones as analgesic, anti-inflammatory and antibacterial agents. Med Chem 3: 67-73.
- Alagarsamy V, Revathi S,Kalaiselvi R,Phuvaneshwari R, Revathi S (2003) Indian J Pharm Sci 534-537.
- Alagarsamy V, Pathak U S, Perumal R V, Meena S,Thirumurugan K (2003) Indian J Pharm Sci2003: 293-296.
- Dandia A, Singh R,Sarawgi P (2004)Green chemical multi-component one-pot synthesis of fluorinated 2,3-disubstituted quinazolin-4(3H)-ones under solvent-free conditions and their anti-fungal activity.J Fluor Chem 125(12): 1835-1840.
- Dinakaran M, Selvam P, DeClercq E, Sridhar S K (2003) Biol Pharm Bull26(9): 1278-1282.
- Iemura R, Hori M, Saito T,Ohtaka H (1989)Synthesis and cytotoxic evaluation of some new 3-(2-(2-phenylthiazol-4-yl) ethyl)-quinazolin-4(3H) one derivatives with potential anticancer effects. Chem Pharm Bull 37(10): 2723-2726.
- Botros S, Saad S F (1989)Synthesis, anti-hypertensive and β-adrenoreceptor antagonist activities of 3-[4-[3-(4-aryl-1-piperazinyl)-isopropanoloxy]-phenyl]-4(3H quinazolones. Eur J Med Chem 24(6): 585-590.
- Tiwari A K, Mishra A K, Bajpai A, Mishra P, Sharma R K,et al. (2006) Discovery and biological evaluation of adamantyl amide 11beta-HSD1 inhibitors. Bioorg Med Chem Lett 16(17): 4581-4585.
- Ram V J, Tripathi B K, Srivastava A K (2003)Synthesis and antihyperglycemic activity of suitably functionalized 3H-quinazolin-4-ones. Bioorg Med Chem 11(11): 2439-2444.
- Peng FW, Wu TT, Zeng ZW, Xue JY, Shi L (2015)Hybrids from 4-anilinoquinazoline and hydroxamic acid as dual inhibitors of vascular endothelial growth factor receptor-2 and histone deacetylase. Bioorg MedChem Lett25(22): 5137-5141.
- Peng F W, Xuan J, Wu TT, Xue JY, Ren ZW,et al. (2016) Design, synthesis and biological evaluation of N-phenylquinazolin-4-amine hybrids as dual inhibitors of VEGFR-2 and HDAC. Eur J MedChem 109: 1-12.
- Li J, Zhang Q, Cai W US Patent 2014235633.
- Ding C, Chen S, Zhang C, Hu G, Zhang W,et al. (2017) Synthesis and investigation of novel 6-(1,2,3-triazol-4-yl)-4-aminoquinazolin derivatives possessing hydroxamic acid moiety for cancer therapy. Bioorg Med Chem 25(1): 27-37.
- Zhang X, Su M, Chen Y, Li J, Lu W (2013) The Design and Synthesis of a New Class of RTK/HDAC Dual-Targeted Inhibitors. Molecules 18(6): 6491-6503.
- Mahboobi S,Sellmer A, Winkler M, Eichhorn E,Pongratz H,et al. (2010) Novel Chimeric Histone Deacetylase Inhibitors: A Series of Lapatinib Hybrides as Potent Inhibitors of Epidermal Growth Factor Receptor (EGFR), Human Epidermal Growth Factor Receptor 2 (HER2), and Histone Deacetylase Activity. J Med Chem 53(24): 8546-8555.
- Beckers T,Mahboobi S,Sellmer A, Winkler M, Eichhorn E,et al. (2012)Chimerically designed HDAC- and tyrosine kinase inhibitors. A series of erlotinib hybrids as dual-selective inhibitors of EGFR, HER2 and histone deacetylases. M Med Chem Commun 3(7): 829-835.
- Cai X,Zhai H, Wang J, Forrester J, Qu H,et al. (2010) Discovery of 7-(4-(3-Ethynylphenylamino)-7-methoxyquinazolin-6-yloxy)-N-hydroxyheptanamide (CUDC-101) as a Potent Multi-Acting HDAC, EGFR, and HER2 Inhibitor for the Treatment of Cancer. J MedChem53: 2000–2009.
- Wang J, Pursell N W, Samson M E S,Atoyan R, Ma A W,et al. (2013) Potential Advantages of CUDC-101, a Multitargeted HDAC, EGFR, and HER2 Inhibitor, in Treating Drug Resistance and Preventing Cancer Cell Migration and Invasion. J Mol Cancer Ther 12(6): 925–936.
- Sun J, Li D D, Li J R, Fang F, Du Q R,et al.(2013) Design, synthesis, biological evaluation, and molecular modeling study of 4-alkoxyquinazoline derivatives as potential VEGFR2 kinase inhibitors. Org Biomol Chem 11(44): 7676-7686.
- Yin Y, Sha S, Wang Y T, Wu X, Wang S F,et al. (2015) Discovery of New 4‐Alkoxyquinazoline‐Based Derivatives as Potent VEGFR2 Inhibitors. Chem Biol Drug Des 86(5): 1323-1329.
- Keller P A (2005) In Science of Synthesis.Priyaa M (Ed)Thieme: Stuttgartpp 15: 285-387.
- Breugst M, Mayr H (2010)Ambident Reactivities of Pyridone Anions. J Am Chem Soc 132: 15380.
- Fang YQ, Bio M M, Hansen K B, Potter MS, Clausen A (2010)Magnesium Coordination-Directed N-Selective Stereospecific Alkylation of 2-Pyridones, Carbamates, and Amides Using α-Halocarboxylic Acids. J Am Chem Soc132: 15525.
- Hieu D T, Anh D T, Hai P T,Thuan N T, Huong L T T, et al. (2019)Quinazolin‐4(3H)‐one‐Based Hydroxamic Acids: Design, Synthesis and Evaluation of Histone Deacetylase Inhibitory Effects and Cytotoxicity. Chem Biodiversity 16(4): e1800502.
- Hieu D T, AnhD T, Tuan N M, Hai P T, Kim J, et al. (2018)Design, synthesis and evaluation of novel N-hydroxybenzamides/N-hydroxypropenamides incorporating quinazolin-4(3H)-ones as histone deacetylase inhibitors and antitumor agents. Bioorg Chem 76: 258-267.
- Paronikyan E G, Dashyan S S,Noravyan A S, Minasyan N S (2014)Synthesis of Derivatives of Cyclopenta[4',5']Pyrido-[3',2':4,5]Thieno[3,2-d]Pyrimidines and Pyrimido[5',4':2,3]-Thieno[2,3-c]Isoquinolines. Chem HeterocyclCompd 50(8): 1188-1194.
- Paronikyan E G,Dashyan S S, Minasyan N S,Stepanyan G M,Babaev EV (2017) Synthesis and neurotropic activity of the derivatives of fused triazolo[4,3-c]- and triazolo[1,5-c]pyrimidines. Chem HeterocyclCompd 52(5): 337-345.
- Deau E,Hédou D,Chosson E,Levacher V, Besson T (2013)Convenient one-pot synthesis of N3-substituted pyrido[2,3-d]-, pyrido[3,4-d]-, pyrido[4,3-d]-pyrimidin-4(3H)-ones, and quinazolin-4(3H)-ones analogs. Tetr Lett 54(27): 3518-3521.
- Zuo S J, Li S, Yu R H, Zheng G X, Cao Y X,et al. (2014) Discovery of novel 3-benzylquinazolin-4(3H)-ones as potent vasodilative agents. Bioorg Med Chem Lett 24(24): 5597-5601.
- Zheng Y G, Su J, Gao C Y, Jiang P, An L, et al. (2017)Design, synthesis, and biological evaluation of novel 4-anilinoquinazoline derivatives bearing amino acid moiety as potential EGFR kinase inhibitors. Eur J Med Chem 130: 393-405.
- Chang J, Ren H, Zhao M, Chong Y, Zhao W,et al. (2017) Development of a series of novel 4-anlinoquinazoline derivatives possessing quinazoline skeleton: Design, synthesis, EGFR kinase inhibitory efficacy, and evaluation of anticancer activities in vitro. Eur J Med Chem 138: 669-688.






























