Synthesis Condition and Treatment Assisted Tuning of Structural, Magnetic Properties and Bandgap of Ni Nano Ferrite
R Verma and S N Kane*
Magnetic Materials Laboratory, School of Physics D A University, India
Submission: June 01, 2020; Published: June 24, 2020
*Corresponding author: S N Kane, Magnetic Materials Laboratory, School of Physics, D A University, Khandwa Road, Indore, India
How to cite this article: R Verma, S N Kane. Synthesis Condition and Treatment Assisted Tuning of Structural, Magnetic Properties and Bandgap of Ni Nano Ferrite. Organic & Medicinal Chem IJ. 2020; 9(4): 555768. DOI: 10.19080/OMCIJ.2019.09.555768
Abstract
We report synthesis condition, and thermal treatment assisted tuning of structural, magnetic properties, and bandgap of Ni nano ferrite. Experimental techniques of x-ray diffraction ‘XRD,’ vibration sample magnetometry, Ultraviolet-visible (UV-Vis) diffuse reflectance measurements, Scanning Electron Microscopy ‘SEM,’ Energy Dispersive spectroscopy ‘EDS’ measurements, were used to study the synthesized samples. XRD verify the formation of nano spinel ferrite phase (grain diameter: 38.3-39.2nm), and incorporation of Ni, Fe in spinel lattice, with lattice parameter (0.8345-0.8352nm), which also shows the presence of α-Fe2O3 phase. Results reveal that synthesis condition, and thermal treatment show:
a) SEM images with non-homogeneous particle size dispersion, particle agglomeration, while EDS confirms the presence of all elements in studied NiFe2O4 samples,
b) non-equilibrium cation distribution, modification of inversion parameter, oxygen parameter,
c) strengthening of A-O-B, A-O-A and weakening of B-O-B interaction super-exchange interactions,
d) fine-tuning of bandgap (1.39 eV-1.68 eV), and depends on structural properties,
e) observed magnetic properties are a collective effect of non-equilibrium cationic distribution; modification of A-O-B, A-O-A, B-O-B super-exchange interactions, and surface spin-canting. Prospective applications based on synthesized nano ferrites are also discussed.
Keywords:Sol-gel preparation; XRD; Nano ferrites; Cationic distribution; Magnetic properties; Bandgap
Introduction
Spinel ferrites display fcc structure with two inter-penetrating A (tetrahedral), B(octahedral) sub-lattices [1]. Ferrimagnetic spinel ferrites having the formula: Me2+O.Fe3+2 O3, display face-centered cubic ‘fcc’ structure, Fd3m space group [2]. Cationic occupation on A, B site is a complex process controlled by synthesis technique, composition, etc., and can control structural, magnetic properties [3]. As per the cationic distribution, one can have normal, inverse, mixed spinel is, depends on the type of ions occupying A, B sites [1]. Site A fully occupied by the divalent metal ion is normal spinel. In contrast, divalent metal ions on B site are inverse spinel, and divalent metal ions on both A, B sites are mixed ferrites. Distribution of cations can be modified to tune the properties (structural, magnetic, bandgap), can be successfully done by synthesis conditions e. g. microwave-assisted synthesis [4], specific composition [5], thermal treatment [6], is valuable for their usage for magnetic resonance imaging [5], hyperthermia [7] for cancer treatment, photocatalysis for water purification [8].
Literature reports the synthesis of Ni ferrite by co-precipitation [5], via mechanical milling [9], hydrothermal synthesis [10], by microwave-assisted sol-gel synthesis, which has distinct advantages e. g. rapid heating, shorter time (as compared to conventional double sintering technique), rapid reaction, easy reproducibility, control over grain diameter [4] and is energy efficient synthesis protocol [11]. Among the mentioned synthesis techniques, a sol-gel auto-combustion method has distinct advantages, e. g. synthesis done at a relatively lower temperature ~110oC (helpful in gaining control over grain diameter, is a key factor to tune structural, magnetic properties), spinel phase is obtained without sintering, is an energy-efficient protocol with lower thermal costs although the material quality is maintained.
Thus, in this work we report, sol-gel auto-combustion synthesis of NiFe2O4: by varying synthesis conditions (via microwaveassisted sol-gel synthesis, conventional sol-gel synthesis in dry gel form), and post-preparation thermal annealing, to tune structural, magnetic properties and bandgap energy. Prepared samples are studied by x-ray diffraction ‘XRD,’ vibration sample magnetometry, Ultraviolet-visible (UV-Vis) diffuse reflectance measurements, Scanning Electron Microscopy SEM,’ Energy Dispersive spectroscopy ‘EDS’ measurements.
Material Synthesis, Characterization, and Analysis of the Data
NiFe2O4 samples were synthesized by the sol-gel autocombustion method, by utilizing AR grade -nitrate/acetatecitrate precursors [Nickel acetate - Ni(CH₃CO₂)₂·4 H₂O, Ferric nitrate (Fe(NO3)3.9H2O), Citric acid - C6H8O7]. The synthesis is done by mixing precursors in the stoichiometric ratio, keeping pH at 7 by adding ammonia solution and keeping metal salts to fuel (citric acid) ratio as 1:1. The solution was heated at ~110°C for 1 hour in the air till fluffy power is formed called ‘dry gel’ or ‘as-burnt powder,’ which was ground to get a fine powder. Dry gel samples were thermally annealed for two hours at 450 and 550°C. Microwave-assisted sol-gel auto-combustion synthesis is performed in a domestic microwave oven (Samsung, India, model number: MW73D-B/XTL) operating at 2.45 GHz with 800 W power for 20 minutes [11]. Four different NiFe2O4 samples were synthesized:
a) microwave-assisted synthesis (labeled as S1),
b) dry gel (labeled as S2),
c) thermally annealed for 2 hours at 450 °C (labeled as S3), and
d) thermally annealed for 2 hours at 550 °C (labeled as S4). Structural information was obtained by Cu-Kα (wavelength: 0.15405 nm) X-ray diffraction ‘XRD’ measurements (θ-2θ configuration) by Bruker D8 diffractometer. Surface morphology and elemental analysis were done by scanning Electron Microscopy ‘SEM,’ Energy Dispersive spectroscopy ‘EDS.’ Vibrating sample magnetometer ‘VSM’ 7T SQUID VSM (Quantum Design, USA) was used to get magnetic measurements. Ultraviolet-visible (UV-Vis) diffuse reflection spectra were obtained by Perkin Elmar Uv-Vis spectrometer (Lambda 950), wavelength: 200-1500 nm.
Full-profile XRD analysis was done by MAUD Rietveld refinement software [12] to obtain the lattice parameter. XRD analysis gives structural parameters: experimental lattice constant (aexp.), Scherrer’s crystalline size D (calculated by subtracting instrumental branding from the integral width of 311 peak, implicitly described by Chaucy function [13]), specific surface area (S).inversion parameter (δ), oxygen parameter (u). XRD data was also analyzed to get cationic distribution [14]. It gives the best information on the cationic distribution by comparing experimental and computed intensity ratios for: (220), (311), (400), (422) reflections. The intensity ratio of planes I(220)/I(400) and I(400)/I(422) is susceptible to cation distribution. The best cationic distribution among tetrahedral ‘A,’
octahedral ‘B’ sites for which aexp., theoretical lattice parameter noticeably agree, were taken. Obtained cation distribution is used to calculate oxygen positional parameter (u), theoretical/Néel magnetic moment at 0K (Ms(th)) and theoretical lattice parameter (ath.), bond angles (θ1, θ2, θ3, θ4, θ5) are computed as shown in [3].
Hysteresis loops were used to compute coercivity (Hc), saturation magnetization (Ms), remanence (Mr), squareness ratio (Mr/Ms).
Particle size is obtained by analyzing SEM images by using ImageJ software [15]. By following expressions S, ath, u, and theoretical saturation magnetization Ms(th) at 0 K were computed:Specific surface area S is obtained by: S = [6 / (D × ρXRD)], where D - particle size, ρXRD - x-ray density,
where λ - Wavelength of x-ray used, β - Line width, θ - Peak position (in 2θ scale).
Theoretical lattice parameter (ath.) was estimated by using expression:
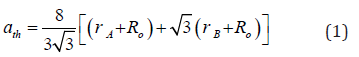
Where, rA – A-siteionic radius, rB – B-site ionic radius, Ro = 0.138 nm (ionic radius of oxygen ion)
Oxygen parameter (u) was obtained by:
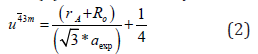
Theoretical saturation magnetization Ms(th) at 0 K by using the expression Ms(th) = MB-MA, where MB,MA - magnetic moments at B, A site.
Optical reflectance values were used to calculate energy bandgap b Kubelka-Munk relation:
F(R) = [(1-R)2] / R, where R is diffused reflectance [2,16]. TAUC plot provides bandgap energy by straight-line fitting to diffusereflectance data, extrapolating to 0 on the energy axis.
Results and Discussion
Figure 1(a) depicts XRD-patterns of NiFe2O4 samples, confirms the occurrence of the spinel phase. Figure 1(a) inset shows the zoom part of the 311 planes, to show changes in peak position. Presence of α-Fe2O3 phase is also observed, attributable to sample synthesis at a somewhat lower temperature (~110 °C), observed in [17] when sintering temperature was low. At higher sintering temperatures, it disappears. Representative Rietveld refined XRD pattern (Figure 1b) of sample S2 also confirms the cubic spinel ferrite phase formation.
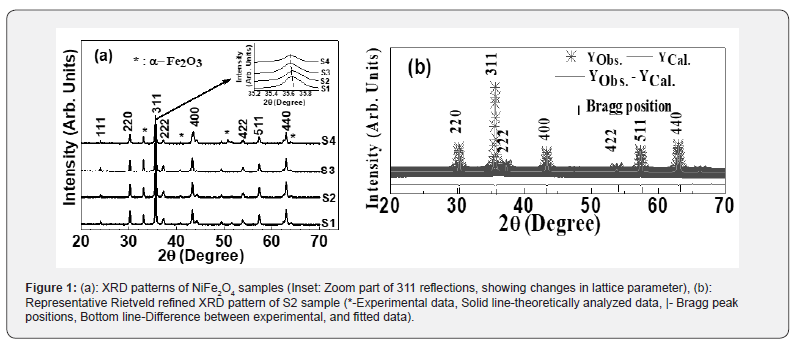
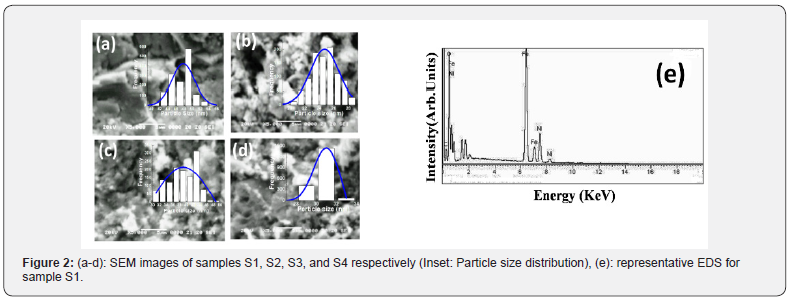
Figure 2(a-d) shows SEM images, and Inset shows corresponding particle size distribution. Perusal of Figure 2(a-d) depicts the non-homogeneous particle size dispersion, particle agglomeration showing different shapes, ascribable to the magnetic nature of spinel ferrites [6]. The observed difference in SEM images indicates the role of preparation in shaping microstructure, reflected in the particle size distribution. Mean agglomerate size for samples S1-S4 are respectively 48.67±0.60nm, 24.73±0.13nm, 39.05±0.99 and 30.95±0.4nm. Distribution width for S1-S4 are respectively 6.49nm, 4.54nm, 12.76nm, and 2.84nm. Sample S1 (microwave-assisted synthesized sample) shows the highest mean agglomerate size, and thermally annealed samples (S3, S4) show reduction of agglomerate size, ascribable to synthesis condition dependence of microstructure. Figure 2(e) gives representative EDS pattern of sample S1, shows peaks of elements, i.e., Ni, Fe and O. Table 1 gives atomic, weight %, experimental Me/Fe molar ratio (Me/Fe)exp., theoretical Me/Fe molar ratio (Me/Fe)th of Ni, Fe, O obtained from EDS analysis. Close agreement between (Me/Fe)exp and (Me/Fe)th values reveals the homogeneous distribution of the elements and formation of the desired ferrite phase [17].
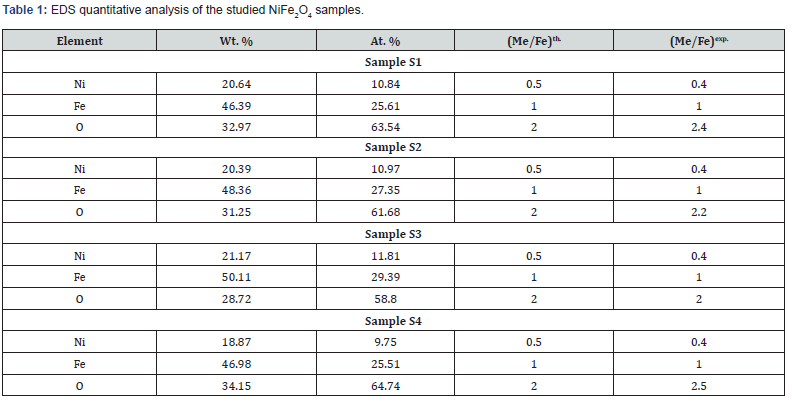
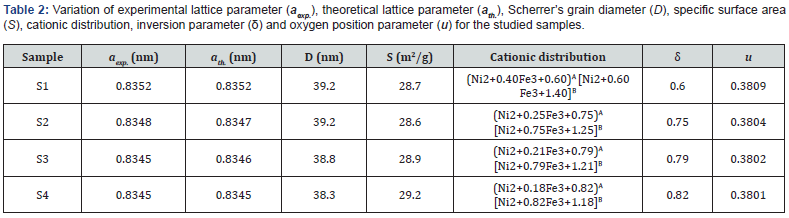
2 depicts the variation of experimental, theoretical lattice parameter (aexp., ath.), grain diameter (D), cationic distribution on A, B site, specific surface area (S), inversion parameter (δ) and oxygen position parameter (u) obtained by XRD analysis. Minor reduction of aexp. is ascribable to changes in cationic distribution and variation of the degree of inversion (δ), and close agreement between observed, calculated aexp., ath. suggests that the computed cationic distribution agrees well with real distribution [18]. Scherrer’s crystallite size (D) shows nanocrystalline ferrite, D varies between 38.3-39.2 nm, and is reflected in specific surface area values range between 28.6-29.2m2/g.
The cationic distribution shows that as we move from sample S1 to S4, on B-site Fe3+ ion concentration continuously decreases from 1.40 to 1.18 with a concurrent increase of Ni2+ ions from 0.60 to 0.82. It is of value to note that NiFe2O4 is inverse spinel with cationic distribution: (Fe3+) [Ni2+ Fe3+] [19], but in present studies, NiFe2O4 shows mixed spinel structure. In the ideal spinel structure, Ni2+ prefers to be on the B site [1]. However, in studied samples, it is on A, B site, demonstrates non-equilibrium cation distribution (a consequence of preparation condition variation), as seen in [18], also referred to as cation disorder [20], would lead to changes in magnetic properties.
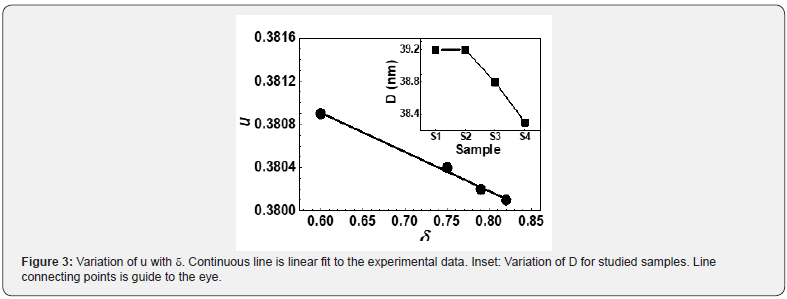
Observed changes in cationic distribution (changes in δ) lead to minor shrinkage of the unit cell, and there is a displacement of oxygen ions in such a way, that there is lowering to lattice distortion, showing minor changes in oxygen positional parameter u (range between 0.3803-3809). Observed u values are more than its ideal value: 0.375, gives the qualitative measurement of Oxygen displacement in the lattice [18], ascribable to variation in preparation conditions. Figure 3 shows a correlation between u and δ, showing linear variation between them. Figure 3 (Inset) shows the variation of grain diameter (D), where for the studied samples, only minor lowering of D is observed.
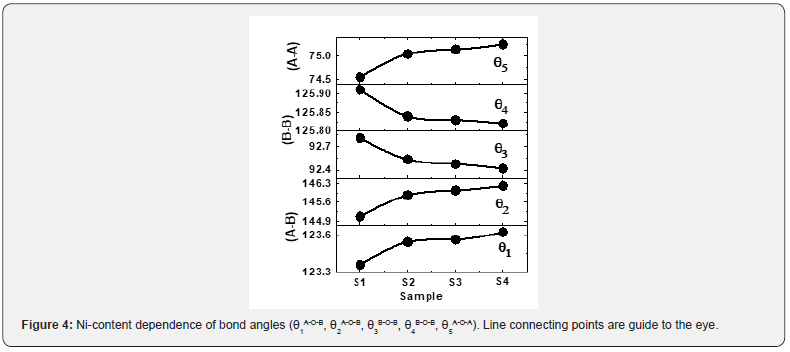
Bond angles between cations, cation-anion depend on the overall strength of Oxygen-mediated magnetic super-exchange interaction (A-O-B, A-O-A, B-O-B). Variation of bond angles are shown in Figure 4. Figure 4 depicts that as we move from sample S1 to S4, an increase of θ1, θ2, and θ5, whereas θ3, θ4 decreases. Bond angles increase reveal strengthening of A-O-B, A- O-A interactions, while the decrease of bond angles reveal weakening of B-B interaction [18,21], and would alter magnetic properties.
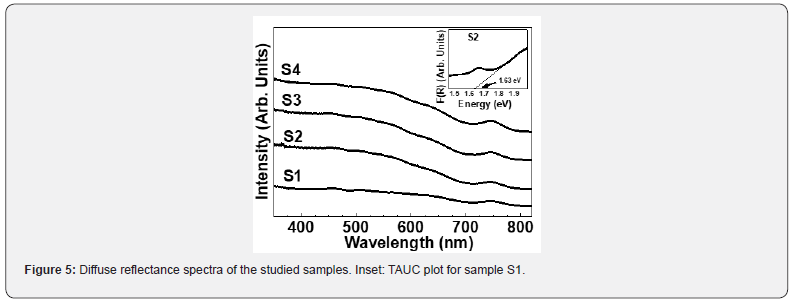
Diffuse reflectance spectra of studied samples are given in figure 5, while inset shows TAUC plot for sample S2, used for calculating bandgap. For the studied samples, bandgap varies between 1.39 eV-1.68 eV, ascribable to preparation condition assisted fine tuning [18]. Observed changes in bandgap with varying sample synthesis, and post-preparation condition is believed to be to a combined effect of changes in
a) lattice parameter [22],
b) cationic distribution [23] e. g.- Fe3+ ions on B-site [24],
c) degree of inversion δ, and
d) oxygen parameter u
This conjuncture is established via dependence of bandgap on aexp., Fe3+ ions on B-site, δ and u, shown in figure 6(a,b). Based on figure 6(a,b), following four empirical relations can be given:
a) [Bandgap] = A1 + B1 [ aexp.], (A1, B1: Fitting constants with values 325.37, -387.88).
b) [Bandgap] = A2 + B2 [ Fe3+ ions on B-site], [24] (A2, B1: Fitting constants with values 3.31, -1.37).
c) [Bandgap] = A3 + B3 [u] (A3, B1: Fitting constants with values 142.74, -371.058).
d) [Bandgap] = A4 + B4 [δ] (A4, B1: Fitting constants with values 0.58, 1.37).
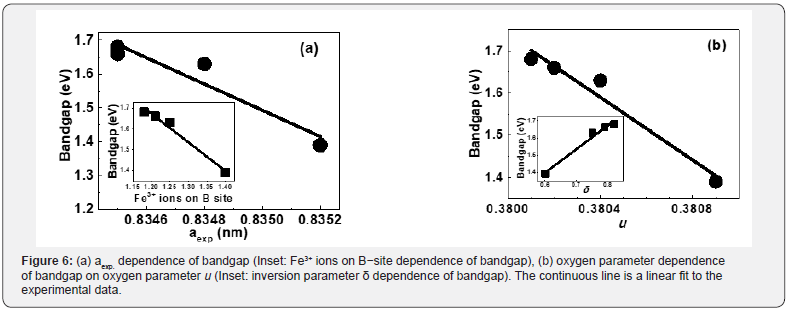
The purpose of the above mentioned empirical relations is to demonstrate strong correlation via linear variation between Bandgap and aexp., Fe3+ ions on B-site, δ and u. Fine-tuning of bandgap (ranging in the visible region) via structural parameters, as demonstrated in Figure 6(a,b), is interesting for application of studied samples in the fabrication of semiconducting devices, in water purification for degrading organic pollutants [8].
Table 3 shows coercivity (Hc), experimental, theoretical saturation magnetization (Ms(exp.), Ms(th)), remanence (Mr), reduced remanence (Mr/Ms) for the studied samples. Squareness ratio indicates that upon removal of the magnetic field, in which direction the magnetization will align itself [25]. Obtained lower squareness ratio values (Mr/Ms) (range between 0.07- 0.33) are attributable to stronger interactions among grains [6], which implies the isotropic behavior of material [26] making them multi-domain particles with no preferred magnetization direction. Hysteresis loops (Figure 7a) clearly show changes in Ms(exp.) while Inset shows the linear increase of Ms(exp.) on u (given in Table 2) shows an increase in disorder-induced change in Ms(exp.) as observed in [27].
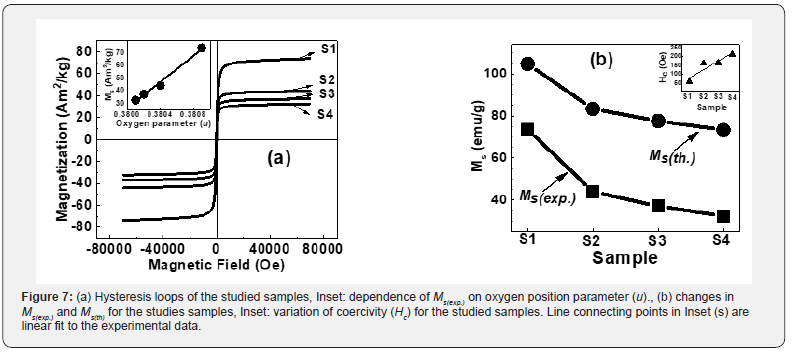
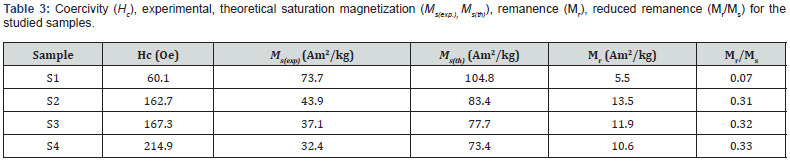
For samples S1 to S4, observed reduction of Ms(exp.), Ms(th.) (shown in Figure 7b) is attributed to the modification of B-O-B, A-O-B and A-O-A interaction controlled by:
a) Cation distribution,
b) Bond angle and
c) Canting angle.
While the canting angle is under consideration, the magnetization behavior is governed by Yafet-Kittel three sublattice model, described in [28]. This is confirmed by computed canting angle values: for S1, S2, S3, and S4 respectively as 32.7°, 38.2°, 37.2° and 51.0° (gives information on spin canting at the surface), known to change Ms(exp.), observed in [18,21].
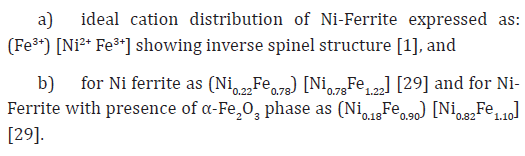
It is of value to note that minor changes in the cation distribution can interrupt exchange-coupling between the Fe3+ ions, resulting in enhanced magnetic properties as compared to bulk samples [20]. Such disruption of the Fe3+ arrangement in the spinel lattice may play the main role in observed magnetic behavior, as suggested in [20]. Sample S1, which is synthesized by microwave-assisted sol-gel auto-combustion synthesis, shows non-equilibrium cation distribution, and shows the minor presence of α-Fe2O3. Table 2 gives cationic distribution of sample S1 as: (Ni2+ 0.40Fe3+0.60)A[Ni2+ 0.60 Fe3+1.40]B shows presence of Ni2+ ions on A, B site, and higher population of Fe3+ions on B-site, is quite different than:
Observed Ms(exp.) value of 73.7Am2/kg for sample S1, higher than bulk magnetization ~ 55Am2/kg for the Ni Ferrite [30], is ascribable to non-equilibrium cationic distribution. For samples, S2 to S4 obtained Ms(exp.) values are respectively 43.9, 37.1, and 32.4Am2/kg, which are lower than bulk magnetization ~55Am2/ kg for the Ni Ferrite [30] are ascribed to surface spin canting, responsible for reducing Ms(exp.) values. Increase of dislocation density from 1.38×1015 lines/m2 (for sample S1) to 1.54×1015 lines/m2, (for sample S4) is ascribable to the observed increase of Hc for the studied samples, shown as Figure 7(b) inset.
Perspective Applications
For virus detection
An epidemic with coronavirus (Covid-19) in 2019 began since 31 December 2019, has created an urgent requirement:
a) Firstly, to detect it, and
b) secondly to destroy it. For the same, there is a vital need for materials with virucidal activity (i. e. capacity to destroy or inactivate viruses)
.Patients infected with the Covid-19 virus were diagnosed with a respiratory infection (influenza) [31]. MNP (magnetic nanoparticles)-GMR sensor [32] has shown that binding of the influenza virus to MNP’s is proportional to virus concentration. Thus, specific magnetic nanoparticles of composition exhibiting needed magnetic properties can play a vital role in virus detection, and eventually to destroy it via the usage of appropriate virucidal material [33]. In this context, tunability of structural, magnetic properties of magnetic nanoparticles (NiFe2O4) reported in this work is relevant, especially useful for detecting influenza virus.
For foodborne pathogens/bacterial inactivation in food packaging industries
Staphylococcus aureus bacteria are present in the atmosphere and is harmful to human beings by causing skin infection (including abscesses), respiratory infections e. g. -sinusitis and food poisoning. Ni-based nano ferrites have shown considerable antibacterial activity for foodborne Staphylococcus aureus bacteria [16]. Antibacterial activity of nanomaterials primarily depends on photogeneration (via light irradiation on the cell culture) by reactive oxygen species (•OH, •O2ˉ, and HOO•) on the ferrite nanoparticles surface (depends upon the crystallite size, larger surface area, and increased oxygen vacancies) would break organic bio-molecules, lipids, nucleic acids, proteins, carbohydrates, DNA, and amino acids: Bacteria: Bacteria + •OH→Bacterial inactivation [16].
Magnetic properties of ferrite nanoparticles are also suitable for ‘magnetic-separation,’ intended for recycling. From the discussion, as mentioned above, for optimum performance of ferrite nanoparticles as an anti-microbial agent, it is necessary to tune and optimize their structural, magnetic properties and bandgap, as reported in present studies. It is of value to note that bandgap ~2eV in studied Ni ferrite nanoparticles have strong absorption in visible-region (~50% of solar-radiation) can generate reactive oxygen species by solar radiation which is a non-hazardous, cost-effective, clean-green-energy driven advanced-oxidation-process for bacterial inactivation, and has vast possibilities in food packaging industries.
In hyperthermia for cancer treatment
In magnetic particles hyperthermia, magnetic nanoparticles are used owing to their ability to utilize an electric and magnetic component of the electromagnetic field used to excite them. [34] Nowadays, hyperthermia is regarded as an alternative therapy for cancer treatment. Although, various modes of hyperthermia are accessible for anticancer therapeutics today, nearly all suffer from limitation: targeting the tumor. The ideal delivery method for hyperthermia would be noninvasive, tissue-specific, localized, high-intensity heating in deep tissues. Hyperthermia, based on magnetic nanoparticles, can meet these requirements. Magnetic nanoparticles (e. g. - spinel nano ferrites of various Ni-based, Co-Zn, Mg-Zn, Li-based compositions [7,34] in a stable colloidal solution can be delivered non-invasively to specific tissues via external magnetic fields. On delivery, may be heated with AC magnetic fields by frequencies which do not affect healthy tissues.
In this context, a parameter called specific absorption rate (SAR) is essential, which measures the heat loss generated by magnetic nanoparticles in the AC magnetic field. SAR value should be high to minimize the amount of magnetic material for hyperthermia. SAR strongly depends on structural (e. g. - grain diameter, cationic distribution, and magnetic properties. A specific composition, synthesis condition, and post-preparation treatments are of use used to tune structural, magnetic properties of the nano ferrites needed for their optimum performance in hyperthermia applications. Non-equilibrium cation distribution strongly influences ferrites static, dynamic magnetic [7,34], thereby affecting SAR values. Thus, present results on NiFe2O4 magnetic nanoparticles are of interest for their potential usage in hyperthermia applications.
Visible light water purification for degradation of organic pollutants
In photocatalysis, the light activates a solid catalyst (e. g. spinel nano ferrite) that, in turn, can increase chemical reaction’s rate without itself being consumed. Magnetic nano ferrites (bandgap~2eV), have strong absorption in visible-region (~50% of solar-radiation) is advantageous, show improved efficiency in photocatalytic applications [8], can be magnetically recovered for reuse has shown significant efficiency towards the degradation of organic, inorganic pollutants [35]. Literature shows that in ferrites, cations occupying octahedral (B-site) influence catalytic reaction [35,36], as metallic ions on octahedral sites usually exposed on spinel ferrite surface, thus can easily participate in reactions to produce strong oxidant hydroxyl radicals (•OH radicals) for degrading organic pollutants into the harmless end-products (CO2; H2O), with no secondary-pollutants [37,38].
Solar visible-light assisted photocatalysis utilizing ferrites is a non-hazardous, cost-effective, clean-green-energy driven advanced-oxidation-process ‘AOP’: production of in-situ highly oxidizing radical species such as OH•). Mixing of oxidants: e. g. -H2O2 to the reaction mixture, improves reactive oxygen spices formation to enhance the degradation process [39]. Figure 8 illustrates the schematic of ferrite visible-light photo-degradation. From this work for sample S1 highest population of Fe3+ on B-site, would result in enhanced catalytic activity (as Fe3+ ions are almost fully placed at surface, accelerates degradation reaction).
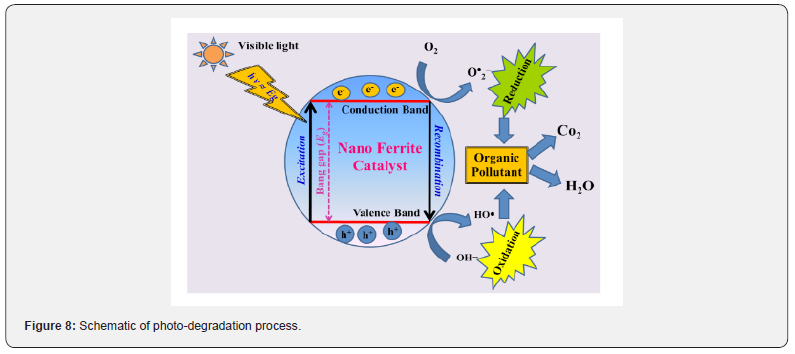
Presence of α-Fe2O3 is of use because, in α-Fe2O3, Fe3+ ions are located on the octahedral site (001 plane of hexagonal closed pack structure) also contributes towards visible-light photocatalysis, as it absorbs in visible-region (bandgap ~2.2eV). While magnetic properties are appropriate for ‘magnetic-separation,’ intended for recycling purpose. Good visible-light photocatalytic activity, re-usability of the photocatalyst is a combined effect of S, cation distribution, bandgap, magnetic properties. However, good visiblelight photocatalytic activity, re-usability of the photocatalyst is a combined-effect of S, D, cation distribution, bandgap, magnetic properties of nano spinel ferrites.
Spinel ferrites as MRI contrast agent
In medical science, magnetic resonance imaging (MRI) is one of the most potent tools [40], providing a noninvasive technique in clinical medicine for the assessment of anatomy, function of tissues. MRI has many advantages: multidimensional topographic capabilities, excellent temporal, and spatial resolution, lack of radiation exposure, and rapid in-vivo image acquisition [5]. It has been a preferred tool for imaging the brain, the central nervous system, for assessing cardiac function, and for detecting tumors. Since it can give anatomic images of soft tissue with high resolution [41], contrast agents are used in MRI for better diagnostic accuracy, by enhancing the image contrast. In MRI, the image contrast is linked to the relaxation process of hydrogen nuclei in water molecules, characterized by T1 and T2 relaxations [5].
The T1 contrast agents are based on paramagnetic ions e. g.- gadolinium (III) complexes, as they can decrease longitudinal relaxation time and increase the T1 signal intensity, thus giving a positive contrast. T2 contrast agents generally represent the superparamagnetic compounds of iron oxide, and the iron content of a T2 contrast agent produces intense local disruptions in the magnetic field of MRI scanners, leading to increased T2 relaxation, decreased signal intensity [5]. Following ferrite nanoparticles: MnFe2O4, NiFe2O4, and CoFe2O4 have been checked as T2 contrast agents [41]. For an efficient T2 contrast agent, nanoparticles should have large magnetization, which depends on their structural properties, size, surface properties [41], so for an efficient T2 contrast agent, tuning magnetic properties is required. Therefore, present studies on NiFe2O4 demonstrate suitable structural, magnetic properties via synthesis, thermal annealing, and have the potential for the development of efficient T2 contrast for MRI.
Conclusion
Modification of synthesis condition in the sol-gel autocombustion protocol and post-preparation thermal treatment was used to prepare nano spinel NiFe2O4. Observed minor changes are due to non-equilibrium cationic distribution, and mirrors in inversion parameter and oxygen parameter. Correlation between structural properties and bandgap energy is observed. It is demonstrated that synthesis condition and thermal treatment can be profitably used to tune structural, magnetic properties and bandgap of Ni nano ferrite, have prospective applications include combating viruses (including COVID-19), bacterial inactivation in food packaging industries, in hyperthermia for cancer treatment, water purification, and as MRI contrast agent
Acknowledgment
Authors thank Dr. M Gupta L Behra, Dr. R J Chaudhary-A Jana, Dr. U P Deshpande, Dr. D M Phase-V K Ahire, UGC-DAE CSR, Indore respectively for XRD, magnetic, Uv-Vis; SEM-EDS measurements. Work supported by UGC-DAE CSR, Indore project (No.: CSR-ICISUM- 25/CRS-308/2019-20/1360, dated March 5, 2020).
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
Conceptualization: SNK; Preparation of the samples, RV, SNK; Characterization and data analysis: SNK, RV; Project Administration, Supervision, Resources: SNK; Writing the manuscript: SNK, RV. All authors approve the draft and participate in reviewing.
References
- J Smit, H P J Wijn (1959) Ferrites Philips, Technical Library, Eindhoven, Holland, p. 137.
- R Verma, F Mazaleyrat, UP Deshpande, SN Kane (2020) Ni addition induced modification of structural, magnetic properties and bandgap of Ni-Zn nano ferrites, Materials Today: Proc.
- S Raghuvanshi, F Mazaleyrat, S N Kane (2018) Mg1-xZnxFe2O4 nanoparticles: Interplay between cation distribution and magnetic properties. AIP Adv 8(4): 047804.
- M Venkatesh, GS Kumar, S Viji, S Karthi, EK Girija (2016) Microwave assisted combustion synthesis and characterization of nickel ferrite nanoplatelets. Mod Electro Mater 2: 74-78.
- T Ahmad, I Rhee, S Hong, Y Chang, J Lee (2011) Ni-Fe2O4 Nanoparticles as Contrast Agents for Magnetic Resonance Imaging. J Nanosci and Nanotech 11: 5645-5650.
- SN Kane, M Satalkar (2017) Correlation between magnetic properties and cationic distribution of Zn85-x Nix Mg0.05Cu0.1Fe2O4 nano spinel ferrite: effect of Ni doping. J Mater Sci 52: 3467-3477.
- G Barrera, M Coisson, F Celegato, L Martino, P Tiwari, et al. (2020) Specific Loss Power of Co/Li/Zn-Mixed Ferrite Powders for Magnetic Hyperthermia. Sensors 20(7): 2151.
- SK Rashmi, HSB Naik, H Jayadevappa, R Viswanath, S B Patil, et al. (2017) Solar light responsive Sm-Zn ferrite nanoparticle as efficient photocatalyst. Mater Sci Eng B 225: 86-97.
- Y Shi, J Ding, X Liu, J Wang (1999) NiFe2O4 ultrafine particles prepared by co-precipitation/mechanical alloying. J Magn Magn Mater 205: 249-254.
- J Zhou, J Ma, C Sun, L Xie, Z Zhao, et. al. (2005) Low‐Temperature Synthesis of NiFe2O4 by a Hydrothermal Method. J Am Ceram Soc 88: 3535-3537.
- SN Kane, R Verma, P Tiwari, F Mazaleyrat (2019) Preparation condition, composition, and post-preparation thermal treatment assisted control of structural and magnetic properties of spinel nano ferrites. AIP Conf Proc 2142(1).
- L Lutterotti, P Scardi (1990) Simultaneous structure and size-strain refinement by the Rietveld method. J Appl Cryst 23: 246-252.
- T Tatarchuk, M Bououdina, W Macyk, O Shyichuk, N Paliychuk, et al. (2017) Structural, Optical, and Magnetic Properties of Zn-Doped CoFe2O4 Nanoscale Res Lett 12(1): 141-151.
- EF Bertaut (1950) Etude de la nature des ferrites spinelles. Comptes Rendus Hebdomadaires des Séances de l'Academie des Sciences 230: 213-215.
- CA Schneider, WS Rasband, KW Eliceiri (2012) NIH Image to Image J: 25 years of image analysis. Nat Methods 9: 671-675.
- MM Naik, HSB Naik, N Kottam, M Vinuth, G Nagaraju, et al. (2019) Multifunctional properties of microwave-assisted bioengineered nickel doped cobalt ferrite nanoparticles. J Sol Gel Sci Tech 91: 578-595.
- ER Kumar, R Jayaprakash (2013) Effect of combustion rate and annealing temperature on structural and magnetic properties of manganese substituted nickel and zinc ferrites. J Magn Magn Mater 348: 93-100.
- R Verma, SN Kane, P Tiwari, SS Modak, T Tatarchuk, et al. (2018) Ni addition induced modification of structural, magnetic properties and antistructural modeling of Zn1-xNixFe2O4 (x = 0.0 - 1.0) nanoferrites. Mol Cryst Liq Cryst 674(1): 130-141.
- B Rabi, A Essoumhi, M Sajieddine, J M Greneche, E K Hlil, et al. (2020) Structural, magnetic and magnetocaloric study of Ni0.5Zn0.5Fe2O4 Appl Phys A 126(174).
- S Seifikar, T Rawdanowicz, W Straka, C Quintero, N Bassiri Gharb (2014) Structural and magnetic properties of sol-gel derived NiFe2O4 thin films on silicon substrates. J Magn Magn Mater 362: 255-261.
- R Verma, SN Kane, S Raghuvanshi, M Satalkar, SS Modak, et al. (2018) Synthesis, structural and magnetic properties of cadmium substituted Li-ferrite. AIP Conf Proc 2142(1).
- M Sundararajan, LJ Kennedy, P Nithya, JJ Vijaya, M Bououdina (2017) Visible light driven photocatalytic degradation of rhodamine B using Mg doped cobalt ferrite spinel nanoparticles synthesized by microwave combustion method. J Phys Chem Solids 108: 61-75.
- EC Devi, I Soibam (2018) An investigation on the optical band gap and Ac conductivity of Mn-Zn nanoferrites. J Supercond Nov Magn 31: 1183-1188.
- SN Kane, P Tiwari, Deepti, R Verma, UP Deshpande (2020) Study of structural, magnetic properties and bandgap of spinel Co1-xFe2+xO4 ferrite. Mater Today Proc.
- NLA Rodin, MR Sahar, F Mohd Noor (2020) Magnetic analysis of cobalt oxide nanoparticles comprised boro-tellurite glass with erbium lanthanide. J Magn Magn Mater 496: 165931.
- SE Shirsath, B Toksha, K Jadhav (2009) Structural and magnetic properties of In3+substituted NiFe2O4. Mater Chem Phys 117: 163-168.
- S Raghuvanshi, P Tiwari, SN Kane, DK Avasthi, F Mazaleyrat, et al. (2019) Dual control on structure and magnetic properties of Mg ferrite: Role of swift heavy ion irradiation. J Magn Magn Mater 471: 521-528.
- NSS Murthy, MG Natera, SI Youssef, RJ Begum, CM Srivastava (1969) Yafet-kittel angles in zinc-nickel ferrites. Phys Rev 181: 969-977.
- KC Verma, N Goyal, M Singh, RK Kotnala (2019) Hematite α-Fe2O3 induced magnetic and electrical behavior of NiFe2O4 andCoFe2O4 ferrite nanoparticles. Results in Phys 13: 102212.
- K Maaz, S Karim, A Mashiatullah, J Liu, MD Hou, et al. (2009) Structural analysis of nickel doped cobalt ferrite nanoparticles prepared by co-precipitation route. Physica B 404: 3947-3951.
- Z Sedaghat, N Karimi (2020) Guillain Barre syndrome associated with COVID-19 infection: A case report. J Clin Neurosci 76: 233-235.
- VD Krishna, K Wu, AM Perez, JP Wang (2016) Giant magnetoresistance-based biosensor for detection of influenza A virus, Front. Microbiol 7: 400.
- ST Jones (2020) How materials can beat a virus. J Mater Sci 55: 9148-9151.
- M Coisson, G Barrera, F Celegato, L Martino, SN Kane, et. al. (2017) Hysteresis losses and specific absorption rate measurements in magnetic nanoparticles for hyperthermia applications. Biochimica et Biophysica Acta 1861: 1545-1558.
- S Gul, MA Yousuf, A Anwar, MF Warsi, FO Agboola, et al. (2020) Al-substituted zinc spinel ferrite nanoparticles: Preparation and evaluation of structural, electrical, magnetic and photocatalytic properties. Ceramics Int 46: 14195-14205.
- X Han, T Chen, R Li, F Cheng, M Zhang, M Guo, et al. (2019) Hydrothermal temperature effect on microstructure evolution and Fenton-like catalytic performance of spinel ferrite (Mg, Ni) (Fe, Al)2O4 synthesized from saprolitic nickel laterite. Colloids and Surfaces A 568: 11-19.
- MR Hoffmann, S Martin, W Choi, DW Bahnemann (1995) Environmental applications of semiconductor photocatalysis. Chem Rev 95: 69-96.
- K Jahanara, S Farhadi (2019) A magnetically separable plate-like cadmium titanate-copper ferrite nanocomposite with enhanced visible-light photocatalytic degradation performance for organic contaminants. RSC Adv 9: 15615-15628.
- L George, C Viji, M Maheen, EM Mohammed (2020) Synthesis, characterization of Mg/Mn substituted Ni-Zn ferrites and mechanism of their visible light photo catalysis of Methylene Blue and Rhodamine B dyes under magnetic influence. Mater Res Exp 7(1): 015014.
- MA Brown, RC Semelka (2003) MRI: Basic Principles and Applications, Wiley Liss, New York, USA, pp. 39-40.
- HB Na, IC Song, T Hyeon (2009) Inorganic Nanoparticles for MRI Contrast Agents. Adv Mater 21: 2133-2148.






























