Synthesis, Characterization, Biological and Antitumor Activity of Co(II) , Ni (II) , Cu(II) and Zn (II) Complexes of N-(2-Chlorophenyl)-N’-Benzoyl Thiourea
Ali M Hassan1, Zaghloul I Elbialy2 and Khalid M Wahdan3*
1Department of Chemistry, Al Azhar University, Egypt
2Egyptian Organization for Standardization and Quality
3 Researcher at the Faculty of Science, Al Azhar University, Egypt
Submission: May 20, 2020; Published: June 04, 2020
*Corresponding author: Khalid M Wahdan, Researcher at the Faculty of Science, Al Azhar University, Egypt
How to cite this article: Ali M H, Zaghloul I , Khalid M W. Synthesis, Characterization, Biological and Antitumor Activity of Co(II) , Ni (II) , Cu(II) and Zn (II) Complexes of N-(2-Chlorophenyl)-N’-Benzoyl Thiourea. Organic & Medicinal Chem IJ. 2020; 9(4): 555767. DOI: 10.19080/OMCIJ.2019.09.555767
Abstract
A derivative of thiourea ligand N-(2-chlorophenyl)-N’-Benzoyl thiourea (CBT) in equimolar ratio 1:1and its transition metal complexes CoII, NiII, CuII and ZnII were synthesized by microwave (green chemistry). The structure of ligand and its complexes have been characterized by using elemental analysis, mass Spectroscopy, FT-IR, UV-Vis,1HNMR and 13CNMR. The geometry of the proposed structures of the chelates based on their electronic spectra, electron spin resonance (ESR) and magnetic susceptibility. The stability of complexes was studied by TGA analysis (Thermal studies). The free derivative thiourea ligand (CBT) and its complexes were Studied for antibacterial and antifungal activity. Also the anticancer activity of ligand and its complexes were studied against breast cell cancer (MCF-7). It was noticed that the NiII complex showed the highest cytotoxic activity (IC50=122) more than the free CBT ligand.
Keywords:Thiourea; Transition metals complexes; Biological activity; Antitumor effect; Microwave irradiation
Introduction
Thiourea (NH2)2C=S is a compound where the oxygen atom of urea compound replaced by a Sulphur atom. The compounds bearing carbonyl and thiocarbonyl groups are used as potential donor ligand for the preparation of complexes [1,2]. Among these, Thiourea and its derivatives are versatile ligands that coordinate to form stable compounds. They are able to coordinate with metal either as neutral or monoanion or dianion ligand [3,4]. These thiourea ligands and their metal complexes exhibit a wide range of biological activity. They are reported to act as antimicrobial, antibacterial, antifungal, antimalarial, antituberculosis and anticancer activities [5,6]. They also form a variety of complexes of different symmetries with various metal ions [7,8]. In view of the importance of thiourea and their derivatives it was worth interesting to synthesize N-substituted thiourea ligand and their complexes with transition metal elements because it was observed that this activity was enhanced by complexion with certain transition metal elements [3,9].
Materials and Experimental Method
Materials and physical measurements: All purchased chemicals were of Annular AR grade and were obtained from Sigma Aldrich and all Metals salts were purchased from ADWIC .The Microwave-assisted synthesis was carried out in a domestic microwave energy output 900W. Purity of Schiff base ligand and its complexes were detected by using thin-layer chromatography (TLC) technique. Melting points were recorded in open capillaries with Barnstead Thermolyne Mel-temp 1001D Electrothermal Melting Point. Elemental analysis was done on automatic analyzer CHNS Vario El III-Elementar, Germany. The FT-IR spectra samples were ground with (CsBr) powder. Then pressed into a disk and recorded on Shimadzu FTIR spectrometer. Mass spectra were determined by using Mass GC-2010 Shimdazu instrument. Metals content were determined by complexometric titration using xylene orange (XO) as indicator and hexamine as a buffer (pH = 6).
Electronic absorption spectra in DMF were measured using automated UV/Vis-NIR 3101 PC Shimadzu spectrophotometer ranged from 200-900nm. 1HNMR spectra for Schiff base ligand was recorded in 300MHz Varian-Oxford Mercury in DMSO-d6 as solvent and the chemical shifts were recorded in ppm relative to TMS as an internal standard. Magnetic susceptibility of complexes was measured on powdered samples using the faraday method. Thermal analysis measurements (TGA) were carried out with Shimadzu thermal analyzer model 50 at Micro analytical .The ESR spectra of the powdered Cu(II) complex recorded at room temperature by X-band EMX spectrometer (Bruker, Germany) using a standard rectangular cavity of ER 4102 with 100KHz frequency.. Schiff base ligand and their metal complexes were screened for in-vitro antibacterial activity against two species of Gram-positive bacteria and two species of Gram-negative bacteria as well as two species of fungi. Also, Cytotoxicity evaluation was applied against breast cell cancer (MCF-7). All of these were carried out in faculty of Science, Cairo University.
Chemicals
All consumed chemicals were from analytical grade and were used as received without further purifications. Chemicals used are Benzoyl chloride, ammonium thiocyanate, o-chloro analin, acetone, cobalt acetate, nickel acetate, copper acetate, zinc acetate and methanol.
Synthesis of the organic ligand
0.1m of ammonium thiocyanate(7.6gm) dissolved in 50ml of acetone then added drop by drop to 0.1m of benzoyl chloride (14.06gm) (11.62ml) taken in 3 neck flask with continuous stirring. The mixture is refluxed for 1 hour with continuous stirring. After 45 minutes white ppt (ammonium chloride) appeared and then disappeared at 1hr. The mixture left in room temp until the precipitate appears again completely. Filtration done and precipitate washed by acetone to get all the filtrate (Benzoyl thiocyanate). The filtrate added drop by drop in a 3-neck flask contains 0.1m (10.5ml) of ortho chloro analin with continuous stirring. The mixture refluxed for 6hrs with continuous stirring. The mixture transferred to a baker and covered for two days for complete precipitation. Then the Precipitate was filtrated and washed by ethanol and acetone to give the pure ligand (CBT).
Synthesis of metal complexes
The prepared ligand and the acetate salts of the metal Co(CH3COO)2.4H2O, Ni (CH3COO)2.4 H2O,Cu(CH3COO)2.H2O and Zn (CH3COO)2.2H2O were mixed in (1:1) ratio. The reaction mixtures were then irradiated by the microwave oven by using drops of methanol as a solvent. The reaction was completed in a short time (3-5min) with higher yields. The resulting product washed by hot methanol and ether and finally dried under reduced pressure over anhydrous CaCl2 in a desiccator. The progress of the reaction and purity of the product was monitored by TLC using silica gel (yield: 80-85%). The synthetic route of the prepared compounds is illustrated in Scheme 1.
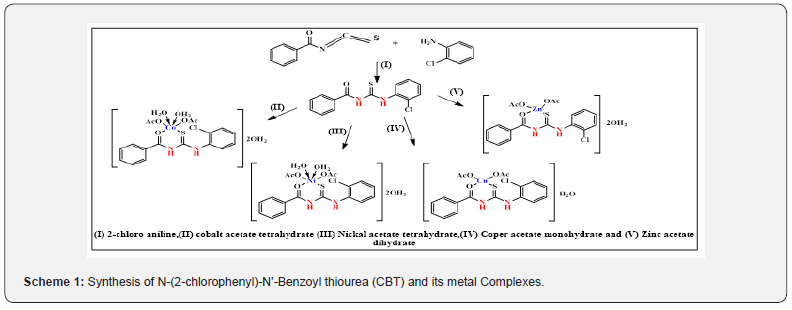
Results and Discussion
The structure of the prepared ligand N-(2-chlorophenyl)- N’-Benzoyl thiourea (CBT) was characterized by melting point, elemental analysis, IR, mass spectra, UV-vis and NMR studies. This data is compatible with the required product. Analytical and physical properties of prepared compounds tabulated in Table 1.
IR spectra
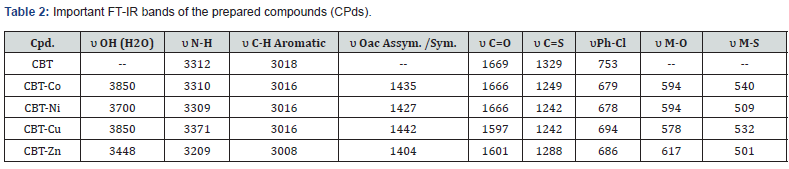
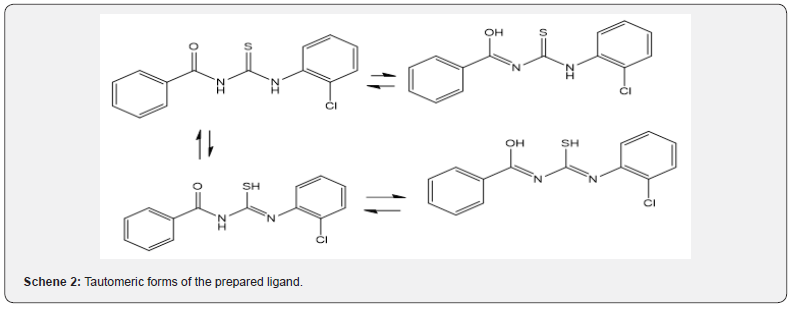
The characteristic IR bands of all thiourea ligands showed the expected frequencies of υ(C=O), υ (N-H), υ(C-N) and υ(C=S). The coordinative behavior of present ligand, towards Zn(II) and Ni (II) ions to form complexes is difficult to establish, as this ligand is capable of exhibiting three tautomeric forms as clear from Scheme 2 due to presence of [‒NH‒C(=S)] and [‒NH‒C(=O)] functional groups. However, the lack of the characteristic vibrations of υ(S–H) around 2500‒2600cm-1 [10] and presence of a peak at 3312cm-1 characteristic of υ(N–H) [11] confirmed the absence of tautomeric form (N=C‒SH) and (N=C-OH). Two sharp intense bands observed at 1669 and 1329 cm-1can be ascribed to the stretching vibration of carbonyl group υ(C=O) and thionyl group υ(C=S) respectively [12,13].
These observations confirmed the ketonic-thion form of the ligand in the solid-state [14]. Moreover, the υ(C–Cl) stretching frequency was observed at 753cm-1, while this band appearing at 678-686cm-1 assigned to the usual modes of phenyl ring vibration, respectively [15,16]. On the other hand and upon coordination of the metal center to ligand, the characteristic bands of υ(C=O) and υ(C=S) present in the spectrum of the free ligand at 1669 and 1329cm-1 were found to be shifted to a lower frequency and appear from 1597 to 1666 for C=O and from 1242 to 1288cm-1 for C=S. This finding may be taken as an evidence for the coordination of the carbonyl oxygen and thionyl sulphur atoms with the metal ions. The IR spectra for ligand and complexes are shown in Figure 1-3 and the data are tabulated in Table 2.
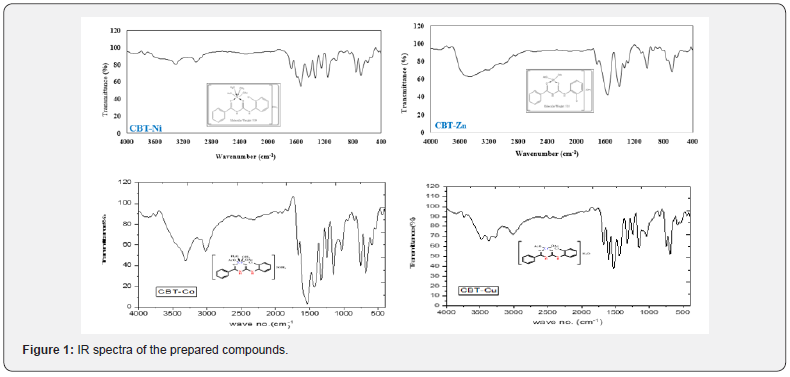
Mass Spectra
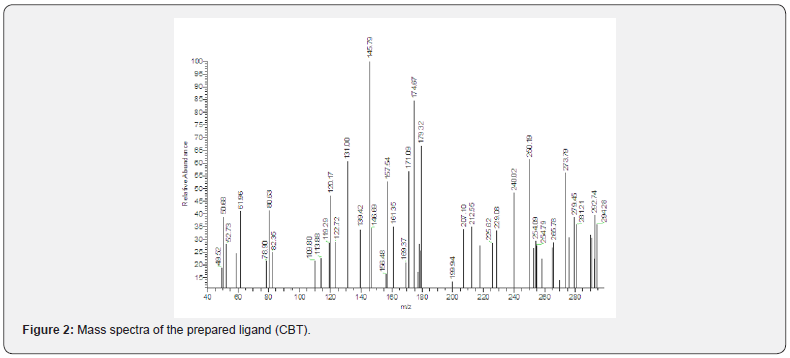
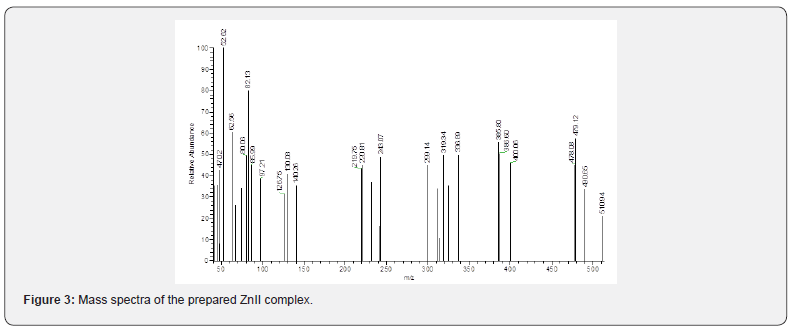
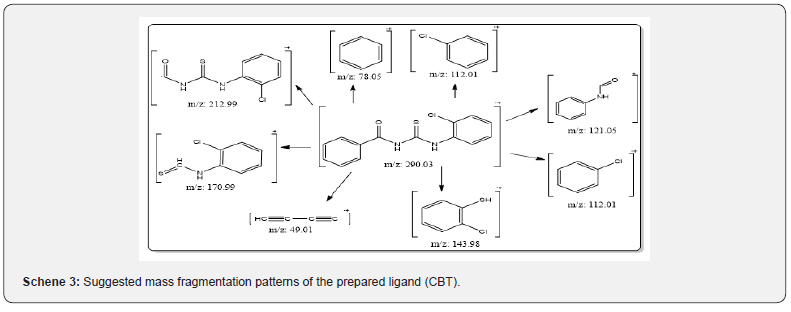
The recorded mass spectra of the ligand and its complexes (Scheme 3) showed a peak at m/z (relative abundance) 2902.7(39.57%) which is the molecular peak of (C14HH1N2OSCl). The ion peak at m/e = m/z 212.55(42%) is due to M+(C8H5N2OSCl), while the ion peak at m/e = 145.79 (100%) corresponds to M+(C6H6SCl) which is the base peak, the ion peak at m/e = 120.17 (47%) corresponds to M+(C7H6ON), the ion peak at m/e = 171 (56.75%) is due to M+(C7H5NSCl), the ion peak at m/e = 113.88 (22.77 %) points to M+(C6H5CL). The ion peak at m/e = 50.68(38.79%) refers to M+ (C4H2), the ion peak at m/e = 78.9 (21.5%) corresponds to M+ (C6H6). The fragmentation pattern of N-(2-chlorophenyl)-N’-Benzoyl thiourea is shown in Scheme 3 and Figure 2.
The mass spectra of the CBT-Zn complex (Scheme 4) showed peak at m/z (relative abundance) 510.9 (21.06%) as the molecular peak of (Zn.C18H21N2O7S). The ion peak at m/e = m/z 400 (45.9 %) is due to M+(Zn.C16H14NO3S), while the ion peak at m/e 325 (35.48%) corresponds to M+(ZN.C10H9O3S), the ion peak at m/e = 385.5 (55.5%) corresponds to M+(Zn.C16H17ON2S), the ion peak at m/e = 336.89 (49.9%) is due to M+(Zn.C15H14ON2S), the ion peak at m/e = 319.34 (49.6 %) points to M+( Zn.C14H11ON2S). The ion peak at m/e = 243(48.9%) refers to M+ (Zn.C8H6ON2), the ion peak at m/e = 97.2(38.58%) corresponds to M+ (Zn-S) M+ (C6H6SCl), the ion peak at m/e =52.6(100%) REFERS TO (C2N2) which is the base peak, the ion peak at m/e =44.7(35.63) REFERS TO (CS). The fragmentation pattern of ZnII complex is shown in Scheme 4 and Figure 3

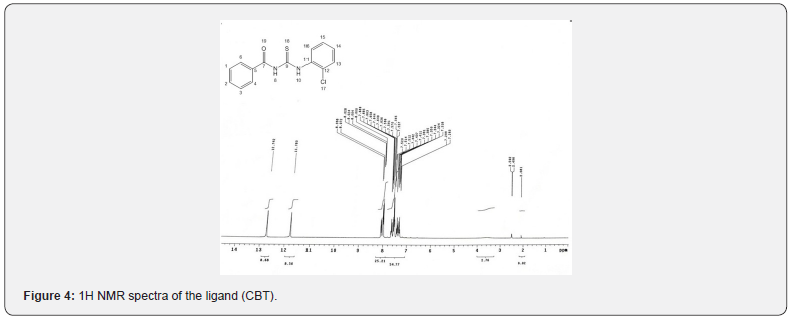
HNMR spectra [1]
The assignments of the main single in the [1] HNMR spectra of the ligand given in Figure 5 recorded in DMSO-d6 as a solvent with TMS internal standard, displays some groups of signals corresponding to the various protons [17]. The chemical shifts were expressed in ppm. The spectrum of the ligand (CBT) give two singlet signals, one at δ 11.78ppm assigned to (S,1H, N8-H) and the other at δ 12.742ppm assigned to(S,1H, N10-H) [18] (which were also identified by D2O exchange) . The multiplets observed at δ 7.293 - 8.098ppm are attributed to the phenyl protons [19].
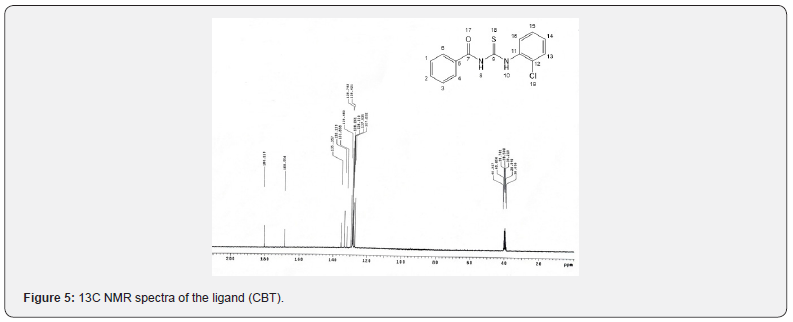
CNMR Spectra [13]
The 13CNMR spectra of the synthesized ligand (CBT) showed peaks at 180.219 ppm assigned to (C=O), 168.556ppm assigned to (C=s), 135.357ppm (C11) 133.239ppm(C16), 131.858 ppm (C5), 129.483ppm(C2) and at 128.793ppm(C12), 128.261ppm(C13), 128.110ppm(C1 and C3), 127.935 ppm(C4 and C6), 127.222 ppm(C15).
Electronic and magnetic properties of the prepared compounds
The stereochemistry of the metal ions in the complexes can be assigned via the electronic spectral measurements. The diffuse solid reflectance spectra of the ligand and its complexes in solidstate showed a number of bands in the UV -Vis region (200- 800nm).
In the spectrum of the free ligand
Three absorption bands were observed at 257.5, 275 and 310nm. The first band can be assigned to n −π * transitions originated from aromatic moieties, and the third band can be assigned to n −π * transitions originated from C=O and C=S groups. The latter band is due to the intermolecular charge transfer interaction from aromatic groups to C=O and C=S group [20,21].
The cobalt (II) complex spectrum showed
Absorption bands from 425 to 650 nm assigned to
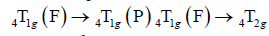
The nickel (II) complex spectrum showed
Three electronic bands from 450 to 750nm due to the transition
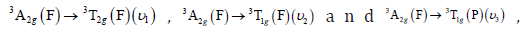
The copper (II) complex spectrum showed
The electronic spectra of the copper complexes revealed bands from 425 to 750nm assigned to the transitions ( ) 2 2 2 B → E [19]. The measured magnetic moments, 1.78B.M, falls in the range reported for tetrahedral geometry.
The zinc (II) complex spectrum showed
Absorption bands from 560 to 720 (nm) which can be assigned to metal ligand charge transfer MLCT in a low spin tetrahedral geometry of Zn (II) complex confirmed by the diamagnetic properties. The assignments of the observed electronic transitions apart from that together with the geometry and the magnetic moment values [23, 24] are listed in Table 3.
The most essential features of the spectrum of the complex are entirely different when compared to that of the free ligand. Upon interaction of the ligand with metal ions, there are some shifts in bands positions in the spectra of the complexes. This change can be taken as a positive evidence of complex formation. In addition, appearance of new bands at longer wavelength assigned to ligand metal charge transfer (LMCT) and d-d transitions is another confirmation to complex formation, which gives evidence for the coordination of the ligands to the metal ions. All spectra data were tabulated in Table 3 and represented in Figure 6.
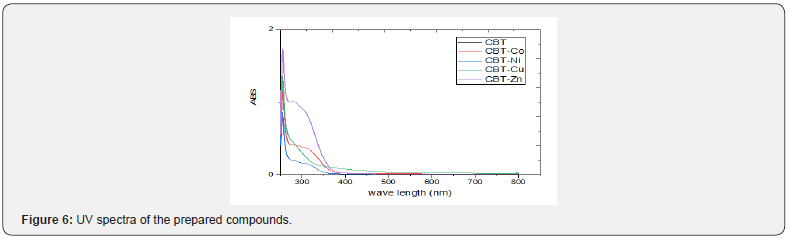
Electron spin resonance spectrum (ESR)
ESR spectroscopy is a technique for studying chemical species that have one or more unpaired electrons through measuring the absorption of electromagnetic radiation by a molecular system containing one or more unpaired electron, such as organic and inorganic free radicals or inorganic complexes possessing a transition metal ion and rare earth metals. The spectra of copper complex exhibit single broad signal with hyperactive structure indicating the contribution of free acetate ligand with complex formation. The spectra showed broad signals with two “g” values (g\\, g┴) Figure 7. For all complexes the value of g\\ < g┴ < 2.3, characteristic of complexes with 2B1 (dx2-y2) orbital ground state. The average g values were calculated according to the equation gav = 1/3[g\\ +2g┴] and it was equal to 1.397 for CBT-Cu.
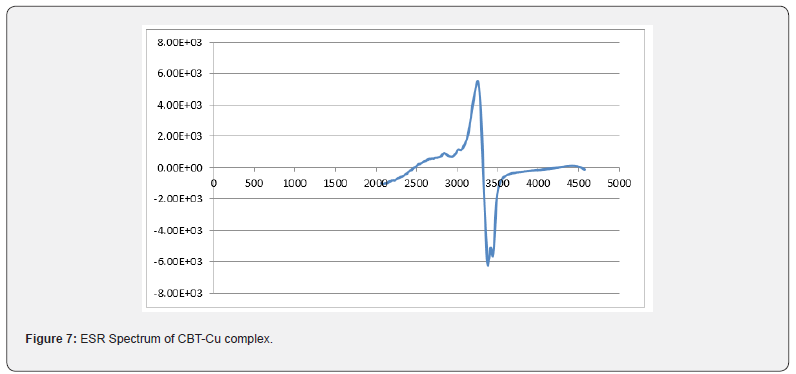
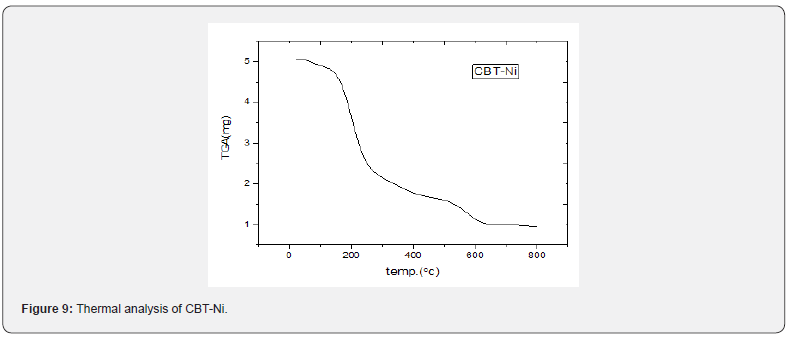
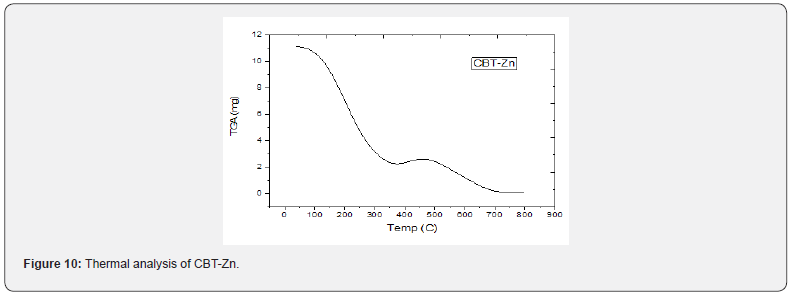
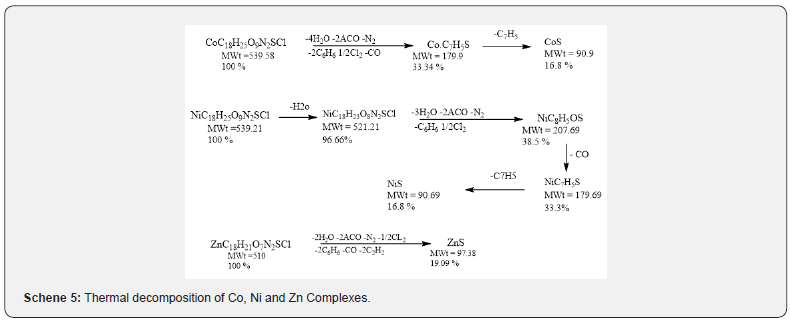
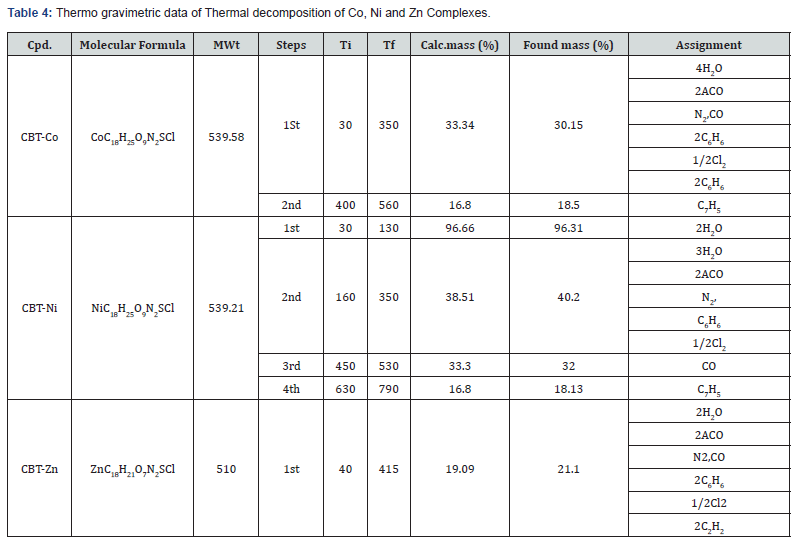
Thermal analysis (TGA)
TGA data of the thermal decomposition of the prepared complexes are shown in Table 4, Figures 8- 10 and Scheme 5. The TGA curves indicate that the loss of weight starts around 205°C and continues to about 300°C at which point most of the organic part of the compounds have been lost. This sharp decomposition period brings about 78% weight loss in the complexes and led to the complete formation of sulphide.
Biological activity
Antibacterial and anti-fungal effect
Some chelates exhibited a moderate inhibitory activity of complexes than that of the corresponding free ligands. The free ligand (CBT) and its metal complex ZnII in addition to the standard drugs were screened separately for their antibacterial activity against Staphylococcus aureus (ATCC:6538), streptococcus mutans (ATCC:25175)(Gram-positive bacteria), Escherichia Coli (ATCC:9637) and Klebsiella Pneumonia (ATCC:10031) (Gramnegative bacteria) and antifungal activity against Aspergillus Nigar (ATCC:32856) and Candida albicans (ATCC:6538) fungi. The antimicrobial activity against the growth of various microorganisms was determined by measuring the inhibition zone in millimetersaround the well, also the activity index data was calculated [25]. The result is recorded in Table 5.
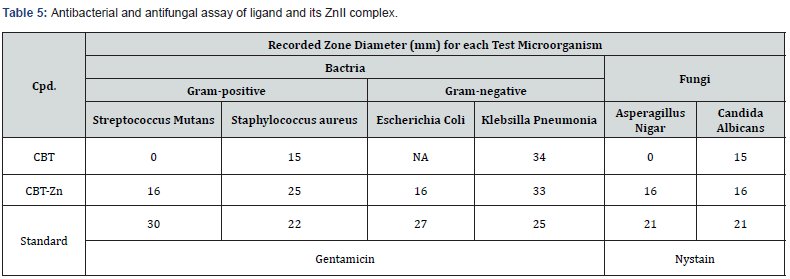
As one can see from the observation results that the growth of microorganism was more inhibited by the metal complexes as compared with the organic ligand (CBT) and the standard drugs. The metal complexes act as more powerful bactericides and fungicides agents and they may serve as a vehicle for activation of ligand where the metal ions being more hypersensitive against the microbial cells. This behavior of the metal complexes may be a result of modification in structure upon coordination and formation of metal organic framework and can be explained based on the overtone concept and chelation theory. In general, the easy penetration of the metal complexes into lipid membranes, disturbance of the respiration process of the cell and blocking the synthesis of proteins are restrict further growth of the organism and lead to enhance of activity of metal complexes compared with the organic ligand [26,27].
Anticancer effect
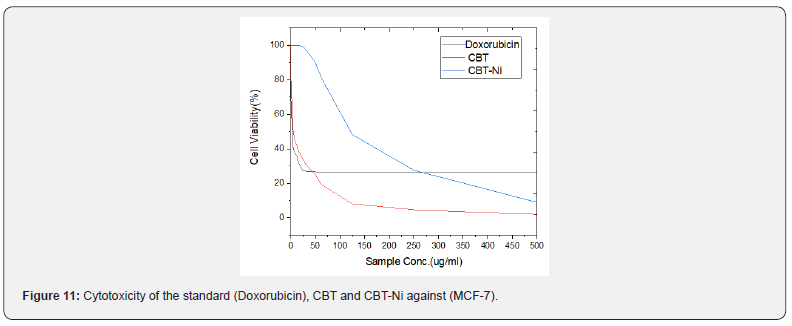
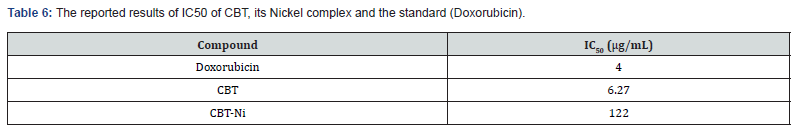
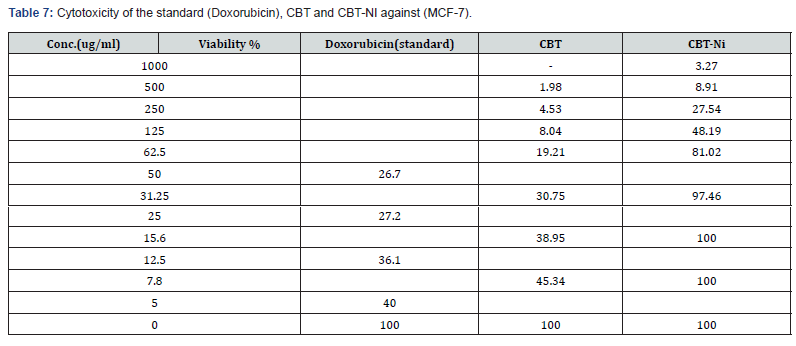
The cytotoxicity activities of free ligand (CBT) and Ni (II) complex were tested against (MCF-7) human tumor cell lines. For comparison purpose, the cytotoxicity of Doxorubicin as stander antitumor drug was evaluated and produced (IC50 ml) under the same conditions. The reported results in terms of IC50 value was recorded in Table 6. The cytotoxic activity was evaluated by using different concentrations and reported in Table 7 & Figure 11. As depicted, the ligand and its complex has very good cytotoxic activity compared with the standard. These finding could be explained by the solubility effect as good relationship could be seen between activity and solubility of compounds. The activity of the complex could be explained by its greater solubility and lipophilicity increase with increasing bulkiness and may be facilitate transport through the cellular membrane [28].
Conclusion
To conclude, complexes were successfully synthesized and fully characterized by chemical and spectroscopic methods. Then the antibacterial and antifungal effect studied and compared between the ligand itself and the ZnII complex and the anticancer effect studied of its NiII complex and compared with the ligand and standard drugs. The study showed that the ligand and its metal complexes possess an appreciable activity and can consider as an effective inhibitor towards the different microbial strains and cancer. Generally, such activity enhanced upon complexation where metal complexes show better activity than their parent ligand.
References
- Arslan H, Külcü N, Flörke U (2003) Synthesis and characterization of copper (II), nickel (II) and cobalt (II) complexes with novel thiourea derivatives. Transition Metal Chemistry 28(7): 816-819.
- Mansuroglu DS, Arslan H, Flörke U, Külcü N (2008) Synthesis and characterizationof nickel and copper complexes with 2, 2-diphenyl-N-(alkyl (aryl) carbamothioyl) acetamide: the crystal structures of HL1 and cis-[Ni (L1) 2]. Journal of Coordination Chemistry 61(19): 3134-3146.
- Shadab M, Aslam MJ (2014) Synthesis and Characterization of some Transition Metal Complexes with N-Phenyl-N’-[Substituted Phenyl] Thiourea. Material Science Research India 11(1): 83-89.
- Arslan H, Flörke U, Külcü N, Emen M (2006) Crystal structure and thermal behaviour of copper (II) and zinc (II) complexes with N-pyrrolidine-N′-(2-chloro-benzoyl) thiourea. Journal of Coordination Chemistry 59(2): 223-228.
- Karakuş S, Rollas SJ (2002) Synthesis and antituberculosis activity of new N-phenyl-N′-[4-(5-alkyl/arylamino-1, 3, 4-thiadiazole-2-yl) phenyl] thioureas. Il Farmaco 57(7): 577-581.
- Solomon V, Haq W, Smilkstein M, Srivastava K, Puri SK, et al. (2010) 4-Aminoquinoline derived antimalarials: synthesis, antiplasmodial activity and heme polymerization inhibition studies. European journal of medicinal chemistry 45(11): 4990-4996.
- Holt SL, Carlin RL (1964) Some Transition Metal Complexes of Substituted Thioureas. II. Nickel (II). American Chemical Society 86(15): 3017-3024.
- Puglisi C, Levitus R (1967) Some substituted thiourea complexes of nickel (II) thiocyanate. Nuclear Chemistry 29(4): 1069-1077.
- Ke S Y, Xue S (2006) Synthesis and herbicidal activity of N-(o-fluorophenoxyacetyl) thioureas derivatives and related fused heterocyclic compounds. Arkivoc 10: 63-68.
- Elhusseiny AF, Eldissouky A, Al Hamza AM, Hassan HH (2015) Metal complexes of the nanosized ligand N-benzoyl-N′-(p-amino phenyl) thiourea: Synthesis, characterization, antimicrobial activity and the metal uptake capacity of its ligating resin. Journal of Molecular Structure 1100: 530-545.
- Yeşilkaynak T, Özpınar C, Emen FM, Ateş B, Kaya K(2017) N-((5-chloropyridin-2-yl) carbamothioyl) furan-2-carboxamide and its Co (II), Ni (II) and Cu (II) complexes: Synthesis, characterization, DFT computations, thermal decomposition, antioxidant and antitumor activity. Journal of Molecular Structur 1129: 263-270.
- Yeşilkaynak T, Muslu H, Özpınar C, Emen FM, Demirdöğen RE, et al. (2017) Novel thiourea derivative and its complexes: Synthesis, characterization, DFT computations, thermal and electrochemical behavior, antioxidant and antitumor activities. Journal of Molecular Structure 1142: 185-193.
- Fouda M, Abd Elzaher M, Shakdofa M, El Saied F, Ayad M, et al. (2008) Synthesis and characterization of transition metal complexes of N′-[(1, 5-dimethyl-3-oxo-2-phenyl-2, 3-dihydro-1H-pyrazol-4-yl) methylene] thiophene-2-carbohydrazide. Transition Metal Chemistry 33(2): 219-228.
- Chernyavskaya A, Loginova N, Polozov G, Shadyro O, Sheryakov A, et al. (2006) Synthesis and antimicrobial activity of silver (I) and copper (II) complexes with 2-(4, 6-di-tert-butyl-2, 3-dihydroxyphenylsulfanyl) acetic acid. Pharmaceutical Chemistry Journal 40(8): 413-415.
- Yusof MSM, Khairul WM, Yamin BM (2010) Synthesis and characterisation a series of N-(3, 4-dichlorophenyl)-N′-(2, 3 and 4-methylbenzoyl) thiourea derivatives. Journal of Molecular Structure 975(1-3): 280-284.
- Ali OA, El Medani SM, Serea MRA, Sayed AS (2015) Unsymmetrical Schiff base (ON) ligand on complexation with some transition metal ions: Synthesis, spectral characterization, antibacterial, fluorescence and thermal studies. Molecular and Biomolecular Spectroscopy 136: 651-660.
- Person WB (1965) A criterion for reliability of formation constants of weak complexes. Journal of the American Chemical Society. Journal of the American Chemical 87(2): 167-170.
- Sharma R, Ambwani J (1995) Synthetic, Structural and Antimicrobial Studies of some Macrocyclic Ligands and their Copper (II) Complexes. Journal of the Indian Chemical Society. Journal of the Indian Chemical Society 72(8): 507-509.
- Singh V, Singh S, Singh D, Singh P, Tiwari K, Mishra M, et al. (2013) Synthesis, spectral and single crystal X-ray diffraction studies on Co (II), Ni (II), Cu (II) and Zn (II) complexes with o-amino acetophenone benzoyl hydrazone. Polyhedron 56: 71-81.
- Kurt G, Sevgi F, Mercimek BJCP (2009) Synthesis, characterization, and antimicrobial activity of new benzoylthiourea ligands. Chemical Papers 63(5): 548-553.
- Gunasekaran N, Bhuvanesh N, Karvembu R (2017) Synthesis, characterization and catalytic oxidation property of copper (I) complexes containing monodentate acylthiourea ligands and triphenylphosphine. Polyhedron 122: 39-45.
- Massoni M, Clavijo JCT, Colina Vegas L, Villarreal W, Dias JS, et al. (2017) Propyl gallate metal complexes: Circular dichroism, BSA-binding, antioxidant and cytotoxic activity. Polyhedron 129: 214-221.
- Abd El Wahab ZH (2008) Complexation of 4-amino-1, 3 dimethyl-2, 6 pyrimidine-dione derivatives with cobalt (II) and nickel (II) ions: synthesis, spectral, thermal and antimicrobial studies. Journal of Coordination Chemistry 61(11): 1696-1709.
- Mahapatra BB, Chaulia S, Sarangi AK, Dehury S, Panda J (2015) Synthesis, characterisation, spectral, thermal, XRD, molecular modelling andpotential antibacterial study of metal complexes containing octadentate azodye ligands. Journal of Molecular Structure 1087: 11-25.
- Heidari F, Fatemi SJA, Ebrahimipour SY, Ebrahimnejad H, Castro J, Dušek M, et al. (2017) Six-coordinate oxo-vanadium (V) dimer complex with methoxy bridging: Synthesis, crystal structure, biological activity and molecular docking. Inorganic Chemistry Communications 76: 1-4.
- Gull P, Malik MA, Dar OA, Hashmi AA (2017) Design, synthesis and spectroscopic characterization of metal (II) complexes derived from a tetradentate macrocyclic ligand: Study on antimicrobial and antioxidant capacity of complexes. Microbial pathogenesis 104: 212-216.
- Reedijk J (1996) Improved understanding in platinium antitumour chemistry. Chemical Communications (7): 801-806.
- Qiao L, Haung J, Hu W, Zhang Y, Guo J, et al. (2017) Synthesis, characterization, and in vitro evaluation and in silico molecular docking of thiourea derivatives incorporating 4-(trifluoromethyl) phenyl moiety. Journal of Molecular Structure 1139: 149-159.






























