Synthesis, Crystal Structure and Photoluminescence Properties of Diaquabis (1,10Phenanthroline, κ,N,N’)(Benzoato-κ,O) Manganese(II)Dihydrate
Idongesit Justina Mbonu1,2*, Olusegun Kehinde Abiola1, Kenneth Abara Ibe1, Doris Fovwe Ogeleka1 and Elias E Elemike1
1Department of Chemistry, Federal University of Petroleum Resources, Nigeria
2Department of Chemistry, University of Utah, United States
Submission: July 17, 2020 Published: July 28, 2020
*Corresponding author: Idongesit Justina Mbonu, Department of Chemistry, Federal University of Petroleum Resources, P.M.B.1221 Effurun, Delta State Nigeria
How to cite this article: Idongesit J M, Olusegun K A, Kenneth A I, Doris F O, Elias E E. Synthesis, Crystal Structure and Photoluminescence Properties of Diaquabis (1,10Phenanthroline, κ,N,N’)(Benzoato-κ,O) Manganese(II)Dihydrate. Organic & Medicinal Chem IJ. 2020; 9(5): 555773. DOI: 10.19080/OMCIJ.2020.09.555773.
Abstract
A new complex, Mn3(C7H4O2)2(C12H8N2)(CH3OH)2(H2O)4, has been synthesized under slow solvent evaporation method. The compound was characterized by photoluminescence and single crystal X-ray diffraction techniques. The compound gives triclinic structure with a space group P-1(No. 2). The observed unit cell dimensions are a = 7.4369(3)Ǻ, b= 12.9898(6)Ǻ, c = 14.1176(6)Ǻ, α =88.847(2)°, β =82.518(2)°, γ = 89.975(2)° , V = 1351.93(10)Ǻ³, and Z = 2. Its asymmetric unit consists of centrosymmetric trinuclear MnII cation, four water molecules, four oxygen atoms from benzoate dianion and 1, 10- phenanthroline molecule. Each of the MnII cations is six-coordinated forming octahedral geometry. The crystal packing of the prepared compound is stabilized by strong intermolecular hydrogen bonds forming 2D structure which further engaged in a weak C —H…O, C—N---O, O—H…O and N—H….O hydrogen bonding to create a 3D network. The determined molar conductance in DMSO solution was 9.35Ω-1cm2mol-1 indicating a non-electrolyte behavior of this compound. Mn-MOF shows fluorescence in solution ranging from 324-460nm revealing its efficient room-temperature phosphorescence in chloroform with a remarkable weak quenching by molecular oxygen, an indication that this compound can be used in fields such as drugs industries, energy storage systems, as luminescence sensors to analyze water, air and chemical pollutions.
Keywords:Crystal structure; 1, 10-phenanthroline; Benzoate anion; MnII complex; Photoluminescence
Introduction
Colossal works have been done towards the design and preparation of coordination polymers that are porous because of their captivating structures and their imminent applications in luminescence, electronics, catalysis, energy storage, sensors, and partitioning [1,2]. Metal-carboxylates and metal-o-phenanthroline with their derivatives are outstanding due to their interesting highlights and properties [3,4]. Our past work sums up detailed synthesis, structural impact variables, and properties of Mn-MOF. Over the last two decades coordination chemists have been devoted to the study of artificial systems by trying to mimic the natural photosynthesis processes. All aspirations has been on how to exploit electronic states of metal complexes and develop them into supramolecular systems with various electron acceptors and donors for applications in photo catalysis, sensoring, luminescence probes, biomedical applications ,and energy storage in order to reduce pollution in our society, In situations whereby the fluorescence emission red-shift with regards to the wavelength of excitation source, information from the signal gives the intensity, wavelength, lifetime and polarization as in regards to the environment of the fluorophore.
Organic compounds are limited in their use because they are thermally unstable and limited in their capacity in terms of electron transfer and transport processes [5,6]. Inorganic compounds even when they are thermally stable cannot be easily processed because of this they are limited in their versatility When inorganic and organic compounds are combined together, new framework with improved and enhanced properties are formed towards high capacity participation in “electron transfer and transport” leading to versatility in applications. In continuation of our past work, we report new manganese coordination metal-organic framework [Mn3(C7H4O2)2(C12H8N2) (CH3OH)2(H2O)4], resulting from the reaction of benzoic acid and MnII ions with 1,10- phenanthroline. The asymmetric unit of Mn-MoF consists of centrosymmetric trinuclear MnII cation, four water molecules, four oxygen atoms from benzoate dianion and 1,10 phenanthroline molecule (Figure1).
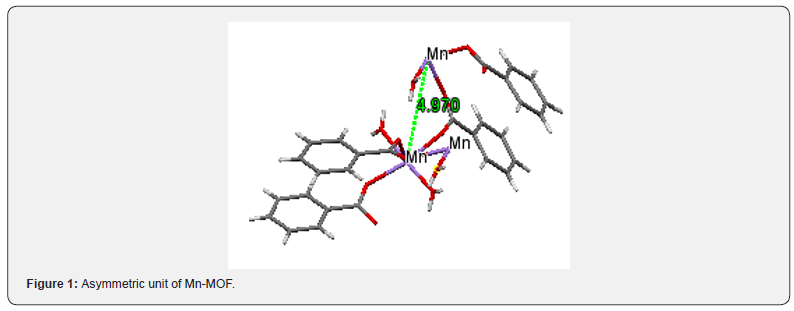
Materials and Methods
The different coordination modes of the manganese transition metal with 1,10 phenanthroline and carboxylic acids was determined [7,8,9]. Bond distance, bond angle values were determined [9]. The identification of chiral center(s) and their (R/S)-configuration were also carried out [10]. Selected bond lengths (Ǻ) were used for elucidating the structure of Mn-MOF [11,12].
The following crystallographic tools were used to calculate and determine the geometrical properties of the crystal structure of Mn-MOF. The cell dimensions, space group symmetry, structure refinement and data reduction for Mn-MOF were computed by APEX2, SAINT, SHELXL-2018/3, respectively. The molecular graphics was prepared using SHELXL and PLATON (V-260918) software [13,14]. The Mn-MOF atom refinement was done by invariom refinement and molecular electron-density distribution constructed from ‘Hansen/Coppens’ multipole-mode parameter values as given in Table 1. The electron density of the asymmetric unit is obtained by a single-point energy calculation.
Preparation of Mn-MOF
The mixture of MnCl2.4H2O (0.5 mmol) and benzoic acid (0.1 mmol) in 10 mL aqueous solution was stirred for about [15] 5 minutes to give a red solution at 50 °C. The mixture was kept at a pH 7 by the slow addition of a NaOH solution (1mol/L). A 5 mL methanol solution of 1, 10-phenanthroline (0.5 mmol) was added and the solution was stirred and heated for 3h at 50 °C, followed by filtration. The pale-yellow crystals were separated by slow evaporation at room temperature after 5 days [16]. The results of elemental analyses show that the C, H, Mn, and O are 50.50, 4.20, 16.50, and 28.80 (calc.) and 50.48, 4.27, 16.40, and 28.85 (found), respectively.
Single-crystal X-ray diffraction
Plate-like yellow single-crystals of Mn-MOF of 0.05 x 0.54 x 0.60 mm3 crystal size dimensions (Table 1) were chosen for X-ray diffraction measurement. Cell dimensions, space group symmetry, structure refinement (SAINT program: Bruker, 2009) and data reduction for Mn-MOF were computed using SHELXL-2018/3 software program [17] and results summarized in Tables 1-11. The structure of Mn-MOF was solved via SIR92 program application to be triclinic, P-1(No.2) space group and calculated density of 1.637g/cm3 primarily based on five molecules packed in the unit-cell volume of 1351.93(10) Ǻ3 and this information used to evaluate the position of each elements in the molecule.
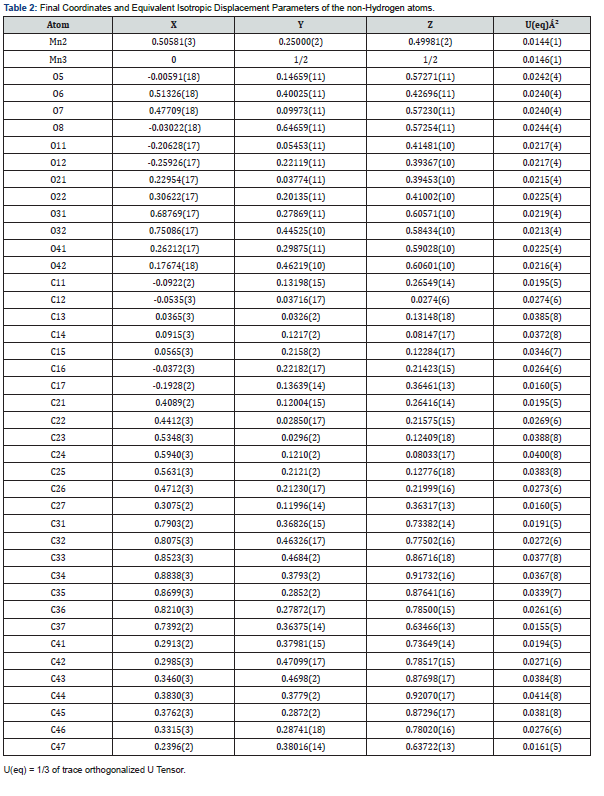
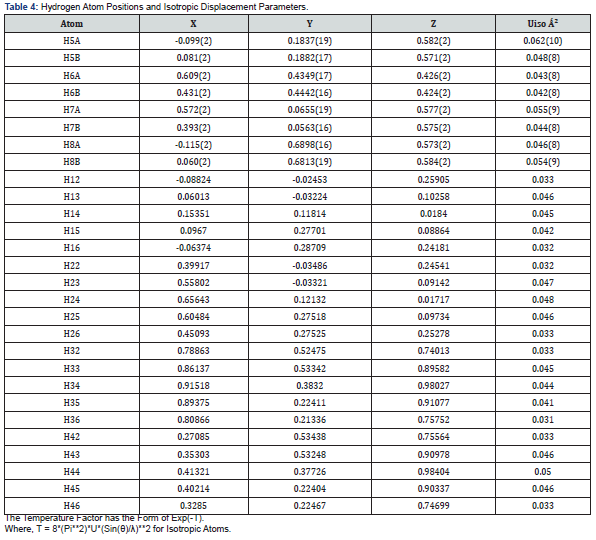
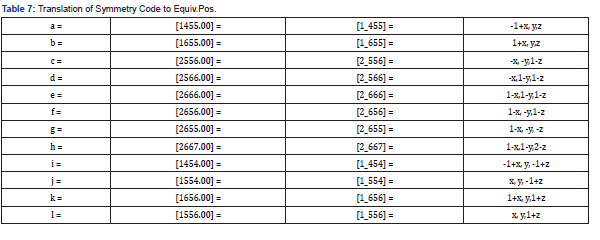
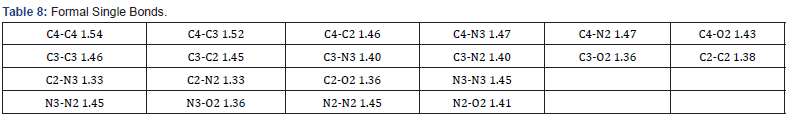
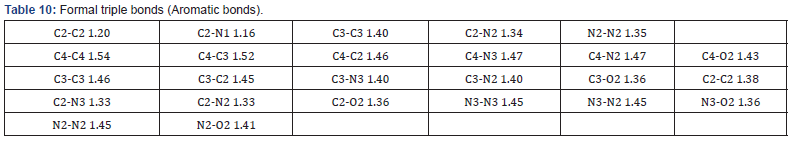
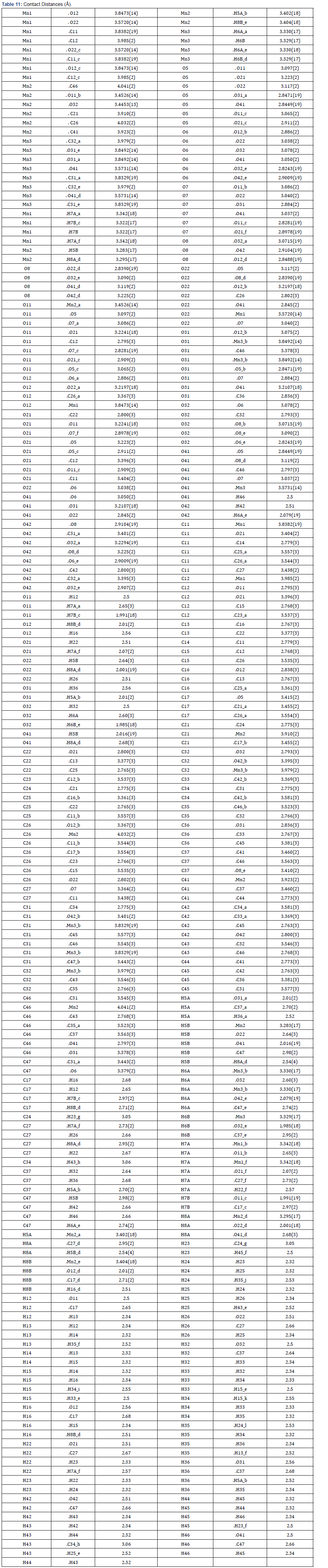
Final Coordinates and Equivalent Isotropic Displacement Parameters were used for the non-Hydrogen atoms (Table 2). The hydrogen atoms coordinated to benzene rings (C—H = 0.9500Ǻ) in Mn-MOF (Table 3) have been fixed in a geometrical order. The refinement on the crystal structure of Mn-MOF was achieved by applying the’ riding model’ with isotropic displacements (Tables 2,4,5). All information on the geometric parameters used in characterizing the molecular structure of Mn-MOF is found in supporting information, Tables 2-11. SHELXL [17] and PLATON (V-260918) have been used to prepare the molecular graphics of the compound for publication. The construction of Mn-MOF structures is proven in Figures 1-3.


Photoluminescence spectrum Mn-MOF
The luminescence spectrum of Mn-MOF was measured in the range 200-800nm, utilizing PerkinELmer Luminescence Spectrophotometer by absorption and emission modes.
Results and Discussion
Structural commentary
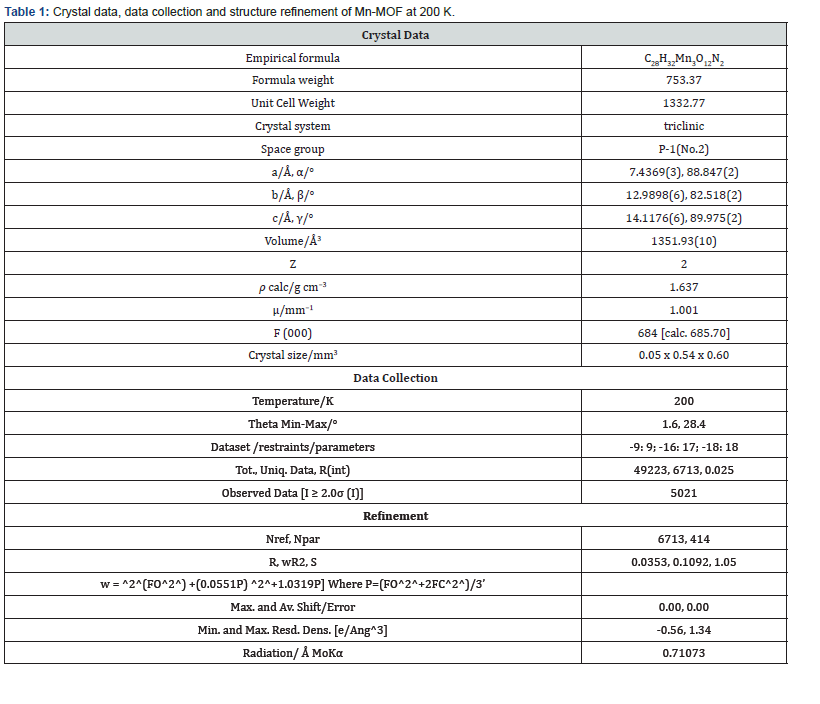
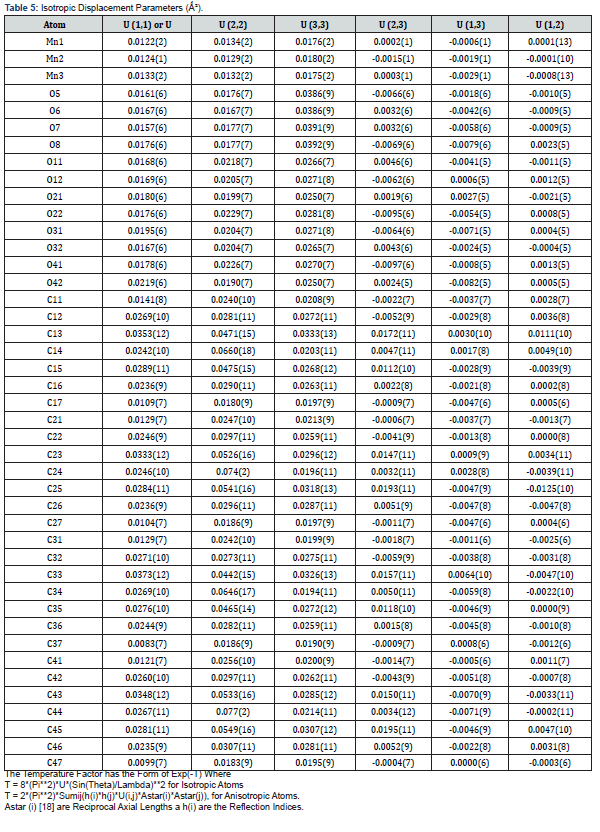
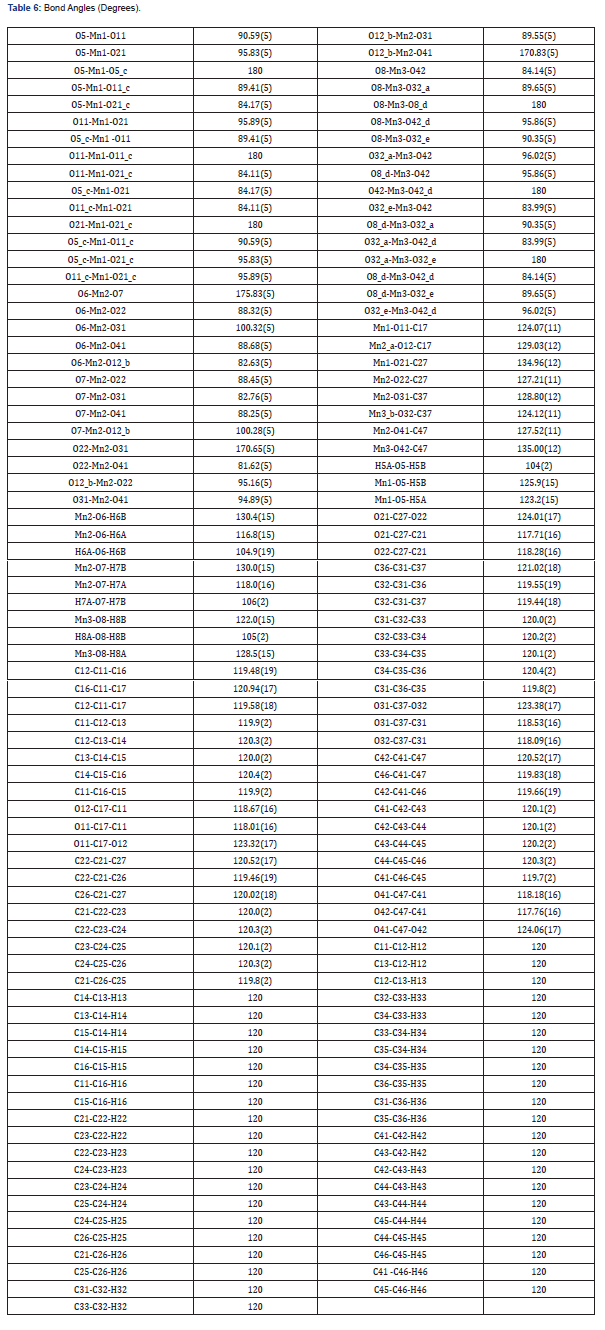
The asymmetric unit consists of three [19] MnII, two water molecules, and four carboxylate anions. The MnII is six-coordinated with octahedral geometry by six oxygen atoms. The apical position of Mn1 is occupied by two oxygen atoms at O22-Mn-O22 angle of 180.00 with a contact distance of 2.1635(13). The apical positions of Mn2 are occupied by two oxygen atoms at O22-Mn2-O31 angle of 89.95(5) with a contact distance of 2.1754(14): 2.1802(14). The apical positions of Mn3 are occupied by two oxygen atoms at O33- Mn-O33 angle of 180.00 with a contact distance of 2.1792(13). Two adjacent MnII ions are connected by two carboxylic oxygen atoms to form [Mn2O2] ring with Mn1…Mn2 distances of 2.16Å and Mn2…Mn3 of distances of 2.18 Å, respectively forming a 3D framework with two types of channels (Figure 2a). Figure 2b is the ball and stick illustration of Mn-MOF displaying hydrogen bonding system.
Supramolecular features
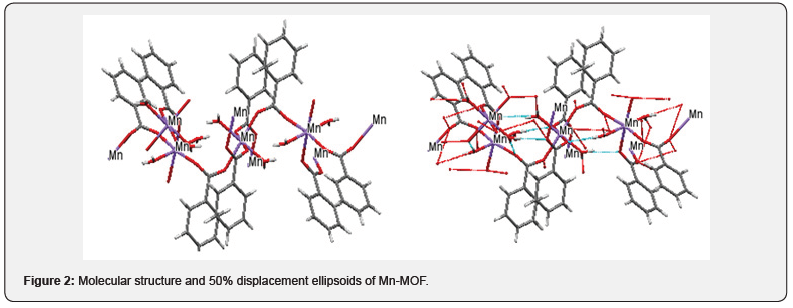
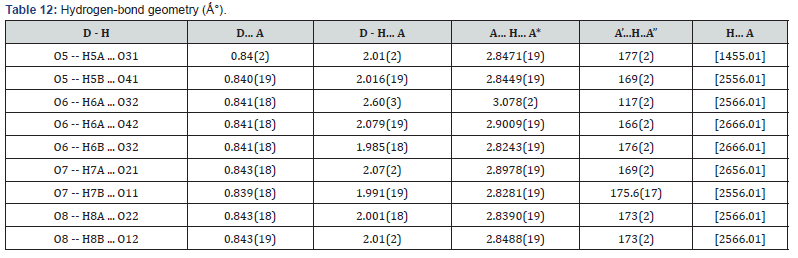
Figure 3 shows the crystal packing of Mn-MOF with the molecules linked into a three-dimensional network via intermolecular C−H…O, C−H…N, O −H…O, and N−H…O hydrogen bonds (Tables 12 & 13).

Refinement details
Table 12 & 13 shows the hydrogen and oxygen and hydrogen bound atoms were refined as O--H..O = 0.839(18) to 0.843(19); A’...H...A” = 117(2) to 177(2).The rest of the hydrogen atoms were calculated geometrically and refined with a riding model U(eq ) = 1/3 of the trace of the orthogonalized U Tensor with bond lengths of C–H being 0.95 Å( see Table 2).
Special geometric details
All the estimated standard deviations (esds), except the esds in the dihedral [20] angle between two l.s. [21] planes were calculated using the total covariance matrix. An isotropic treatment [22] of cell esds was employed for esds estimated standard deviations involving l.s [23] planes. The cell estimated standard deviations considered in this work were treated individually within the esds in distances [24], angles and torsion angles and correlations in cell parameters outlined by the crystal symmetry (Table 3,6 & 11). The coordination mode of manganese (II) with 1,10 phenanthroline and carboxylic acids was determined, results showing octahedral geometry [7,8]. Bond distances and angles, selected bond lengths (Angstrom), identification of chiral center(s) and their (R/S)- configuration were evaluated, and results shown in Tables 2-6 [9,25].
Photoluminescence studies of Mn-MOF
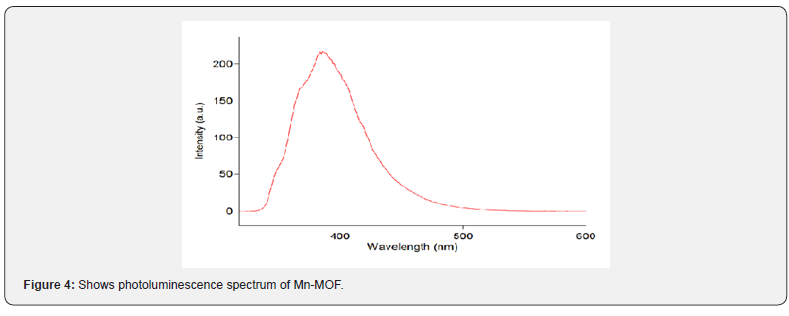
Figure 4 shows the fluorescence intensity of Mn-MOF at 308 nm increased with increase in adsorption time. The investigation of the luminescence properties of this compound revealed its efficient room-temperature phosphorescence in solution with a remarkable weak quenching by molecular oxygen [26-35]. The photophysical result displayed how the electron donor ability of the ligands and the electron–withdrawing character of the manganese(II) results in bathochromic shift of emission maximum (in the 324-460nm) and a decrease in the luminescence quantum yield showing the Mn(II) play a key role in the observed phosphorescence [5,6].
Conclusion
A new Mn(II) compound has been synthesized by our group. The structural arrangement within this compound shows define pores for accommodating other molecules by cation substitution. The investigation of the luminescence properties of Mn- MOF reveals efficient room-temperature phosphorescence in solution with a remarkable weak quenching by molecular oxygen. The pores in the Mn-MOF structure and photophysical results displayed in the observed phosphorescence is an indication that this compound can be used in fields such as drugs industries, as luminescence sensors to analyze water, air, and chemical pollutions. The correlation of the porosity and luminescence activity should be taken into account for designing new potential catalytic and energy storage materials.
Acknowledgment
Tertiary Education Trust Fund (RE: FUPRE/TO/ RESR/2017/01), Nigeria funded this work. Northwest University Summerstrand Campus, South Africa, acknowledged for single crystal structure analysis facility.
References
- Wang Y, Woll C (2018) Chemical reactions at Isolated Single-Sites Inside Metal-Organic Frameworks. Catal Lett 148: 2201-2222.
- He M, Ou X, Wang Y, Chen Z, Li D, et al. (2020) Porous organic frameworks-based(micro)extraction. J Chromatogr A 1609: 1-22.
- Lee S, Kapustin EA, Yaghi O M (2016) Coordinative Alignment of Molecules in Chiral Metal-Organic Frameworks. Science 353(6301): 808-811.
- Inokuma Y, Yoshioka S, Ariyoshi J, Arai T, Hitora Y, et al. (2013) X-ray Analysis on the Nanogram to Microgram Scale Using Porous Complexes. Nature 495: 461-467.
- Bikas R, Hosseini Monfared H, Vasylyeva V, Sanchiz J, Alonso J, et al. (2014) Heteronuclear, mixed-metal Ag(I)-Mn (II) coordination polymers with bridging N-pyridinylisonicohydrazide ligands: synthesis,crystal structures, magnetic and photoluminescence properties. Dalton Trans. 43:163-182.
- Sennappan M, Krishna PM, Ranganathan R, Sundari PS (2019) Synthesis, characterization, nucleic acid interaction and photoluminescence properties of {E}-[2-[2-hydroxybenzylidine]hydrazinyl][pyridine-4-yl]methaniminiumMn(II),Co(II), Ni(II),Cu(II) and Zn(II) complexes. J Molec Struc 1179: 86-91.
- Abebe A, Atlabachew M, Liyew M, Ferede E (2018) Synthesis of organic salts from 1,10-phenanthroline for biological applications. Cogent Chemistry 4(1): 1-10.
- Pook NP, Hentrich P, Gjikaj M (2015) Crystal structure of bis-[tris-(1,10-phenanthroline-k(2)N, N’)cobalt(II)]tetra-nitrate N,N’-(1,4-phenyl-enedicarbon-yl)diglycine solvate octa-hydrate. Acta Crystallogr E Crystallogr Commun 71(8): 910-914.
- Sairenji S, Kikuchi T, Abozeid MA, Takizawa S, Sasai H, et al. (2017) Determination of the absolute configuration of compounds bearing chiral quaternary carbon centers using the crystalline sponge method. Chem Sci 8(7): 5132-5136.
- Cahn RS, Ingold C, Prelog V (1966) Specification of Molecular Chirality Prelog. Angew Chem Intern Ed Eng 5: 385.
- Ladd MFC, Palmer RA (1985) Structure Determination by X-ray Crystallography. J Chem Soc Perkin II S1-S19.
- Umbakar S, Sekar P, Scheer M (2000) Chromium complexes with mixed Group 15 elements as ligands. Synthesis and characterization of the first cyclo-P2As in [CrCp(CO)2(3-P2As)]. J Chem Dalton Trans1135-1137.
- Fun HK, Quah CK, Nayak PS, Narayanab B, Sarojinic BK (2012) N-(2-Bromophenyl)-2-(naphthalene-1-yl)-acetamide. Acta Crystal Section E E68: o2657.
- Kassim K, Hashim NZN, Fadzila AH, Yusof MSM (2012) 2-(4-Chlorophenyl)-1-phenyl-1H-benzimidazole. Acta Crystal Section E E68: o799.
- Tarai A, Baruah JB (2019) Copper (II), Zinc (II), and Cadmium (II) Formylbenzoate Complexes: Reactivity and Emission Properties. ACS Omega 4(19): 18444-18455.
- Foo K Y Hameed, B H (2010) Insights into the modeling of adsorption isotherm systems. Chem Engine J 156: 2-10.
- Ahmed DS, El Hiti GA, Yousif E, Hameed AS (2018) Design and synthesis of porous polymeric materials and their applications in gas capture and storage: a review. J Poly Rese 25(3): 1-20.
- Munoz Olasagasti M, Telleria A, Pereez Miqueo J, Garralda MA, Freixaa Z (2014) A readily accessible ruthenium catalyst for te solvolytic dehydrogenation of amine-boran adducts. Dalton Trans 1-14.
- Niu Y, Cui L, Han J, Zhao X (2016) Solvent-mediated secondary building units (SBUs) diversification in a series of MnII-based metal-organic frameworks (MOFs). J Sol St Chem 241: 18-25.
- Johnson A, Mbonu J, Hussain Z, Wan Sin L, Hoong Kun F (2015) Crystal structure of tetraaquabis(3,5-diamino-4H-1,2,4-triazol-1-lum)cobalt(II)bis[bispyridine-2,6-dicarboxylateo)-cobaltate(II)dehydrate. Acta Cryst E71: m139-m140.
- Saber A, Sebbar Nk, Hokelek T, Taha ML, Mague JT, et al. (2020) Crystal structure, Hirshfeld surface analysis and DFT studies of 1-benzyl-3-[(1-benzyl-1H-1,2,3-triazol-5-yl)methyl]-2,3-dihydro-1H-1,3-benzodiazol-2-one monohydrate. Acta Cryst E Crystallogr Commun 76: 95-101.
- Asiri AM, Arshad MN, Zayede MEM, Alamrya KA, Bkhari TH (2012) (Z)-Ethyl 2-chloro-2-[2-(4-methylphenyl)-hydrazinylidene] acetate. Acta Cryst E E68: o3420.
- Priya GB, Srinivasa T, Girija K, Chandran NR, Velmurugan D (2011) 3-(4-Chlorophenyl) quinazolin-4(3H)-one. Acta Crystallogr Sect E 67(Pt9): o2310.
- Yotnoi B, Seeharaji A, Chimupala Y, Rujiwatra A (2010) Tris(ethylenediamine)cobalt (II)sulphate. Acta Crystallographica Section E, E66: m628.
- Cahn RS, Ingold C, Prelog V (1982) Basic principles of then CIP-system and proposals for a revision. Angew Chem Intern Ed Eng 21: 567.
- Li X, Pan Y, Ji J, Wang Q (2015) Synthesis, Crystal Structure and Theoretical Calculations of Cadmium (II) Coordination Polymer Assembled by 5-Nitroisophthalic Acid and 1,3-Bis(imidazol-1-ylmethyl)-benzene Ligands. J Inorg Organomet 25: 1069-1076.
- Hofmann DWM (2002) Fast estimation of crystal densities. Acta Cryst B 58: 489-493.
- Fisher R X, Tillmanns E (1988) The equivalent isotropic displacement factor. Acta Cryst C44: 775-776.
- Inokuma Y, Yoshioka S, Ariyoshi J, Arai T, Fujita M (2014) Preparation and guest-uptake protocol for a porous compliex useful for ‘crystal –free’ crystallography. Nature 9(2): 246-252.
- Techert S, Zachariasse T (2004) Structure Determination of the Intramolecular Charge Transfer State in Crystalline 4-(Diisopropylamino)benzonitrile from Picoseconds X-ray Diffraction. J Am Chem Soc 126: 5593-5600.
- Marie Noelle C, Alain D (2011) Manganese: Inorganic and Coordination Chemistry. In book: Encyclopedia of Inorganic and Bioinorganic Chemistry.
- Betz R, Gerber T, Schalekamp H (2011) Bis(2-hydroxyphenyl) methanone. Acta Crystal Section E 67(Pt 8): o1897.
- Asiri AM, Abo Dya NE, Arshard MN, Alamry KA, Shafiq M (2012) 1-(1H-1,23-Benzotriazol-1-yl)-2-(4-methoxyphen-yl) ethanone. Acta Crystallogr Sect E 68(Pt11): o3321.
- Chakrabarty R, Mukherjee PS, Stang PJ (2011) Suramoecular Coordination: Self-Assembly for Finite Two-And Three Dimentional Ensembles. Chem Rev 111: 6810-6918.
- Agthe M, Hoydalsvik K, Mayence A, Karvinen P, Liebi M, et al. (2015) Controlling Orientational and Translational Order of Iron Oxide Nanocubes by Assembly in Nanofluidic Containers. Langmuir 31(45): 12537-12543.






























