Synthesis of a Novel Sensor Electrode Based on Bromothymol Blue as an Indicator Electrode in Potentiometric Acid-Base Titration in Aqueous Solution
Nasser M Abu Ghalwa*, Fawzi kodih, Nader B Farhat and Rewaa J Abu Saelik
Chemistry Department, Al-Azhar University, Palestine
Submission: January 16, 2020; Published: March 06, 2020
*Corresponding author: Nasser M Abu Ghalwa, Chemistry Department, Al-Azhar University, Gaza, Palestine
How to cite this article: Nasser M Abu Ghalwa*, Fawzi kodih, Nader B Farhat and Rewaa J Abu Saelik. Synthesis of a Novel Sensor Electrode Based on Bromothymol Blue as an Indicator Electrode in Potentiometric Acid-Base Titration in Aqueous Solution. Organic & Medicinal Chem IJ. 2020; 9(3): 555764. DOI: 10.19080/OMCIJ.2019.09.555764
Abstract
A modified bromothymol blue BTB electrode was prepared by spin coating of the BTB indicator on conducting glass substrate, and its use as indicator electrode to potentiometric acid-base titration in aqueous solution at 298 K was developed. The change of the open circuit potential with pH (E-pH) curve is linear with slope of 0.052 V/Dec at 298 K. The standard potential of this electrode, E0, was determined with respect to the SCE as reference electrode. The recovery percentage for potentiometric acid-base titration using G/BTB as indicator electrode was calculated
Keywords: Potentiometry; Sensor; Conducting Glass; Titration; Indicator Electrode; Bromothymol Blue
Experimental
At the recent years, there has been an expanding interest for chemical analyzers, which are suited for a quick, accurate and in field ion analysis. There is a great interest for easy to use, sensitive, low-cost and maintenance free chemical analyzers for economical real time analysis of environmentally important ions [1]. Titration is a very useful and reliable technique, which is widely used in different fields such as the food industry, scientific research, and chemical, clinical and pharmaceutical laboratories. Potentiometric titration belongs to chemical methods of analysis in which the endpoint of the titration is monitored with an indicator electrode that records the change of the potential as a function of the amount usually the volume of the added titrant of exactly known concentration and the potentiometric titration have a more selective character when acid-base, complexometric or precipitation reactions are explored [2-5].
The Potentiometric sensors based on the potential gap between a working electrode (WE) and a reference electrode (RE), at zero current [1]. This potential is related to the activity of the analyte in the solution, which is considered an effective concentration, in contact with the electrode surface. In potentiometric sensors, a local equilibrium is established at the sensor interface, where either the electrode or membrane potential is measured, and information about the composition of a sample is obtained from the potential [6].
In acid base titration, the unknown concentration of an acid or base is determined by neutralizing the analyte with an acid or base of known concentration. pH indicators are frequently weak acids or bases which is used in titrations in analytical experiments to determine the extent of a chemical reaction. Because of the subjective determination of color, pH indicators are susceptible to imprecise readings [7,8]. Different types of electrode have been used as acid-base indicator electrodes. Of those, the most frequently used are the titanium, hydrogen–palladium and other electrodes [9-12].
Recently, researchers have been interested in studying the composites of conducting electrode film. Composite materials of sensor electrode have superior properties from where broader potential window, higher signal-to-noise ratio, time and Speed of response mechanical stability enabling their application in flowing systems, and resistance toward passivation. the last requirement is especially important because electrode fouling is probably the biggest obstacle to more frequent applications of electroanalytical methods in environmental analysis [13,14].
Bromothymol blue (also known as bromothymol sulfone phthalein and BTB) is a pH indicator for reactions between strong acids and bases. It is mostly used in applications that require measuring substances that would have a relatively neutral pH (near 7). A solution of bromothymol exhibits two forms acidic forms (yellow color) and basic form (blue color) [15] (Figure 1).
This study focuses on the bromothymol blue indicator BTB to prepare a new modified electrode coated on conducting glass film as sensor (glass / indicator electrode), for used in potentiometric acid base titration.
Experimental
Chemicals
The chemicals used in potentiometric titrations and preparation the electrode was tetraethyl orthosilicate (TEOS), bromothymol blue (BTB), hydrochloric acid, ammonia, Acetic acid, phosphoric acid, sodium hydroxide, sulfuric acid, citric acid and disodium phosphate. The chemicals are of analytical pure grade.
Synthesis of Materials
Preparation of Hydrolyzed TEOS
A mixture of 2.5 ml of absolute ethanol, 0.86 ml of 0.1 M of HCl were added to 2.5 ml of TEOS under stirring. The obtained solution was kept under stirring at room temperature until a homogeneous clear solution was obtained. The solution was aged at least for 24 hours before used in the coating process. The hydrolyzed TEOS solution was used as a host matrix for the indicators.
Preparation of Indicators
Indicators solution (0.001 M) of bromothymol blue, prepared using absolute ethanol as solvent.
Stock Solution of Indicators
The sample solution was prepared by mixing 1 ml of blank hydrolyzed TEOS solution and 1 ml for each indicator.
Preparation of Silica-immobilized Thin Films
Substrate Cleaning
Glass were activated by concentrated H2SO4 for 24 hours, then washed with distilled water and ethanol. The surface was finally rubbed with cleaning paper.
Preparation of Glass/BTB Electrodes using Spin Coating Method
All thin films layers prepared in this work were made by spinning three drops of the solutions onto a clean glass slide. The coating process was performed using the spin coater machine at 900 rpm spinning speed for 1 min. period time. To obtain multilayers of thin films a subsequent spin coating method was performed after gradually drying of the previous layer at room temperature for 24 hours, then dried at 80oC for another 48 hours. And repeat the spin coating two or three time. Where the conducting substrate is usually conducting glass, consisting of glass coated with a thin layer of F-doped SnO2
Sensor Design of Potentiometric Cell
The potential of the indicator electrode relative to that of the reference electrode was measured on a digital multimeter model YDM 302C (China). Potentials were measured to ±5 mv. The potential of Bromothymol blue, Thymol blue, sensor indicators electrodes was measured vs. a saturated calomel electrode (SCE). The error in the measurement of the potential due to liquidjunction potentials in these electrolytes is estimated to be about 0.001 V.
The solution in a beaker is stirred by means of a magnetic stirrer. The electrodes (indicator and reference) were dipped slowly into aqueous solution (acid or reductant). After the steady state potential was attained, the titration of the acid was carried out by addition of 1 ml of the base to the acidic solution, waiting until the steady potential is established and then measured. The potential variation depends on the type of the base, the progress of neutralization process and on the initial concentration of the acid to be titrated. The results were reproducible to satisfactory value of ±5 mV for potential measurements. The process of addition of the titrant was repeated until the equivalence point was reached.
Result and discussion
The E-pH relation of BTB electrode
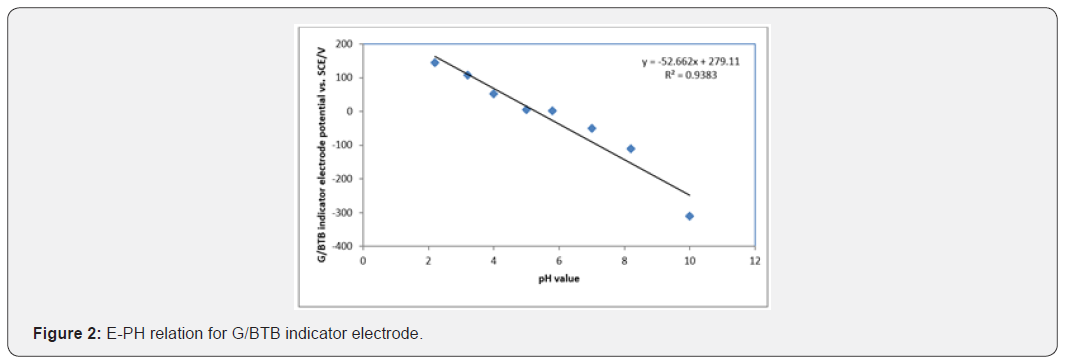
Figure 2 show the change of the open circuit potential (E) of the glass/ Bromo thymol blue (G/ BTB) indicator electrodes with PH. The relation between potential and pH (E-pH) plot of the G/ BTB indicator electrode fits straight line with slope of 52.66 mV and 53.11 mV at 298 K. This value is close to the magnitude of the term 2.303 RT/F (where: R gas constant, T absolute temperature and F Faraday constant) at the corresponding temperature (59.1 mV at 288 K). From Figure 2 the E0 value of the sensor electrode, i.e. the potential at [H+] =1, is computed as 279.1 mV relative to the saturated calomel electrode and can determination by:
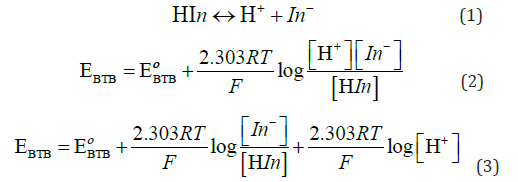
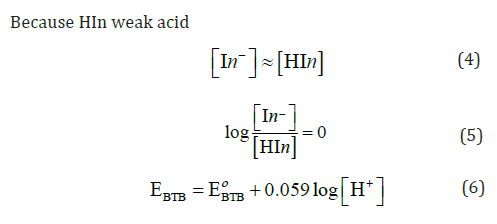

This equation is applicable for the reversible behavior of working electrode. From the developed Nernst equation, we indicate that working electrodes can be used as pH-indicator. At high or low pH, the electrode indicates pH less than true value as pH glass electrode, it may be due to damage in electrode or existence of alkali metal ions in solution too.
Potentiometric Acid-Base Titration
Effect of Concentration of Acid on Potentiometric Titration
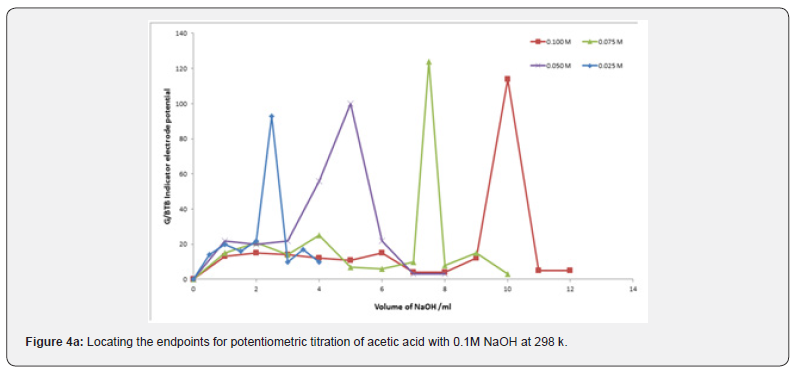
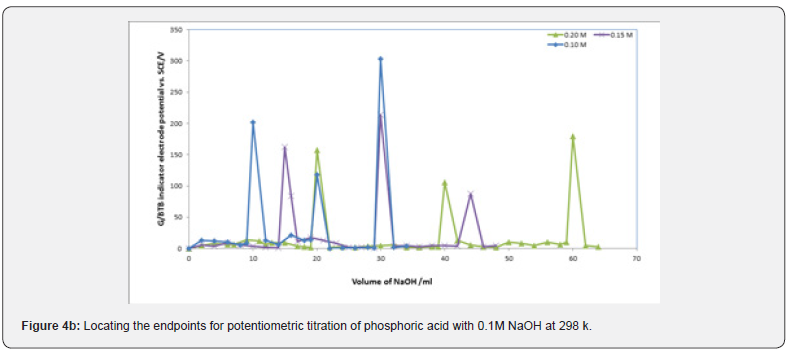
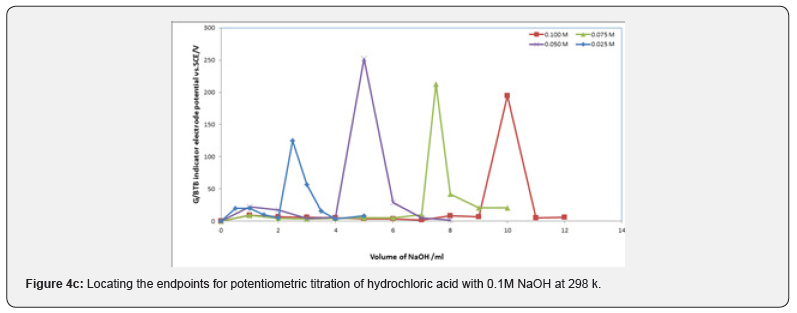
Figures 3a,b,c represent the relation between the volume of 0.1 M NaOH with potential shift in the titrations of different concentrations of acetic acids, phosphoric acid and hydrochloric acid for G/BTB electrodes, where the relation between the volume of 0.1 M HCl with titrations of different concentrations of ammonia represent in figure 3d. The variation of G/BTB electrode potential at 288 K with the different volumes of standard NaOH and HCl followed typical potentiometric titration curves. These curves show slight decrease in potential (to more negative values) with the addition of the titrant.
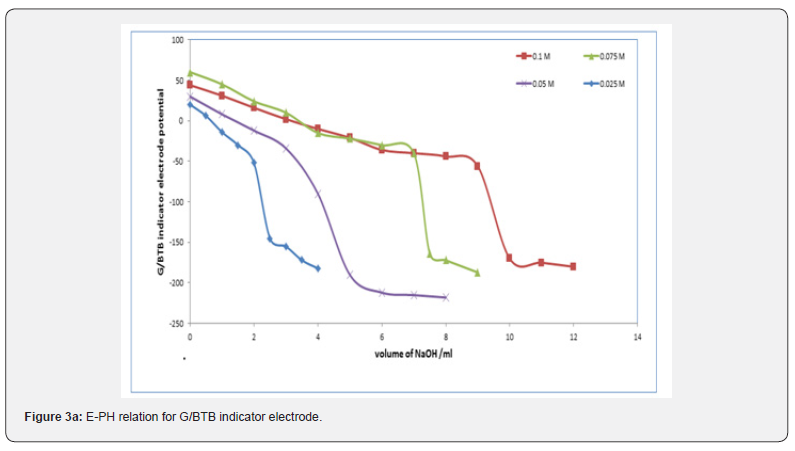
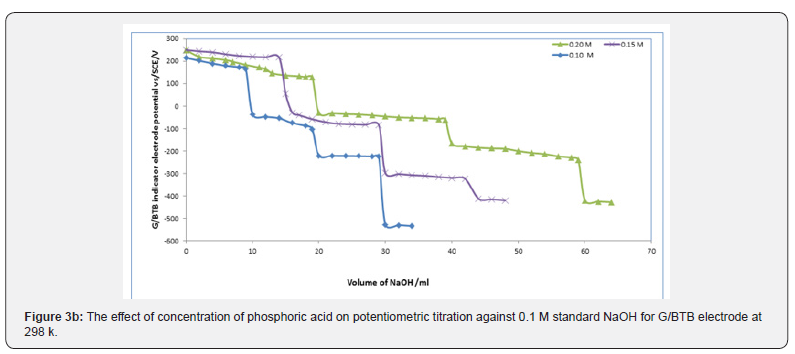
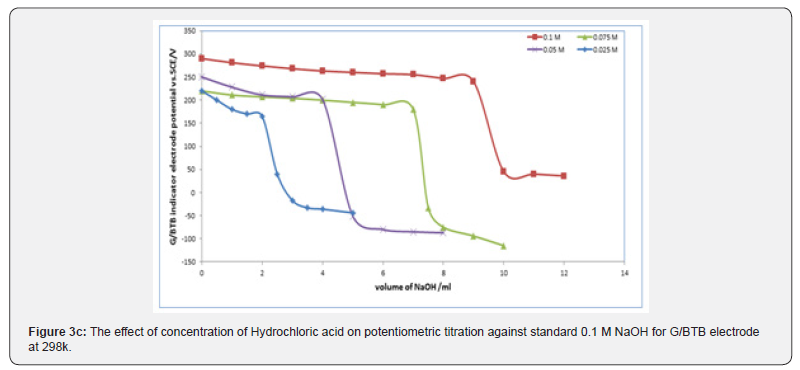
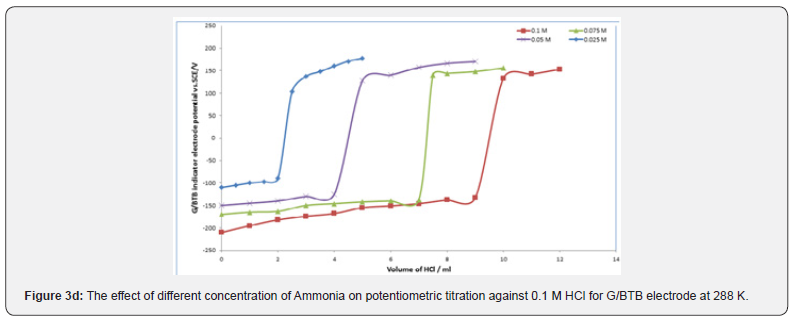
Location of Endpoints
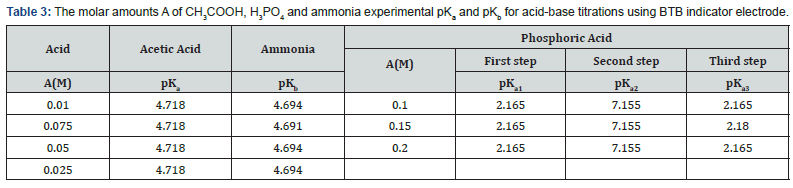
Location of Endpoints The locating endpoints shown in Figure 4. Figure 4 represents ΔE/ΔV against V for the potentiometric titrations of hydrochloric acid, Acetic acid and phosphoric acid, against 0.1 M NaOH where the ammonia against 0.1 HCl. From the plots the values of endpoints are determined. The molar amounts A of CH3COOH acid, HCl acid, CH3COOH acid, experimental and theoretical amounts of standard NaOH, Be, Bt and recovery percentage (R%) for acid-base titrations using BTB indicator electrode are listed in Table 1.
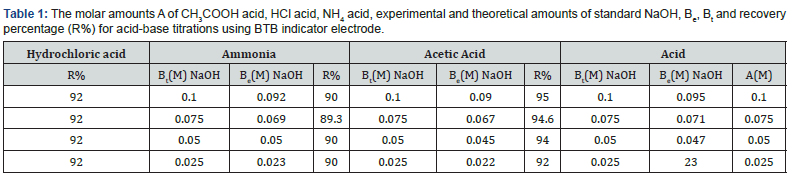

Where table 2 represent the molar amounts of Phosphoric acid experimental and theoretical amounts of standard NaOH, Be, Bt and recovery percentage (R%) for acid-base titrations using BTB indicator electrode.
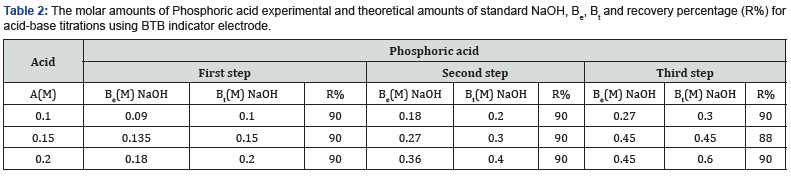
The values of the recovery percentage, R%, for all above titrations are calculated from equation (8). From the plots the values of endpoints and the values of the recovery percentage, R%, are determined as
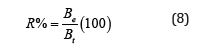
where Be is the experimental amount of base and but is the theoretical amount of base calculated from the stoichiometric equations of neutralization reactions. It is clear from these data that the working electrode can be used as indicator electrode with the satisfactory percentage recovery not less than 88% in potentiometric titrations. These differences in the recovery percentage may be attributed to the impurities in the reagents. The values of pKa (acid equilibrium constant) and pKb (base equilibrium constant) for different acids can be determined using the method of half neutralization [16]. They are close to the previously reported values listed in Table 3 for the tested acids.
The Response Time of the Sensor
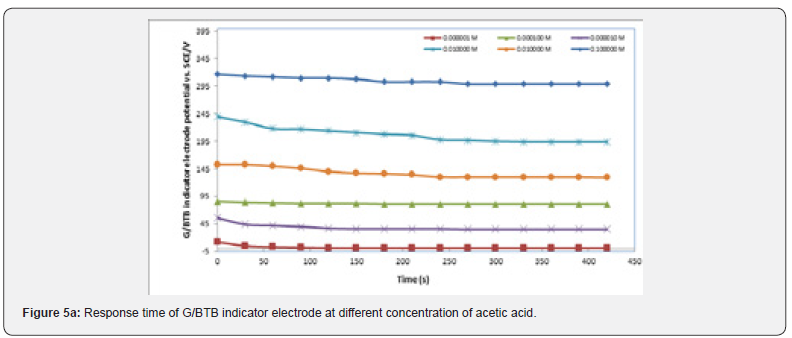
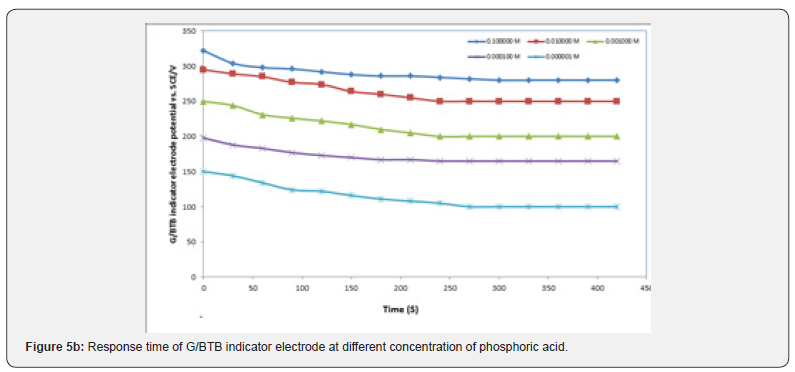
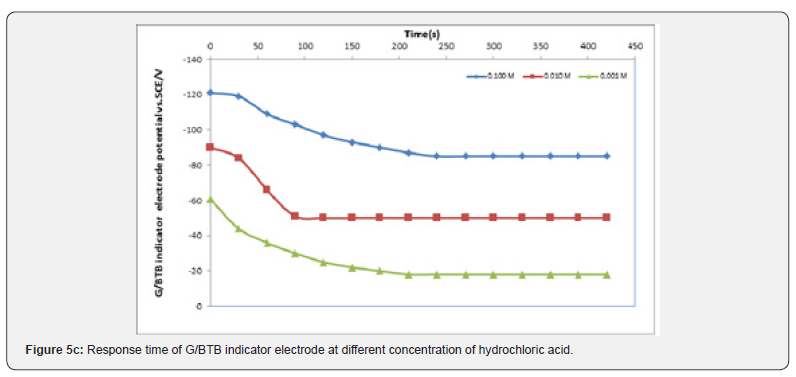
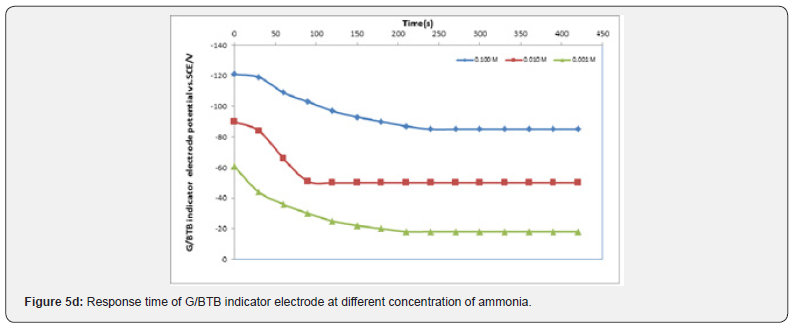
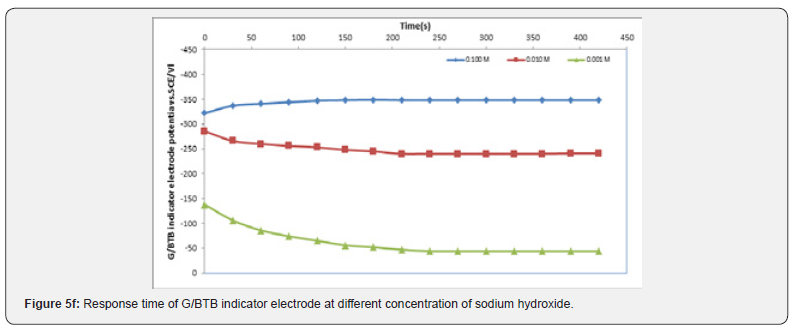
It is well known that the response time of the sensor is one of the most important factors in its evaluation and is defined as the time between the addition of analyte to the solution and the time when a limiting potential has been reached [17,18]. Figure 5 show the response time of the G/BTB sensor at different concentration of phosphoric acid, acetic acid, Hydrochloric acid, ammonia and NaOH respectively. Response time, in the range of (100-450) seconds was achieved, which rendered the sensor highly practical.
Effect of Temperature on the Response Characteristics
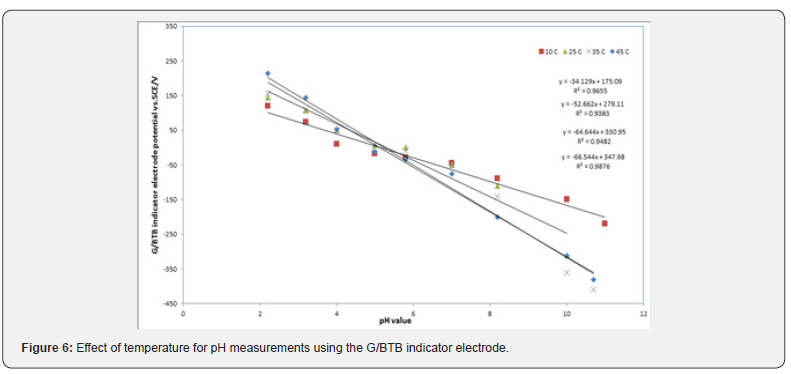
The pH of the electrolyte solution depends on the degree of dissociation of the acids and bases present, which is temperature dependent. And this effects on the behavior of the sensor [19]. To study the thermal stability of the sensor, calibration graphs were constructed at different test solution temperatures 15, 25, 35 and 45 ̊C. According to Figure 6. The sensor would be used for pH measurements in the range from (2-11). At lower temperatures, like 283 K, the slope of the sensor was about 34.12 mV/decade. However, when the temperature of the test solutions was adjusted to 298 K, the slope significantly increased to 52.66 mV/decade. By raising the temperature to 308 K and 318 K the slope increased to 64.64 mV/decade and 66.54 mV/decade respectively.
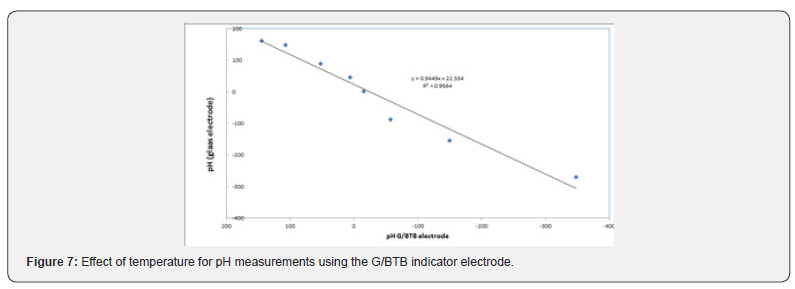
Figure 6 shows the square of the correlation coefficient (r2) for pH measurements using the sensor, at different temperatures, as compared to pH values obtained by a conventional pH electrode (Hanna Instruments HI 1131 pH combination electrode) was found to change as the temperature increases where as r2 values for measurements at 283 K, 298 K, 308 K, and 318 K were 0.9655, 0.9383, 0.9482, 0.9876, respectively. This indicates that better results could be obtained at 298 K. Where figure 7 represent the Correlation between the conventional glass electrode (pH meter) and G/BTB indicator electrode.
Conclusion
a. In the present study the trials were made for the preparation of the modified electrodes of type glass/ Bromothymol blue G/BTB and their use as sensor indicator electrodes in the potentiometric acid-base titrations in aqueous solution at 298 K
b. The recovery percentage for potentiometric acidbase titration using G/BTB as indicator electrode was calculated.
c. The E-pH curve is linear with slope of 0.052 V/decade for the G/BTB electrode at 298 K. This value is close to the theoretical value 2.303 RT/F (0.059 V at 298 K).
d. The standard potential of the tested electrode, E0, is computed as 279.11mV with respect to SCE as reference electrode. Acetic acid, phosphoric acid, hydrochloric acid and ammonia were successfully potentiometric titration with NaOH as titrant in aqueous medium at 298 K.
e. In this study applied the different temperature like 283 K, 298 K, 308 K, and 318 K were the correlation coefficient (r2) 0.9655, 0.9383, 0.9482, 0.9876, respectively.
References
- Schwarz J, Trommer K, Gerlach F, Michael M (2018) All-Solid-State Screen-Printed Sensors for Potentiometric Calcium (II) Determinations in Environmental Samples. American Journal of Analytical Chemistry 9(3): 113-123.
- Skoog DA, West FJ, Crouch ST (2013) Fundamentals of Analytical Chemistry (9th Edn): Cengage Learning Boston.
- Jakubowska M, Bas B, Kubiak WW (2009) End-point detection in potentiometric titration by continuous wavelet transform. Talanta 79: 1398–1405.
- Van Hulle SWH, De Meyer S, Vermeiren TJL, Vergote A, Hogie J, et al. (2009) Practical application and statistical analysis of titrimetric monitoring of water and sludge samples. Water SA 35(3): 329-333.
- Ghalwa NA (2012) Development of a Novel Solid-State Sensor Electrode Based on Titanium Thin Film as an Indicator Electrode in Potentiometric and Conductometric Acid-Base Titration in Aqueous Solution. Journal of Sensors.
- Afkhami A, Shirzadmehr A, Madrakiana T, Bagheri H (2014) Improvement in the performance of a Pb2+ selective potentiometric sensor using modified core/shell SiO2/Fe3O4 nanostructure. Journal of Molecular Liquids 199: 108–114.
- Pradeep DJ, Dave K (2013) A Novel Inexpensive and Less Hazardous Acid-Base Indicator. Journal of Laboratory Chemical Education 1(2): 34-38.
- Jennings P A, Mullen CA, Roy M (2010) Titration and pH Measurement. University of California San Diego, California, USA P 1-6.
- Vasilyeva MS, Rudnev VS, Arefieva OD, Lapina AS (2016) Ti/TiO2 indicator electrodes formed by plasma electrolytic oxidation for potentiometric analysis. International Journal of Environmental Analytical Chemistry 96: 1128-1144.
- Jokić A B, Džudović R M (2013) The application of hydrogen–palladium electrode for potentiometric acid–base determinations in tetrahydrofuran. J Serb Chem Soc 78 (8): 1203-1212.
- Mihajlović Lj, Nikolić-Mandić S, Vukanović B (2009) Use of the sulfide minerals pyrite and chalcopyrite as electrochemical sensors in non-aqueous solutions. The potentiometric titration of weak acids in alcohols. Anal Sci 25: 437.
- Mihajlović R P, Stanić ZD (2005) Natural monocrystalline chalcopyrite and galena as electrochemical sensors in non-aqueous solvents. Part I: Potentiometric titrations of weak acids in γ-butyrolactone and propylene carbonate. J Solid State Electrochem 9: 558.
- Balamurugan A, Chen ZW, Chen SM (2008) Electrochemical Preparation of Bromo Thymol Blue-PEDOT Composite Electrode and Characterization. Journal of The Electrochemical Society 155: 151-156.
- Stani Z, Girousi S (2011) Carbon paste electrode in potentiometry: the state of the art and application in modern electroanalysis (a review). Sensing in Electroanalysis 9: 89-128.
- Issa MN, Shehata MZ, Fawzi SK, Fatma HA (2014) Sol–gel encapsulation of bromothymol blue pH indicatorin presence of Gemini 12-2-12 surfactan. J Sol-Gel Sci Technol 71: 16–23.
- Illingworth JA (1981) A common source of error in pH measurements. Biochemical Journal 195: 259-262.
- Buck RP, Lindner E (1994) Recommendations for nomenclature of ion selective electrodes (IUPAC Recommendations). Pure and Applied Chemistry 66(12): 2527-2536.
- Buck PR, Lindner E (1994) IUPAC Recommendation for Nomenclature of Ion-Selective Electrodes. Pure and Applied Chemistry 66(12): 2527-2536.
- Buck RP, Cosofret VV (1993) Recommended procedures for calibration of ion-selective electrodes. Pure Appl Chem 65(8): 1849-1858.






























