Anti-Microbial Properties of C-benzylated Dihydro Chalcone Derivatives: 2’, 4’-Dihydroxy-3’,5’-( 2’’, 2’’’- Dihydroxy Dibenzyl) - 6’-Methoxy Dihydro Chalcone, 2’,4’-Dihydroxy-3’- ( 2’’- Hydroxy Benzyl) –6’- Methyl Dihydrochalcone and 2’, 4’, 6’ – Tri Hydroxy- 3’- ( 2’’-Hydroxy Benzyl) Dihydro Chalcone Isolated from Stem Bark of uvaria chamae P. Beav
Ado K1*, Salihu L1, Garba S1, Nwaedozie JM1 and Aliyu AB2
1Department of Chemistry, Nigerian Defence Academy, Kaduna
2Department of Chemistry, Ahmadu Bello University, Zaria
Submission: August 11, 2020 Published: September 28, 2020
*Corresponding author: Ado K, Department of Chemistry, Nigerian Defence Academy, Kaduna
How to cite this article: Ado K, Salihu L, Garba S, Nwaedozie J, Aliyu A. Anti-Microbial Properties of C-benzylated Dihydro Chalcone Derivatives: 2’, 4’-Dihydroxy-3’,5’-( 2’’, 2’’’- Dihydroxy Dibenzyl) - 6’-Methoxy Dihydro Chalcone, 2’,4’-Dihydroxy-3’- ( 2’’- Hydroxy Benzyl) –6’- Methyl Dihydrochalcone and 2’, 4’, 6’ – Tri Hydroxy- 3’- ( 2’’-Hydroxy Benzyl) Dihydro Chalcone Isolated from Stem Bark of uvaria chamae P. Beav. Organic & Medicinal Chem IJ. 2020; 10(1): 555777. DOI: 10.19080/OMCIJ.2020.09.555777.
Abstract
Two new C- benzylated dihydro chalcones derivatives:, 2’, 4’- dihydroxy- 3’ – (2”, - hydroxyl benzyl)- 6’ –methyl dihydro chalcone , 2’, 4’, 6’- trihydroxy-3’- (2’’ – hydroxy benzyl) dihydro chalcones and a known compound, 2’, 4’- dihydroxy-3’, 5’ – (2’’. 2”’ –dihydroxy benzyl) – 6’- methoxy dihydro chalcone (diuvaretin) were isolated from the stem-bark of uvaria chamae. The structures of the compounds were elucidated by spectroscopic methods: FTIR, 1HNMR, 13CNMR as well as by comparison with literature data. The isolated compounds were evaluated for their antibacterial and antifungal activities using Disc diffusion method. All the three compounds exhibited high activities against all the tested microbes with diuvaretin exhibiting the highest activities with inhibition zones ranging from 12-30 mm, for bacterial and 14-30mm for fungal organisms, at the highest concentration of 1000μg/cm3. Therefore, U. chamae contain promising antimicrobial compounds with diverse biological activities that can be used in developing drugs for curing life threatening diseases caused by the tested organisms such as typhoid fever, darriahoea and aflotoxins caused by molds.
Keywords: uvaria chamae; Biological activity; Spectroscopic methods; Dihydrochalcones
Recent Technology of siRNA Delivery System
The relationship of humans and animals with plants started with the beginning of life on earth, where plants supplied shelter, oxygen, food, and medicine required by higher living organisms [1]. After a long period of time, and with the beginning of societies, human being learnt to understand, and classified plant materials suited for use in meeting up with the necessities of life. One of these necessities is the use of herbs and herbal extracts for their healing powers which can be traced to earliest of traditions and writings used to codify those plants that can take away pain and treat diseases [2]. According to World Health Organization (WHO), about 70 percent of the world’s population relies heavily on plants for their primary health care. Some 35,000 to 70,000 species of plant have been used as medicines, corresponding to 14-28% of the 250,000 plants species estimated to occur around the World [3]. Globally, more than 50 major drugs are originated from tropical plants. Only 17% of about 250,000 species of higher plants around the world have been scholarly investigated for medicinal potential. The chemical and biological diversities of plants present a potentially limitless renewable source for the use in the development of new pharmaceuticals [4]. Elujoba [5] noted that a plant become medicinal only when its biological activity has been ethnobotanically reported or scientifically established.
Infectious diseases such as typhoid, cholera, diarrhea, dysentery, tuberculosis, and pneumonia are the world leading cause of premature death and these diseases are known to develop resistance against many synthetic drugs. Contrary to synthetic drugs, antimicrobials of plant origin are not associated with side effects and have therapeutic potential to treat diseases. They are also cheap, easily available, and affordable [6,7]. These natural antibiotics should have ingredients that are active against some common microbes that cause life-threatening diseases with attendant economic loss. These microbes include S. typhi, E. coli, S. aureus, C. albicns, A. niger, A. fumigatus, and A. flavus.
Diarrhea is major cause of childhood mortality that results from contaminated food and water sources. It has been estimated by UNICEF that there are about 2.5 million cases of diarrhea in children under the age of five. Approximately, about 1.3 million children less than 5 years die each year from diarrhea which is the second leading cause of death in Asia and Africa [8]. Majority of this death occur in India, Nigeria, Afghanistan, Pakistan, and Ethiopia. The major bacterial pathogens are E. coli, Shigella, Salmonella species and Vibro cholera [8]. A frequent cause of diarrhea in both humans and animals, enterotoxigenic E. coli (ETEC) are estimated to cause 600 million cases of human diarrhea and 800,000 deaths worldwide principally in children under the age of five (5) [9,10]. Typhoid fever was once a major cause of mortality throughout the World. It has been approximately estimated that there are16 million cases of typhoid each year, with 600,000 deaths in less developed nations [11]. This research is aimed at assessing the antimicrobial potentials of the three Compounds isolated from Uvaria chmae P. Beav. Stem bark for the treatment of lifethreatening diseases.
Experimental
Sample collection
Stem bark of uvaria chamae was collected from Rigachikun, Igabi Local Government Area, Kaduna State, Nigeria. The plants were identified and authenticated by Mr. U.S Gallah of Herbarium unit in the department of Biology, Ahmadu Bello University, Zaria. A voucher specimen with number (900264) was collected and specimen was deposited in the herbarium. The Plant materials were air-dried, Pulverized and stored in clean polyethene bags at ambient temperature.
Sample Extraction
A quantity (500g) of the powdered stem bark of Uvaria. chamae was percolated with 4000cm3 of ethanol in a large jar for two weeks. The resultant solution was then filtered and evaporated using rotary evaporator at 40 0C. The residue was extracted again with 2000cm3 of ethanol to obtain maximum extraction. Extract could dry and weighted. A portion (100g) of Uvaria. chamae extract was first placed in a 400cm3 beaker and 200cm3 of n-hexane was added and stirred with a glass rod. The colored solution obtained was then drained and this was repeated several times until the color faded away. The colored solution obtained was then transferred into a clean container and labeled as n-hexane fraction. The same process was repeated separately with dichloromethane and ethyl acetate to obtained dichloromethane and ethyl acetate fractions, respectively. The dichloromethane, ethyl acetate and hexane soluble fractions were separately evaporated and weighted.
Antibacterial assay
The antibacterial screening the fractionated ethanol extracts and of isolated compounds was carried out using agar well diffusion method as described by Navarro et al. [12] & Okeke et al. [13]. The most active crude was subjected to chromatographic separations leading to isolation of three compounds with antimicrobial activities against the tested microbes.
Antifungal assay
Antifungal activity of the fractionated ethanol extracts and isolated compounds were determined by using the method described by Navarro et al. (1996). Potato dextrose agar was used as a growth medium. Isolates of Candida albicans, Aspergillus niger, Aspergillus flavus and Aspergillus fumigatus were used as test organisms.
Results and Discussion
The results of antimicrobial analysis of fractionated crude ethanolic extract of U. chamae were given in (Table 1). Ethyl acetate soluble fraction exhibited high activities against S. typhi and S. flexnerri (inhibition zone of 26.6mm and 21.3mm) respectively. It also recorded low to moderate activities against S. aureus, E. coli, P. aerigunosa, C. albicans and S. pneumonia. The antimicrobial activities of chloroform soluble fraction showed very high activities against all the tested bacteria with inhibition zones ranging from (21.3-30.3mm), at the highest concentration (5μg/cm3). However, it exhibited moderate activities against C. albicans (inhibition zone of 13.3mm).The antimicrobial activities of n-hexane soluble fraction are generally low with exception of U. chamae which recorded high activities (29mm and 26mm) respectively against S. typhi and S. flexnerri at the highest concentration of the extract (5μg/cm3).
The results of antibacterial activity of the three isolated compounds: KB. KC and KD against clinical isolates of Escherichia coli, Salmonella. typhi and Staphylococcus aureus was given in Table 2. The results showed that compound KB exhibited high activities against all the tested organisms, with inhibition zones of 30, 35, and 22 mm for Escherichia. coli, Salmonella. typhi and Staphylococcus aureus respectively, at highest concentration of 100μg/cm3. However, it showed high activities against all the tested microbes even at lower concentration of 500μg/cm3 with inhibition zones of 25, 21 and 20mm against Escherichia. coli, Salmonella typhi and Staphylococcus aureus, respectively.

Compound KC exhibited high antibacterial activity against E. coli S. typhi and S. aureus with inhibition zones of 24, 26, and 20 mm respectively at the highest concentrations. However, it exhibited high activities against Escherichia coli and Salmonella. typhi and moderate activity against Staphylococcus aureus at concentration of 500μg/cm3 and low activities at lower concentration, 250μg/ cm3 against all the tested microbes. Compound KD inhibited the growth of Escherichia. coli, Salmonella typhi and Staphylococcus aureus with inhibition zone of 26, 20 and 19mm respectively, at highest concentration. E. coli being highly inhibited by compound KD. Furthermore, compound KD exhibited high activity against Escherichia coli (21mm inhibition zone), but moderate activity against Salmonella typhi and Staphylococcus. aureus with inhibition zones of 18 and 15 mm respectively at 500μg/cm3. However, at 250μg/cm3 concentration, it showed low activities against all the tested microbes.
The high antibacterial activities exhibited by the compound KB (Diuvaretin chalcone), KC and KD was supported by several research groups identified chalcones and their derivatives possessing great antibacterial activity [14]. Chalcones and their derivatives have been reported to have wide spectrum of activity such as anti-inflammatory, antibacterial, antifungal, antiviral, antioxidant, ant filarial, anti-rheumatoid and antiprotozoal [15,16].This antibacterial activity have been related to the ability of these chalcones compounds to react with cellular nucleophiles such as thiols groups in essential proteins [14]. Pinocembrin chalcone, a compound isolated from Helichrysum trilineatum, exhibited antibacterial activity against Staphylococcus aureus [14], and Escherichia. coli [17]. In a structure activity relationship study, the two hydroxyl groups (OH) attached to ring A and B of a chalcone, OH group attached to ring A is more important to antibacterial activity and OH attached to ring B is for lipophilicity [16].
The results of antifungal activity of the isolated compounds, KB, KC and KD against the fungal isolates: Aspergillus niger, Aspergillus flavus, Aspergillus fumigatus and Candida albicans were presented in (Table 3). The compound KB showed high activity against all the tested fungal organisms: A, Niger, A. flavus, A. fumigatus and C. albicans; with inhibition zones of 25, 30, 22 and 20mm respectively, at the highest concentration. It also showed high activities against Aspergillus. Niger and Aspergillus flavus with inhibition zones of 20 and 24mm respectively, moderate activity against Aspergillus fumigatus and Candida albicans at 500μg/cm3 concentration. However, it also showed moderate activities against all the tested microbes at the lowest concentrations (250μg/cm3).


Compound KD (C-benzylated dihydrochalcone) exhibited high activities against Aspergiluss niger, Aspergillus flavus and Candida albicans at the first two concentrations (100μg/cm3 and 500μg/cm3) with inhibition zones of 23, 28 and 22mm; and 20, 23 and 18mm respectively. However, the growth of Aspergillus flavus was inhibited even at the lowest concentration (inhibition zone of 20mm); while compound KD showed low inhibition against Aspergillus niger and Candida albicans with no inhibition against Aspergillus fumigatus. Compound KC (C-benzylated dihydrochalcone derivative) exhibited high activities against Aspergillus niger and Aspergillus flavus, moderate and low activities against Candida albicans and Aspergillus. fumigatus with inhibition zones of 20, 25, 18 and 12mm at the highest concentration. It also showed high activity against Aspergillus flavus (23mm) and moderate activity against Candida albicans and Aspergillus niger (18 and 15mm), low activity against Aspergillus fumigatus at 500μg/cm3 concentration. However, compound KC showed low activities against Aspergillus niger, Aspergillus fumigatus and Candida albicans; and moderate activities against Aspergillus flavus (18mm) at the lowest concentration.
The strong antifungal activities exhibited by KB, KC and KD was supported by the previously reported work on isobavachalcones isolated from Maclura tinctoria having inhibitory activity against Candida albicans ( IC50 of 15μg/cm3); and Cryptococus neoformans ( IC50 of 7μg/cm3). In a relted study, Kamalachalcone isolated from philippiensis species showed antifungal activity against Aspergillus fumigatus [14]. Many chalcones are known to exhibit antifungal activity due to the presence of α, β- unsaturated carbonyl group that enhances activity [18,19]. The potency against C. albicans depends largely on the ability of hydroxyl groups in chalcones and their derivatives to interact with intracellular thiols in essential protein. This partly contributes to their antimicrobial property [14,18,19].
Column chromatography of the dichloromethane fraction of ethanol extract
A total of one hundred and ten fractions were collected from column chromatography using solvent mixture, hexane: diethyl ether (9.5: 0.5- 5:5). Thin layer chromatography of the fractions indicated that some are similar. Similar fractions were pooled together as one fraction. Fractions F51- F56 with Rf value 0.86 were coded KA, fractions F57-F59 Rf 0.68, were coded KB, fractions F60-64 with Rf value 0.75 were coded KC, F65- F66, Rf value 0.59 were coded KD and fractions F67- F69 with Rf value 0.79 were coded KE. The compounds KB, KC and KD were recrystallized with solvent mixture (hexane: ethyl acetate, 2:1); and the purified crystals were subjected to spectroscopic analyses: FTIR, 1HNMR and 13CNMR. The results of 1HNMR, 13CNMR for the isolated compounds, KB, KC and KD were presented in Tables 4-6 respectively.



Previously isolated Compound: Compound KB
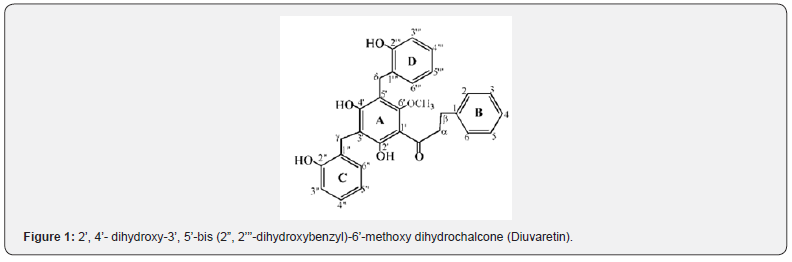
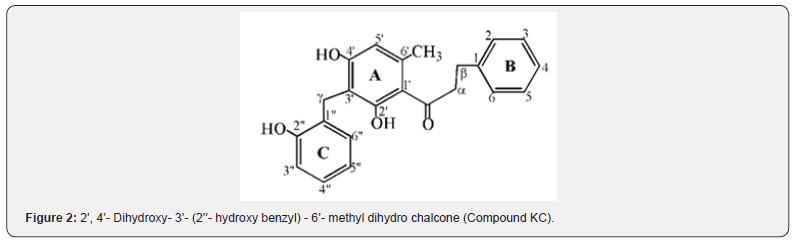
Newly isolated compounds: (Compounds KC and KD) Compound KC was isolated as a Red crystalline solid with melting point of 166-168 oC and has molecular formula (C23H22O4, 362.41838g/mol) deduced from 1H-NMR, 13C-NMR and 2D NMR. The GC-mass spectrum showed fragment ions at m/z 91, 105 and 251 characteristics of dihydro chalcone skeleton having unsubstituted B-ring [20]. The IR spectral analysis showed intense absorptions at 3444cm-1 (-OH), 3034cm-1 stretch (sp2-C), 2922cm- 1 for (sp3-C) and 1710cm-1 (C=O) bond. The 1H and 13C-NMR experiments indicated that compound KC contained twenty-three carbons: one methyl (CH3), three methylene (CH2), ten methine (CH), eight quaternary (C), one carbonyl (C=O) and three hydroxyl groups (OH). The 1H NMR spectrum showed signals attributed to the benzylic methylene protons Hα and Hβ at δH 2.83 (2H, m) and δH 3.09 (2H, m) respectively.
This was supported by the reported of Nkunya et al. [21] for benzylated dihydrochalcones isolated from Uvaria leptocladon. The doublets in the aromatic region at δH 7.09 (1H, m) and δH 7.38 (1H, m) are assigned to H2/6 and H3/5 for ortho and meta protons respectively on ring B. The signal at δH 7.37 ppm was assigned to H-4 proton on ring B. The A-ring was totally substituted except at C-5’ where a methine proton appeared as singlet at δH 6.04 (1H, s). The signals at δH 12.69, 6.90 and 7.07ppm (broad singlet each) were assigned to hydroxyl groups as OH-2’, OH-4’ and OH- 2’’ respectively. These were connected to oxygenated quaternary carbon types that appeared at δC 163.21, 152.71 and 152.71ppm for C-2’, C-4’and C-2’’ respectively. The benzyl ring was attached to the chalcone A-ring through a methylene group (γCH2) whose protons appeared at δH 3.89 (2H, s) and connected to the quaternary carbon (C-3’) at δC 108.04. The benzyl C-ring contains four methine protons at δH 6.90, 7.09, 6.88 and 7.54 ppm assigned to H-3”, H-4”, H-5” and H-6” which were connected to the methine carbons at δC 115.61, 128.85, 121.23, and 131.94 ppm for C-3”, C-4”, C-5” and C-6” respectively.
The comparison of spectral data of compound KC with uvaretin is presented (Table 5) where each carbon signal of compound KC matched the corresponding carbon on uvaretin. The spectral information agrees with that of uvaretin except that the methoxy group (OCH3) attached to C-6’ (δC 162.62ppm) in uvaretin was absent in compound KC. This suggests that compound KC is a derivative of uvaretin, a benzylated dihydrochalcone previously isolated from uvaria angolense obtained from Tanzania [22]. It is likely that the methoxy group in uvaretin was due to biosynthetic transformations from compound KC. This is supported by the report of zsuzsanna [14] that there are two major types of chalcones that occur, and they differed by the presence or absence of hydroxyl group at position 6’. When chalcone synthase (CHS) is expressed alone, 6’-hydroxy chalcones are formed. When the second enzyme, chalcone reductase (CHR), is also active at the same time, 6’-deoxygenated chalcones are formed. Thus, it is proposed as 2’, 4’- Dihydroxy- 3’- (2’’- hydroxyl benzyl) - 6’- methyl dihydro chalcone, 3, differing only at C-6’ with methyl rather than the methoxy group in uvaretin (Figure 2) above.
Compound KD was isolated as yellow crystalline solid (yield, 0.073g, 0.73 %) with melting point of 163-164°C and has molecular formula (C22H20O5, 364.3912g/mol) deduced from 1H-NMR, 13C-NMR and 2D NMR. The GC-mass spectrum showed fragment ions at m/z 91, 105 and 251 characteristics of dihydro chalcone skeleton having unsubstituted B-ring [20]. The IR spectral analysis showed intense absorptions at 3444 cm-1 (-OH), 3034 cm-1 (sp2-C) and 1710 cm-1 (C=O) bond. The 1H and 13C-NMR experiments indicated that compound KD contained twenty-two carbons: ten methine (CH), three methylene (CH2), and one carbonyl carbon (C=O), eight quaternary carbon (C) and four hydroxyl groups (OH). The 1H NMR spectrum showed signals attributed to the benzylic methylene protons Hβ and Hα at δH 3.09 ppm (2H, m) and δH 2.84 ppm (2H, m) respectively. This was supported by the report of Nkunya et al., [23] for benzylated dihydrochalcones isolated from Uvaria leptocladon.
The signals in the aromatic region at δH 7.08 (1H, m) and δH 7.37 (1H, m) were assigned to H2/6 and H3/5 for ortho and meta protons respectively on ring B. However, proton signal at δH 7.09ppm was assigned to para H-4 on ring B. The A-ring was totally substituted except at C-5’ where a methine proton appeared as singlet at δH 6.03 (1H, s). The signals at δH 12.73, 6.91 and 5.44 ppm (broad singlet each) were assigned to hydroxyl groups as OH-2’, OH-4’ and OH-6’ respectively. These were connected to oxygenated quaternary carbons that appeared at δC 163.08, 152.57, 161.12 ppm for C-2’, C-4’and C-6’ respectively. The benzyl C-ring was attached to the chalcone A-ring through a methylene group (γCH2) whose protons appeared at δH 3.89ppm (2H, s) and connected to carbon (C-3’) of A-ring at δC 108.03ppm. The benzyl C-ring contains four methine protons at δH 6.82 (1H, ddd), 7.09 (1H, ddd), 6.87 (1H, ddd) and 7.53 (1H, dd) ppm and assigned to H-3”, H-4”, H-5” and H-6” which were connected to the methine carbons at δC 115.60, 128.87, 121.36, and 131.85 ppm for C-3”, C-4”, C-5” and C-6” respectively. The quaternary carbon at δC 152.57ppm was assigned to C-2” connected to hydroxyl group with signal δH 6.91ppm (OH-2”).
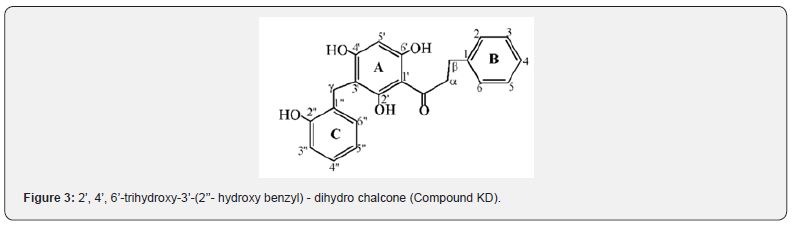
The comparison of spectral data of compound KD with uvaretin is presented (Table 6) where each carbon signal of compound KD matched the corresponding carbon on uvaretin. The spectral information agrees with that of uvaretin except that the methoxy group (OCH3) attached to C-6’ (δC 162.62ppm) in uvaretin was absent in compound KD. This suggest that compound KD is a derivative of uvaretin, a benzylated dihydrochalcone previously isolated from Uvaria angolensis obtained from Tanzania [22]. It is interesting to note that the C-6’ signals (δC 161.12ppm) in compound KD appeared as oxygenated quaternary carbon indicating the presence of a hydroxyl group. It is likely that the methoxy group in Uvaretin was due to biosynthetic transformations from compound KD. Two major types of chalcones occur, differed by the presence or absence of a hydroxyl group at the 6’ position [14]. During the biosynthesis of chalcones, when chalcone synthase is expressed alone, 6’ hydroxy chalcone is formed (Figure 3). Thus, 6’ hydroxychalcones are substrates for biosynthesis of the common groups of Flavonoids: flavonones, flavonols, flavones and anthocyanins [14]. Thus, it is proposed as 2’, 4’-Dihydroxy-3’-(2’’- hydroxy benzyl) - 6’-hydroxy dihydro chalcone (uvaretin derivative) differing only at C-6’ with hydroxyl rather than the methoxy group as in Uvaretin.
Conclusion
The two newly isolated together with already known compounds were isolated from the stem back of uvaria chamae which have been found to have antibacterial and antifungal properties. This confirm the use of uvaria chamae in traditional medicine for the treatment various ailments all over the World. The dihydrochalcones isolated from this study showed wide spectrum of activities against several bacterial and fungal organisms with potentials for the treatment of life-threatening diseases such as typhoid fever, cholera, diarrhea, cancer, tuberculosis, HIV and AIDS. Further research into antiviral screening of the two newly isolated compounds is recommended.
Acknowledgement
The authors wish to thank the Department Biological Sciences, Ahmadu Bello University Zaria, Nigeria for the identification of the plant and Department of Biological Sciences, NDA Kaduna for providing the microorganisms. Authors are also grateful to the University of Kwua Zulu Natal, South Africa or CNMR analysis.
References
- Farnworth NR, Soejarto D (1991) Global Importance of Medicinal Plants. In: Akerele O, Heywood V, Synge H (Eds.), Conservation of Medicinal Plants. Cambridge Universit Press, Cambridge, pp. 25-51.
- Nazim M (2012) Medicinal Plants. Studies: History, Challenges and Prospective. Med Aromat Plants 1(8): 1-8.
- Akerele O (1992) Importance of Medicinal Plants: WHO’s Programme. In: Natural Resource and Human Health: Plants of Medicnal and Nutritional Value. Elselvier, Amsterdam, Netherlands, pp. 63-77.
- Farnsworth NR (1992) Pre -clinical Assessments of Medicinal Plants. Natural Resource and Human Health.Elselvier Science Publishers, pp. 87-91.
- Elujoba AA (1997) The role of pharmacognosy in phytotherapy, the challenges of our time. Nig J Nat Prod And Med 2: 34-36.
- Ciocan D, Bara II (2007) Plant Products as Antimicrobial Agents. SectiuneasiBiologieMoleculara TOM 111: 151-155.
- Shankar EM, Nanda Kumar S, Rao UA (2005) The effect of methanolic extract of Tamarindus indica Linn on clinical isolates of Burkholderiapseudomallei. Indian J Med Res 122: 525-528.
- Sanyaolu A, Groetz, R, Gillam J, Patel P, Oyeleke O, et al. (2018) Global Trends of diarrhea Diseases in Children. Anna Microbi Infect Dise I(1): 24-38.
- WHO (1999) The World Health Report, Making a difference. World Health Organization.
- W O (2019) The World Health Report. World Health Organization.
- Ivanoff B (1995) Typhoid Fever. Global Situation and W.H.O Recommendation. Asian Journal Trop Med and Public Health 26: 1-6.
- Navarro V, Villarreal M L, Rojas G, Lozoya X (1996) Antimicrobial valuation of some medicinal plants used in Mexican traditional medicine for the treatment of infectious disease. J Ethnopharmacol 53: 143-147.
- Okeke MI, Iroegbu CU, Eze EN, Okoli AS, Esimone CO (2001) Evaluation of extracts of the root of Landolphiaowerriene for antibacterial activity. J Ethnopharmacol 78: 119-127.
- Zsuzsanna R, Perjesi P (2016) Naturally Occurring Chalcones and their Biological Activities. Phytochem Rev 15: 87-120.
- Ertan R, Evranos B (2013) Chemical and Structural Properties of Chalcones1. Fabad J Pharm Sci 36: 223-242.
- Gutierrez RMP, Ramirez AM, Sauceda JV (2015) Review: The potential of Chalcones as a source of drugs. Afr J Pharm Pharmacol 9(8): 237-257.
- Thomas A, Rex Ogbuku EM, Idibiye K (2018) A Review: Secondary Metabolites of Uvariachamae P. Beav. (Annonaceae) and their Biological Activities. Interna J Agric Environ FoScienc 2(4): 177-185.
- Yazdan AB, Sagar DV, Shaik AB (2015) Chemical and Biological Potentials of Chalcones: A review. Organic & MedicinalChem IJ 1(1): 1-9.
- Patil S, Rajput S, Hospete S, Ircal S (2019) Synthesis, Biological Activity and Spectral Characterization of Chalcone. J Appli Chem12(17): 38-45.
- Kaouadji M (1989) Two C–methyl -C –Prenyl dihydrochalcones from Plantusacerifolia. Phytochem 28(11): 3191-3192.
- Nkunya MHH, Weenen H, Renneb C, Waibel R, Achenbatch H (1993) Benzylated dihydrochalcones from Uvarialeptocladon. Phytochem 32(5): 1297-1300.
- Mohammad I, Waterman PG (1985) Chemistry of the Annonaceae: Part 18 Benzylated Indoles and Dihydrochalcones in Uvariaangolensis from Tanzania. J Nat Prod 48(4): 571-580.
- F Freiburghaus, R Kaminsky, MHH Nkunya, R Brun (1996) Evaluation of African medicinal plants for their in vitro trypanocidal activity. J Ethanophar 55:1-11.






























