Insight into Interactions of l-Arginine/l-Histidine with Drug Betaine Hydrochloride in Aqueous Medium at Different Temperatures by using Physicochemical Methods
Jyoti Gupta1, Dinesh Chand2 and Anil Kumar Nain1*
1 Department of Chemistry, Dyal Singh College, University of Delhi, India
2 Department of Chemistry, Amity University Dubai Campus, Dubai International Academic City, Dubai, UAE
Submission: February 10, 2020; Published: February 20, 2020
*Corresponding author: Anil Kumar Nain, Department of Chemistry, Dyal Singh College, University of Delhi, New Delhi – 110 003, India
How to cite this article: Jyoti G, Dinesh C, Anil Kumar N. Insight into Interactions of l-Arginine/l-Histidine with Drug Betaine Hydrochloride in Aqueous Medium at Different Temperatures by using Physicochemical Methods. Organic & Medicinal Chem IJ. 2020; 9(3): 555763. DOI: 10.19080/OMCIJ.2019.09.555763
Abstract
Studies on physicochemical properties of l-arginine/l-histidine in aqueous solutions of betaine hydrochloride are beneficial in understanding solute-solvent interactions. The densities, ρ, ultrasonic speeds, u and viscosities, η of l-arginine and l-histidine in water and in aqueous-betaine hydrochloride (1 % and 2 % betaine hydrochloride in water, w/w) solvents have been experimentally measured at six temperatures (293.15, 298.15, 303.15, 308.15, 313.15 and 318.15) K and at atmospheric pressure in nine different concentrations. Related molecular and thermodynamic properties like the apparent molar volume, Vφ, limiting apparent molar volume, Vοφ and transfer volume, ,trVοφ were also evaluated in this study using density data. Likewise from the ultrasonic data, different thermo-acoustical parameters, such as apparent molar compressibility, ,sKφ, limiting apparent molar compressibility, ,sKοφ and transfer compressibility, ,,strKοφ have been evaluated. The trends in transfer properties have been interpreted in terms of cosphere overlap model. In addition, the rheological data have been used to determine Falkenhagen Coefficient, A, Jones-Dole coefficient, B, free energy of activation of viscous flow per mole of solvent, and #1ομΔ solute, #2ομΔ, entropies, # SοΔ and enthalpies, #HοΔ of activation of viscous flow. Hydration number, Hn has also been calculated. Different parameters such as nature of solute, solute-solvent interactions, etc., were categorized by using different analytical and physical loom. The results show that the studied drug is a structure maker compound in all systems of this work. The free energy parameters indicate the formation of transition state in the presence of amino acids. The solute co-solute interactions are strongest in the system containing l-arginine.
Keywords: Density; Ultrasonic Speed; Viscosity; Kosmotropic Behaviour; Betaine Hydrochloride; Acoustical Parameters; Thermodynamic Parameters of Viscous Flow; Electrostriction
Mini Review
The molecular basis of life rests on the functioning of biological macromolecules, generally nucleic acids and proteins which are also economically significant since they are arrayed as nutrition factors and flavor enhancers at a large scale [1]. Nature has chosen basic units of proteins i.e. amino acids that have biological significance in living systems and plays many important functions in maintaining life in the individuals [2]. In addition, amino acids also play a major role in the manufacturing of some pharmaceutical drugs such as antibiotics, anti-HIV and cancer drugs.
This also makes the research involving interactions between biological molecules and other molecules of enormous importance for quite a long time. The current cognizance of biological macromolecules is unbelievable without being heedful of the solvent environment as hydration by water molecules describes a vital role in the assembly of the structure and dynamics of the protein. Hydration of hydrophobic, charged atomic groups and ions which are the constituents of practically every biological system plays an important role in the stability of native conformations of biopolymers [3]. Since most of the biochemical processes includes volume change and hydration of biological macromolecules in aqueous medium, the studies on various thermodynamic and transport properties of biomolecules such as amino acids, peptides and sugars in aqueous and aqueous drug solutions may therefore, proves to be valued in medicinal and pharmaceutical chemistry [4].
Proteins, both in isolated as well as concentrated form, are essential ingredients in many food processes, as they perform many specific functions. The determination of functional properties of properties is most important for the development of noval food products by industry. Physicochemical properties of proteins affect the behavior of proteins and processing in food systems. Rheological parameters are necessary for aggregation process, dough formation etc. The water absorption capacity (and viscosity) of different proteins facilitate modifications in food formulations. Changes in viscosity are used to evaluate thickening influence of proteins which is of practical importance in soups, beverages, fluid soups, etc. [5].
The detailed literature survey reveals that the information is scarce on volumetric, viscometric and acoustic properties of betaine hydrochloride in presence of amino acids with positively charged side chain. This fact prompted us to undertake the measurements of these properties of l-arginine and l -histidine in presence of aqueous and aqueous drug solvents at different temperatures. Investigation of various interactions in these solutions is the main objective of these studies, which is still unaware.
Betaine compounds are inner salts and occupy an idiosyncratic place among organic substances. Free betaines have unusually high melting points and are noteworthy for their high solubility in water and insolubility in non-polar solvents [6]. Betaine hydrochloride (BHC), a co-product of the carbohydrate industry, is a compound of interest because of two reasons. The first one is the unusual radiation sensitivity and other one is its frequent occurrence in biological systems. It is the main component of complex lipids and can also act as transmethylating agents [7]. BHC drug, which is used as the stomach acidifier and digestive aid for the human body, finds applicability in treating peculiarly low levels of thyroid disorders, potassium, food allergies, yeast infection, diarrhea, hay fever, etc. Conversely, more systematic studies are necessary to collect the evidence to amount the effectiveness of the drug for these uses [8].
Amino acids consist of amino and carboxyl groups, along with a side- chain specific to each amino acid. They provide useful data about the nature of protein and the part of solvent structure in the denaturation process. For that purpose, we have chosen l-histidine, an essential amino acid and a non-essential amino acid namely l-arginine that plays a significant role for the treatment of cardiovascular disease as it is antiatherogenic, anti-ischemic, antiplatelet, and antithrombotic.
As part of a comprehensive study of physicochemical properties of drugs, present study embrace measurement of the volumetric, acoustic and viscometric properties of l-arginine and l-histidine in aqueous and aqueous-betaine hydrochloride. In earlier contributions [9-13], we studied the properties of mixture of amino acids and drug solutions. In this paper, an attempt has been made to have an admissible information on the behavior of amino acids in aqueous solutions of betaine hydrochloride by measuring densities, ρ , ultrasonic speed, u and viscosities, η of solutions of l-arginine and l-histidine in aqueous and aqueousbetaine hydrochloride (1% and 2% betaine hydrochloride in water, w/w) solvents at temperatures from 293.15 to 318.15 K and at atmospheric pressure. Investigation of the derived parameters give esteemed information vis-a-vis the intermolecular interactions amid solute and solvent. Thus, keeping these considerations, we have undertaken the present work.
The experimental results were used to calculate the apparent molar volume, Vφ , limiting apparent molar volume, Vο φ and transfer volume, ,tr Vο φ , apparent molar compressibility, s, K φ , limiting apparent molar compressibility, s, Kο φ and transfer compressibility, s, ,tr Kο φ , hydration number, H n , coefficient A, coefficient B and dB/dT have been obtained. Additionally, Gibbs free energy of activation of viscous flow per mole of solvent, # 1 Δμο and solute, # 2 ο Δμ , entropies, ΔSο # and enthalpies, ΔHο # of activation of viscous flow have been computed using transition state theory. The results have been discussed in terms of competing patterns of interactions persisting in the solute and the solvent.
Materials and Methods
Chemicals
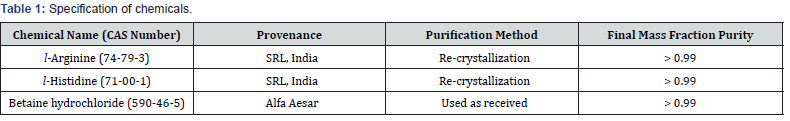
The amino acids used in the experiment were the following from SRL India, l -arginine and l -histidine with reported purities of ≥99 % were used after recrystallization from ethanol-water mixture and dried overnight under vacuum at room temperature for 72 h. Thereafter, these chemicals were stored over P2O5 in desiccator before use. The drug betaine hydrochloride with listed purity ≥99 %, as per supplier specification, acquired from SRL India was dried for about 24 h and used without further purification. The purities and other specifications of the chemicals used in this study have been indicated in table 1.
The triply distilled water having specific conductance less than 1×10−6 S∙cm−1 was used for preparation of binary and ternary solutions. All the solutions were prepared with great caution in glass vials and in molal base concentration by weighing using single pan five digit analytical balance (Model: GR-202R, AND, Japan) with precision in weight up to ±0.01 mg and closed tightly to avoid contamination and evaporation. The standard uncertainties in the molality of the solutions are found to be within ±1×10−4 mol∙kg−1.
Equipment and Procedures
Density and Ultrasonic Speed Measurements
The density and ultrasonic speed measurements of the prepared solutions was carried out with density and sound analyser (DSA-5000 M, Anton Paar, Austria) digital vibrating-tube densimeter with reproducibility of ±1X10−3 kg∙m−3 for density and ±1 X10−2 m∙s-1 for speed of sound. The instrument consists of a builtin thermostat for maintaining temperature. The ultrasonic speed measurement studies are important because of their extensive use in textile industry, leather and pharmaceutical industry. The standard uncertainties associated with the measurements for temperature, density and ultrasonic speed were estimated to be within ±0.01 K, ±0.05 kg∙m−3, and ±0.5 m∙s-1, respectively
Viscosity Measurements
Multi-component systems rather than single component systems are widely used in product formulations and processing in many industrial applications. Amongst the various properties considered in optimization and product and process design, viscosity is one of the most important. The dynamic viscosities were measured by using a microviscometer (Lovis 2000M, Anton Paar, Austria) at T = (293.15, 298.15, 303.15, 308.15, 313.15 and 318.15) K, and at pressure, p = 101 kPa. The working temperature was kept controlled to ±0.02 K with a built in Peltier thermostat. The protocol of the instruments was mentioned elsewhere [10].
Results
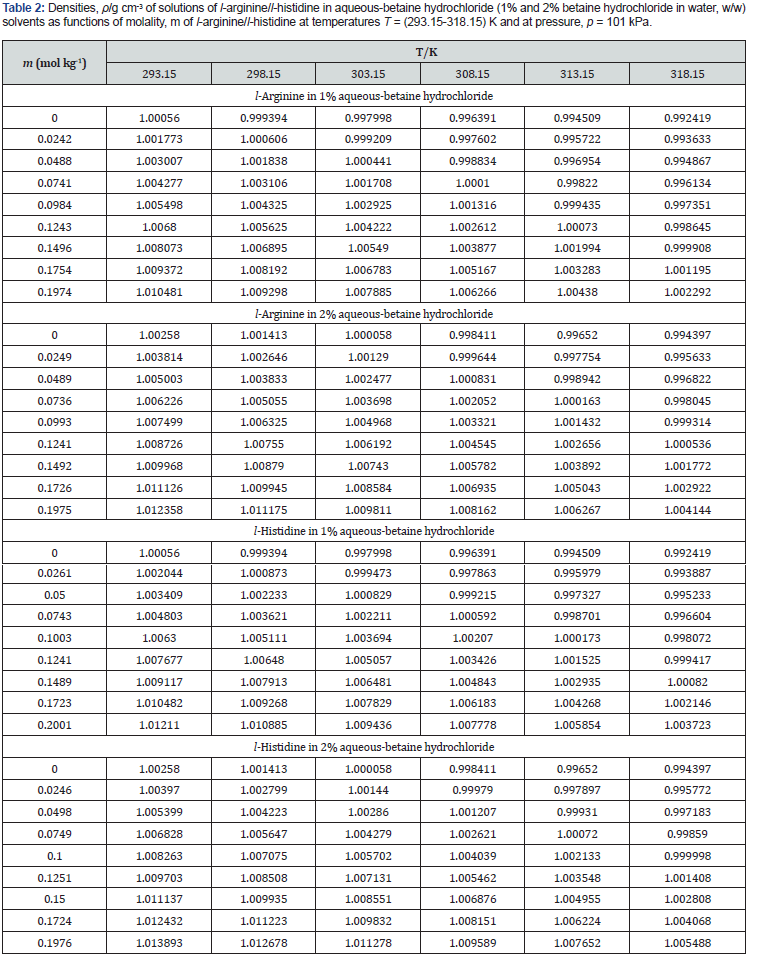
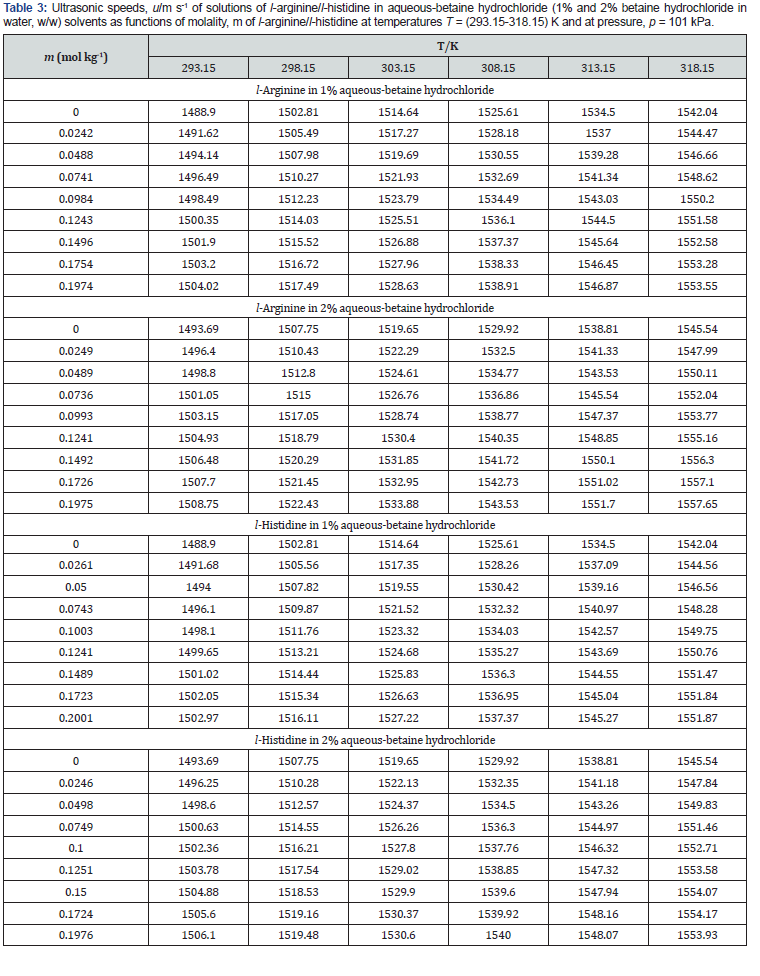
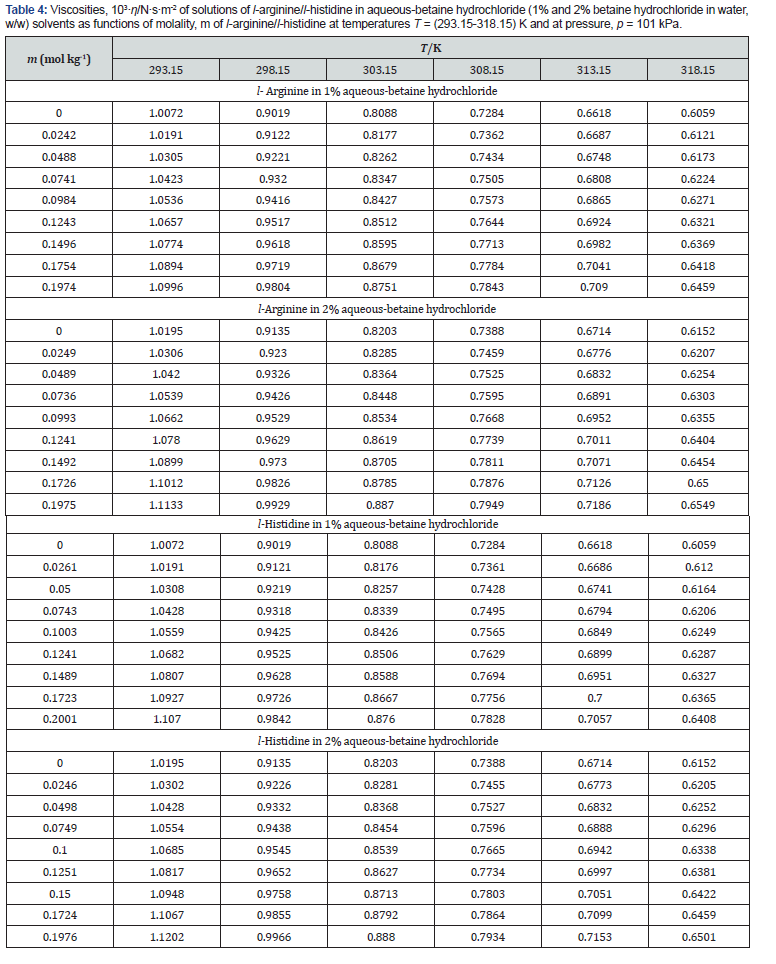
Experimental data of density, ρ , ultrasonic speed, u, and viscosity, η of solutions of l-arginine and l-histidine in water and in aqueous-betaine hydrochloride solvents as a function of l-arginine/l-histidine concentration and temperature have been summarized in tables 2-4, respectively.
Apparent Molar Volume and Compressibility
Apparent molar volume is crucial in the sense that it manifests the behavior of solutes in solution with the change in solution. It is actually the measure of packing of amino acid molecules into the solvent molecules. The calculated values of Vφ quantitatively express the interaction of drug with its environment and therefore give a vivid depiction of the monomer structural changes. The calculated values of densities of solution were used to calculate apparent molar volume, Vφ of solute using the Eq. (1). Apparent molar compressibility, s, K φ can be calculated using Eq. (2) from measured values of ultrasonic speed as
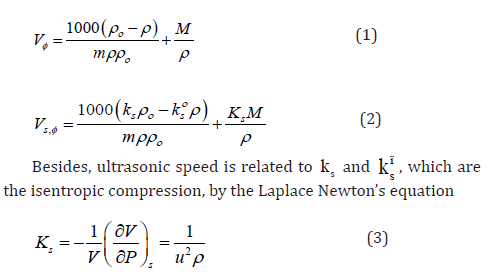
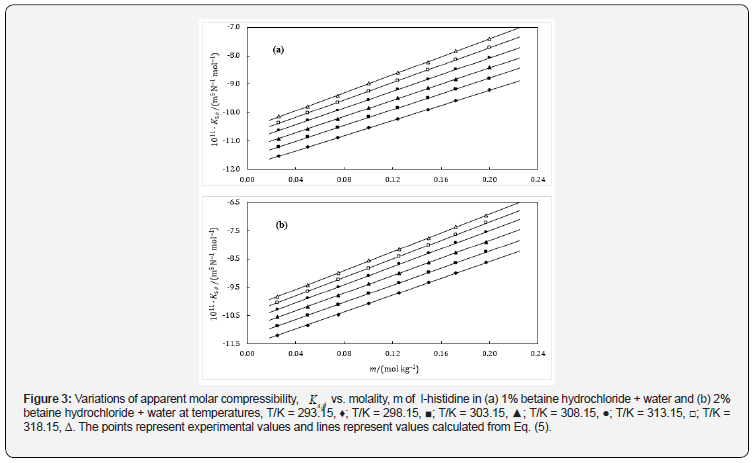
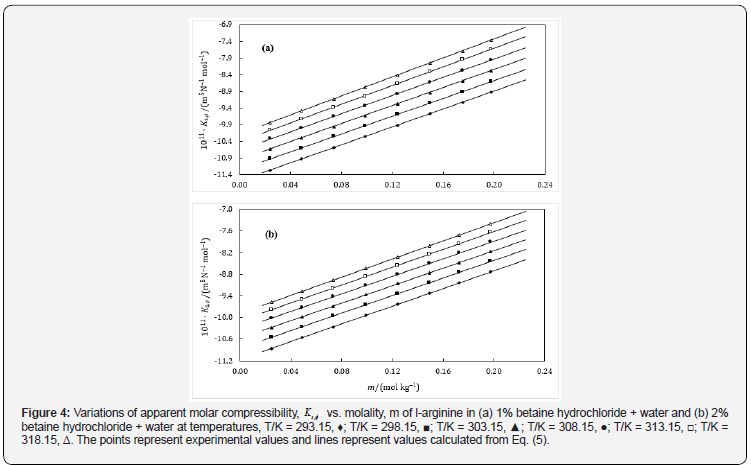
It is also essential for safe equipment design. Isentropic compression always decreases with increase in concentration. The decrease in density is not able to compensate the ultrasonic speed caused by rise in temperature. Figures 1-4 shows concentration dependence of Vφ and s, K φ for all the systems studied. The figures show a linear variation of Vφ and s, K φ against molality throughout the whole concentration range and at each investigated temperature.
Limiting Apparent Molar Volume and Compressibility
A valuable empirical generalization on the change of Vφ and s, K φ with molal concentration is given by Masson’s [14] equation. In the present research work, the involved solvent (aqueousbetaine hydrochloride) is treated as 1:1 electrolyte. The calculated Vφ and s, K φ data were fitted using method of least squares into the Masson [14] equation to get the values of limiting apparent molar volume, Vο φ , limiting apparent molar compressibility, s, Kο φ and the slopes, v S , and k S for these solutions
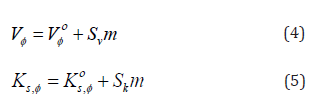
where the intercepts, Vο φ or s, Kο φ are the partial molar properties at infinite dilution. At infinite dilution, m tends to zero, each solute molecule is only surrounded by solvent molecules and there becomes a wide gap between solute molecules, solute-solute interactions vanish, Vο φ and s, Kο φ becomes equal to Vο φ and s, Kο φ , respectively. Tables 5 & 6 contains the values of Vο φ , v S , s, Kο φ , k S and standard deviations of linear regression, σ for all the systems at all the studied temperatures.
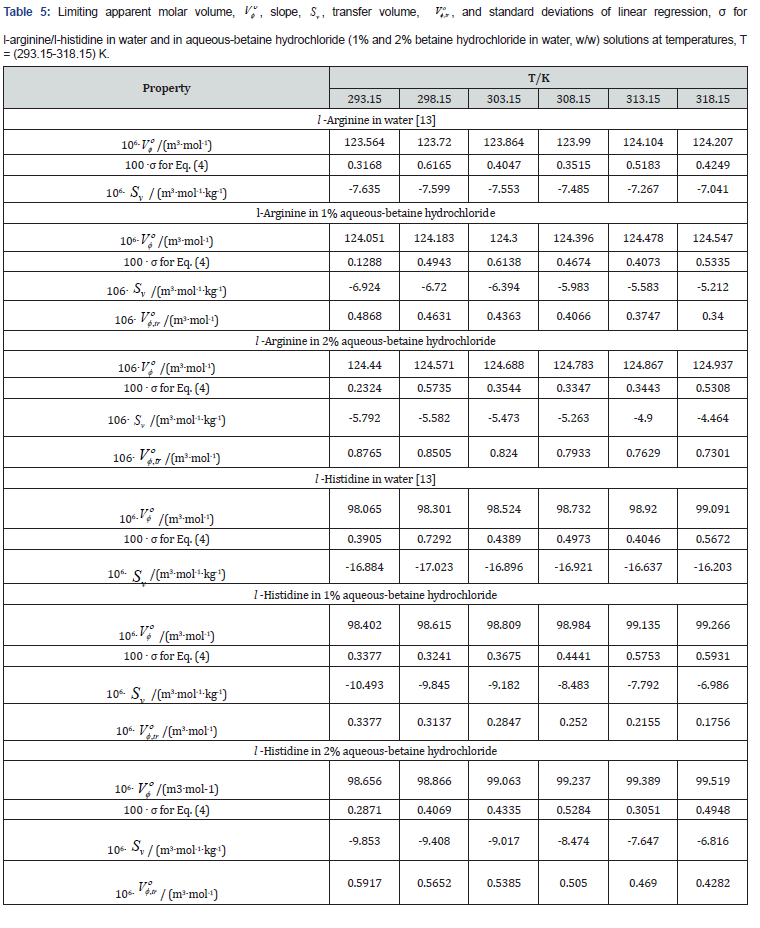
The Vο φ values can be considered to be a sum of the geometric volume of the solute molecule and changes in the solvent occurred while it interacts with the solute molecule. It can also be assessed as the actual measurement of the size of hydrated solute molecule in solution creating a gap among themselves and adjacent solvent molecules [15]. Solute-solvent interactions were witnessed from the positive Vο φ values (Table 5) in all these systems and negative v S values indicate weak solute-solute interactions for l-arginine and l-histidine in aqueous and aqueous-betaine hydrochloride solutions. There is an irregular variation in v S values, but these values demonstrate that several effects [16] influence the solutesolute interactions exist in the solution.
The negative v S values can be interpreted in terms of a negative volume change caused by the overlap of hydration cospheres of both the amino acids and drug molecules. It proves that the solvation layer formed around the molecules at infinite dilution is voluminous. The trends observed in Vο φ values may be due to the hydration behavior [17,18] of solute and may be explained as
a) the terminal groups of zwitterions NH3 + and COO- of amino acids were hydrated in electrostatic manner whereas, hydration of R group depends on its nature (hydrophilic, hydrophobic or amphiphilic); the Vο φ values increase due to drop in the electrostriction at terminals, whereas it declines due to interruption of side group hydration by that of the charged end.;
b) COO- group has 10 times smaller electrostriction than NH3 + group; and
c) volume change ensues as the hydration co-spheres of terminal NH3 + and COO- groups and adjacent groups overlay.
Furthermore, these Vο φ values increase with increase in temperature showing that the structure equilibrium imparted to water by the hydrophobic chain is temperature labile. This further supports the hypothesis that self-association of solute reduces solvation effects characteristic of monomeric compound. This increase proves that the structure of solvent is reinforced to a lesser degree due to the formation of solute-solute agglomerate which is majorly caused by the decrease of surface contact between solute and solvent.
The analysis here offered, shows that, for all the amino acids under consideration, the values of Vο φ increase with increase in concentration of the drug in a predictable manner. The more heavily hydrated molecule has less Vο φ than those molecules which are not densely hydrated. This peroration is also found to be valid as the intermolecular interactions between betaine hydrochloride and amino acids result in a much simpler establishment of relatively small hydrophobic character. As a result, there is expansion of the solution. Comparable trends in Vο φ were also reported by Sawhney et al. [19] for l-arginine in aqueous solutions of drug ketorolac tromethamine.

The negative values of s, Kο φ (Table 6) shows the piercing of water structure in aqueous drug solution and also in water and also that the water molecules in the bulk solution are more compressible than the water molecules around ionic charged groups of amino acids [20,21]. Interactions between drug and amino acid molecules is likely to create a polar environment, which helps in increasing the hydrophobic interactions aroused due to stabilizing the simpler model compounds of proteins.
This can also be thought of the consequence of higher resistance to pressure of the structured water in comparison to the water molecules of the bulk. The observed negative values (Table 6) of s, Kο φ are due to the net combined effect of a foreseen possible interactions/ processes in the binary as well as ternary mixtures. The values of s, Kο φ for all the solutions, obtained using Eq. (5) are presented, at various temperatures, in Figures 3 & 4, shows that the s, Kο φ values become more negative with decrease in concentration of the drug at a particular temperature as well as with increase in complexity of amino acids, resulting strong attractive interactions between solute and solvent interactions.
The negative values of s, Kο φ may be analyzed by the idea of two diverse phenomena i.e. electrostriction and hydrophobic solvation. Since the solute (amino acid) has an intrinsic volume and is observed as incompressible [22], the changes observed come from the relative changes in hydration layer so higher (less positive) s, Kο φ values for l-histidine suggest that R group are more able, as compared to l-arginine, to form the denser, compressible hydration layer that leads to large and negative compressions. The iceberg analogy for describing hydrophobic hydration seems to be appropriate.
In both situations, i.e. in ice as well as in hydrophobic hydration sphere, water is voluminous but less compressible. The negative values of s, Kο φ (loss of compressibility of medium) may be attributed to an overall constriction in the solution volumes, caused by releasing some hydrophobically hydrated water molecules around drug molecule into the bulk water. Hydrophobically hydrated water is known to be an ice-like structure having greater volume but less compression than ordinary water [23]. So, when they are released into normal bulk water structure, their volume decreases and compressibility increase with increase in temperature. The complete knowledge of effect of temperature on the expansion of liquid is necessary for equipment design. When amino acids (solute) gets dissolved in a solvent, the ions present are hydrated to form hydration shell, which would cause re-structuring of water molecules around the shells that would cause a disturbance in the original hydrogen bond network in pure liquid water.
The s, Kο φ values show a very small dependence on temperature. Also, it varies linearly with rise in temperature which may be due to the decrease in the extent of structuring of water with increase in temperature. Increase in temperature witnesses less negative values of s, Kο φ . The less sensitivity of s, Kο φ values to temperature is understandable as they reflect not only the volume itself but also the change in volume with pressure, though the latter is not expected to be influenced from temperature as much as the volume itself. According to the intuition, bonds loosen, and intermolecular distances increase, as the temperature increases, leading to higher compressibility of solvent. Similar trends are observed in literature [24,25].
Transfer Volume and Compressibility
With the aim of assembling more facts about chemistry behind the various solute-solute and solute-solvent interactions, transfer functions specially in water and D2O solvent systems have proven to be very useful tool as it eliminates solid or gaseous state as a reference for the thermodynamic functions. Most of the electrostatic type of interactions are believed to be largely cancelled in calculating transfer properties because of the similarity of two solvents. Therefore the transfer volumes, ,tr Vο φ and transfer compressibilities, s, ,tr Kο φ are calculated from Vο φ and s, Kο φ data of l-arginine and l-histidine from water to aqueousbetaine hydrochloride solutions at infinite dilution by using the mathematical relations
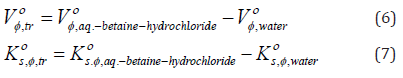
where ,water Vο φ and s, ,water Kο φ are the limiting apparent molar volume and limiting apparent molar isentropic compressibility of amino acids in water. The worked-out values of ,tr Vο φ and s, ,tr Kο φ for l-arginine and l-histidine from water to aqueous betaine hydrochloride solutions are collected in tables 5 and 6, respectively. As the transfer is carried out at infinite dilution, ,tr Vο φ and s, ,tr Kο φ reflects merely differences between the mutual ionsolvent interactions in two solvents. The plus point of calculating ,tr Vο φ and s, ,tr Kο φ rather than Vο φ and s, Kο φ values respectively enables insights to be extended on the effects of solvent properties and removes the dominating effect of the intrinsic size of the ions.
The positive values of ,tr Vο φ for all the systems at all investigated temperatures are reported in Table 5. The increase in volume of solution in presence of amino acid molecules leads to positive values of ,tr Vο φ . To infer this behavior, all interactions in ternary system must be studied. This is due to the fact that amino acids under study, i.e., l-arginine and l-histidine induce a considerable contraction in volume of the peripheral solvent because of electrostriction. This electro strictive effect of amino acids is diminished. A high ,tr Vο φ explores better packing features, hence greater interaction with drug and amino acids. These positive values of transfer may be validated based on ecosphere overlap model [26] developed by Friedman and Krishnan.
Generally, the following types of interactions in the ternary system may be assumed [27-29] and the expansion volume or effects of shrinkage of solute-solute interactions can be interpreted:
a) Ion-ion interactions among [(CH3)3NCH2COOH]+ group of betaine hydrochloride and COO- group of amino acids or may be among Cl- ion of betaine hydrochloride and NH3 + group of amino acids;
b) The ion/hydrophilic-hydrophilic interactions between ionic groups of betaine hydrochloride and zwitterions of amino acids;
c) Hydrophilic-hydrophobic group interaction between the hydrophilic groups of betaine hydrochloride molecule and nonpolar (-CH2) inside chain of amino acids;
d) Hydrophobic-hydrophobic group interactions between the carbon skeleton of betaine hydrochloride and non-polar (-CH2) inside chain of amino acids.
Amino acids exist predominantly as zwitterions in pure water. Additionally, betaine hydrochloride ionises to give [(CH3)3NCH2COOH]+ and Cl- [7], and amino acids in the present study are basic in nature, therefore the interaction of betaine hydrochloride with solute (amino acids) is essentially driven by the interactions of the drug with the polar headgroups of amino acids via hydrogen bonds and hydrophobic forces, especially in the case of the zwitterionic species. The ,tr Vο φ values can also be justified on the basis of co-sphere overlap model. In view of the above said model, it has been claimed that in type (b) and (c) interactions, hydration co-sphere overlap occurs as two molecules come close to each other in aqueous solution, resulting in displacement of cosphere overlap stuff by alteration in its thermodynamic properties making a negative contribution to the transfer volume.
However, ion/hydrophilic-hydrophilic interaction leads to a positive influence on the transfer volume because the electrostriction effect ease due to hydrophilic-ionic interactions and overlapping of their hydration co-spheres leads to enhancement of water structure due to its release from bound state to bulk. This further reinforces the contention that hydrophilic-ionic interactions dominates over other interactions, rendering positive ,tr Vο φ values. This seem to support the view that all the compounds in the present study overwhelmingly disturb the structure of water resulting in voluminous hydration spheres. The solutes (amino acids) are not cubical objects so cannot be placed side by side to completely fill the space.
In order to obtain the detailed structural characterization about the solvation spheres, it is noteworthy to supplement the volumetric data along with compressibility measurements. The positive s, ,tr Kο φ values (Table 6) shows existence of strong solutesolvent interactions, resulting in decrease in compressibility of solutions. From the rendering of data included in Table 6, it is witnessed that s, ,tr Kο φ values are positive and surge with upsurge in concentration of betaine hydrochloride. The depressing trend in values with decreasing concentration, infers that due to strong solute-solvent interactions, readjustment in solution continues in the direction of closer packing of molecules or less compressible phase [30].
Negative values of s, ,tr Kο φ represent the disruption of hydration sphere of charged center of co-solute due to enhancement in type (c) and (d) interactions [31]. In substances having large polar surfaces, hydrophobic hydration is the major form of solutesolvent interactions. This is known as structurally enhanced water or stiffened water, formed when the molecules rearrange in an organized fashion having strong hydrogen bonds between them which is found to be less compressible than bulk water. The overlay of hydration co-spheres of two ionic species results in higher volume as some electro stricted water molecules come back to the bulk water with a greater volume contribution than electro stricted water molecules which also coincides with the above discussion on ,tr Vο φ .
Furthermore, it has been shown that the values of ,tr Vο φ and s, ,tr Kο φ in 1% aqueous-betaine hydrochloride solution are smaller than those in 2% aqueous-betaine hydrochloride solution. In this opinion we want to signalize that the calculations obtained from the experimental data suggest that in 2% aqueous-betaine hydrochloride solution the binding forces among solute and solvent becomes robust and a strong molecular interaction exists between amino acid and solvent molecules. When the concentration of drug increases, the solute-solvent interactions are to be present and there seem to be an exclusion of water molecules from solvation spheres. Also, the lower values in case of 1% aqueous-betaine hydrochloride solution can be explained by the fact that the amino acid residues are not completely hydrophobically hydrated. They do not completely fit to the structure of water but disturbs it or remain partially dehydrated results in the contraction in volume thereby causing lower values of ,tr Vο φ and s, ,tr Kο φ at lower drug concentrations.
Analysis of Viscosity Data
The relative viscosity data has been analyzed and interpreted in terms of the rearranged Jones-Dole equation. The viscosity of a non-electrolytic solution or a dipolar ion can be related to viscosity of solvent (aqueous-betaine hydrochloride) by Eq. (8). The dependence of viscosity on molalities of investigated solutions can be manifested through Jones-Dole equation [32] of the form
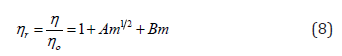
where ηr represents the relative viscosity of the solution, m is the molal concentration (mol·kg-1) of amino acids under study and A and B are the Falkenhagen [33,34] and Jones-Dole coefficients, respectively. These values are valuable since they form the basis for apprehending molecular interactions. The intercept A gives pivotal information regarding solute-solute interactions [35]. The coefficient B is known to be the real hydrodynamic volume of the solvated ion/solute which is administered by the ion-solvent interactions, i.e., the structure of the solvent in the solution [33,34,36].
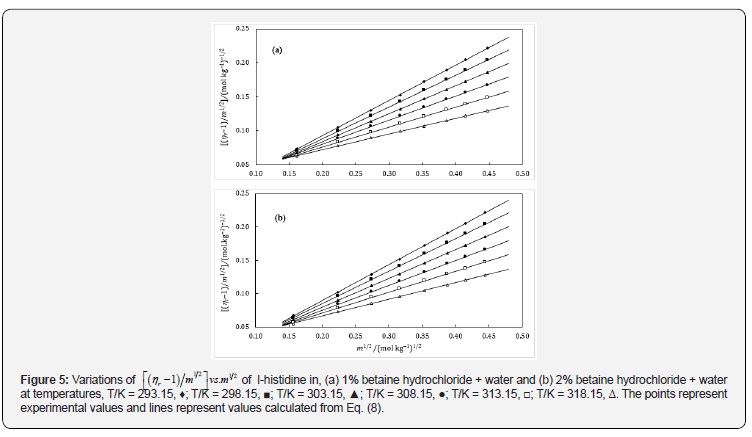
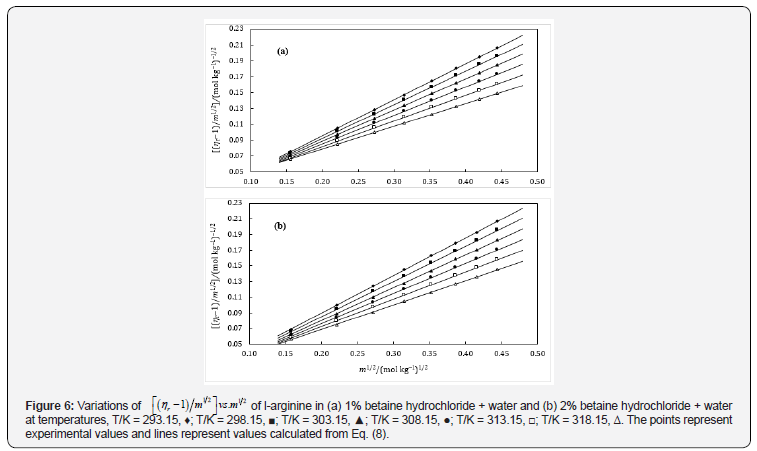
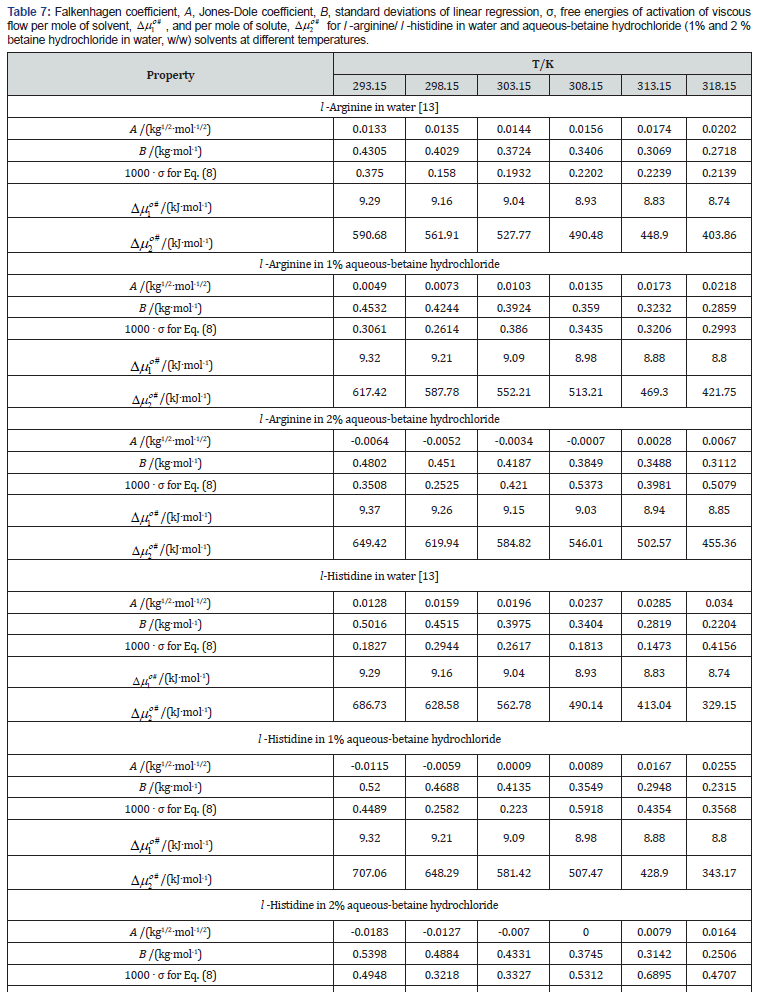
The values of A can also be calculated theoretically but are often overlooked in case of non-electrolytes because of their very small magnitude. The parameters A and B have been acquired as the intercept and slope respectively after plotting ( 1) 1 2 . 1 2 r η − m vs m , which will be conversed in terms of solute-solute and solutesolvent interactions. The plots were found nearly linear in these systems (Figures 5 & 6) and the values of A- and B- coefficients for amino acids in aqueous-betaine hydrochloride along with the standard deviations of linear regression, σ at different temperatures are reported in Table 7.
The perusal of Table 7 reveals that the values of A-coefficients are very close to zero while B-coefficients are positive and large. These observations also indicate a change in viscosity which is due to the disturbance of cation-anion contact hydration pairs and not the free water hydrogen network. The viscosity B-coefficient may be used as a tool to elucidate the solute-solvent interactions and provide information about the solvation of solute in solution and their effects on the structure of solvent in the neighborhood of solvent molecules.
In structured solvents, for example water, a solute, with or without its primary solvation sheath, can affect the structure of solvent molecules, even at larger distances. A ‘structurebuilding’ solute decreases the average effective temperature of solvent molecules and leads to high B-coefficient. Viscosity and temperature have an exponential relationship, therefore a rise in temperature causes B-coefficient to fall and this behavior has been used to identify structure-forming solutes. This point out to the fact that the coefficient B is highly specific property of the individual solute and is strongly temperature dependent.
The dB/dT values are negative for l-arginine and l-histidine in these aqueous-betaine hydrochloride solvents. In the present study, this quantity has been directly interpreted for the evidence of breaking or enhancement of the solvent structure. That is significant given that, whatever are the merits of classification; dB/dT values are widely used to distinguish solutes as structure breakers or makers as suggested by Tyrell & Kennerley [37], in their classical treatise. Solute which shows structure making behaviour are recognized as kosmotropes and chaotropic behaviour is shown by structure making solutes [38]. The negative values of dB/dT shows that amino acids act as structure makers in aqueous betaine hydrochloride solvents.
Hydration Number
The ultrasonic speed, density and viscosity data also allows the calculation of hydration numbers. The standard partial molar volumes Vο φ of amino acids can be articulated as

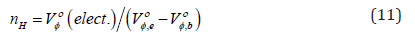
where ,b Vο φ is the molar volume of bulk water and ,e Vο φ is the molar volume of electro stricted water. This model undertakes that for every water molecule taken from the bulk to the region near the amino acids, the volume is decreased by
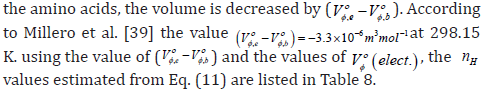
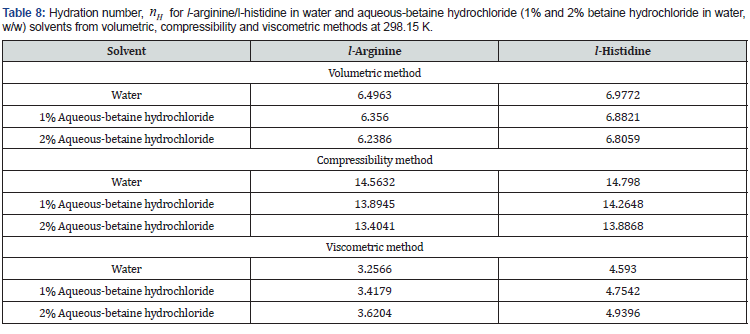
Further, the properties of hydration sphere formed around the investigated solutes have also been characterised based on the method proposed by Millero et al. [39],
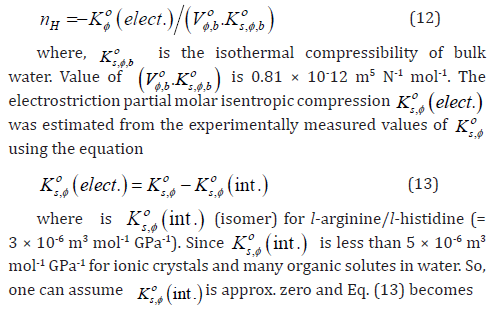

The hydration numbers, H n at 298.15 K, for l-arginine/lhistidine in these systems, estimated from Eqs. (12) and (15) are listed in Table 8.
It can be seen that H n values for the studied amino acids decreases with increasing concentration of betaine hydrochloride from volumetric and compressibility studies while it increases in case of viscometric studies. As we know, water molecules have a tendency to form tetrahedral structure by forming strong hydrogen bonds. When ions are in water solution, water molecules are caged around these ions. In the original tetrahedral structure of water, one position is taken by ions and only three water molecules can be hydrogen bonded which are further counted as hydration number in its primary shell. Therefore, water coordination number for these bonded ions will be less than those for the pure water [24,41].
Further this establishes the fact that the drug has a dehydrating effect on l-arginine/l-histidine solutions. The greater drug concentration makes additional drug molecules to take place of water adjoining amino acid molecules, leading to weakness of hydration and enrichment of solvation. This indicates the increase in solute-cosolute interactions. The hydration numbers chiefly come from the electrostriction effect of the charged end/polar groups of amino acids on water. This indicates there is a boosted hydration of amino acids in these solutions, as water is replaced by the drug, i.e., drug acts as water structure-maker by H-bonding. These trends in values further support our earlier conclusions regarding the behaviour of these systems drawn from values of
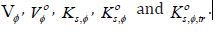
Thermodynamic Activation Parameters of Viscous Flow
On the basis of Eyring’s transition state theory, the viscosity data were analyzed for relative viscosity of amino acid solutions as suggested by Feakins et al. [42,43], using the following equation
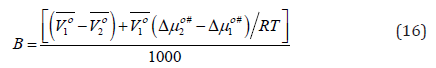
where 1 Vο is the apparent (partial) molar volume of the solvent (aqueous-betaine hydrochloride) and 2 Vο (= Vο φ ) is the limiting apparent (partial) molar volume of the solute at infinite dilution, respectively and R is the universal gas constant. Eyring and co-workers [44] calculated the Gibbs energy change of activation per mole of solvent, # 1 Δμο using the viscosity relation
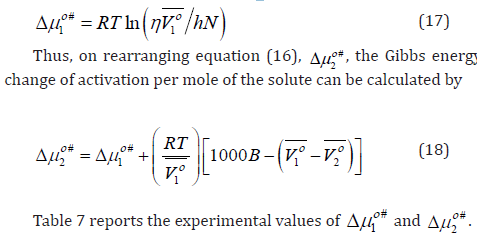
Thus it is clear from the interpretation of data included in table 7, the # 2 ο Δμ values for l-arginine and l-histidine in aqueous and aqueous-betaine hydrochloride solutions, are positive and much larger than those of # 1 Δμο . Also, Samoilov [45] in his work explained that activation energy parameters of exchange water nearby are positive for chaotropes while these are negative for structure breaking ions. This suggests that the interactions between l-arginine and l-histidine and solvent (aqueous-betaine hydrochloride) molecules in the ground state are stronger than in the transition state [34,46].
Henceforth in the transition state, the solvation of the solvent molecules is more preferred in free energy terms. Also, the values of # 1 Δμο and # 2 ο Δμ for these amino acids in aqueousbetaine hydrochloride solvents are larger than those in water and these values increase with increase in concentration of betaine hydrochloride in solution (Table 7). This increase may be due to association occurring between the solute, water molecules and hydrotropic agent (betaine hydrochloride) which additionally supports the occurrence of strong solute-solvent (hydrophilicionic group) interactions in these systems. The # 2 ο Δμ values increase with increase in temperature, indicating a pervasiveness of solute-solvent interaction surge with rise in temperature making the flow of solute molecules difficult [34,46].
It has been conveyed [34,46], # 2 ο Δμ > # 1 Δμο for solutes along with positive viscosity B-coefficients indicates that in ground state, interactions between amino acids and solvent molecules are considerably stronger than those in the transition state. This means that due to cleavage and distortion of the intermolecular bonds, the development of transition state is much less favoured in the presence of the drug molecule. Positive values of # 2 ο Δμ and B-coefficient on the basis of Table 7 indicate that the combined effect of π bonds and the substituent groups result in overall disorder in the solvent. Also, positive magnitude intensifies from l-arginine to l-histidine which further indicates that the disorder has some relation with substitution. Thus, the observed trends drawn from #
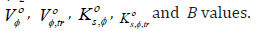
To get a better vision into the molecular atmospheres, the Gibbs energy of activation of viscous flow of solutions, ΔGο # was calculated by using the equation [43]
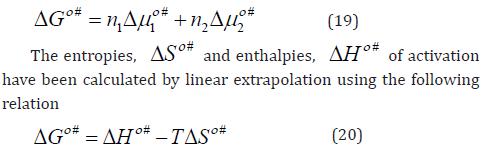
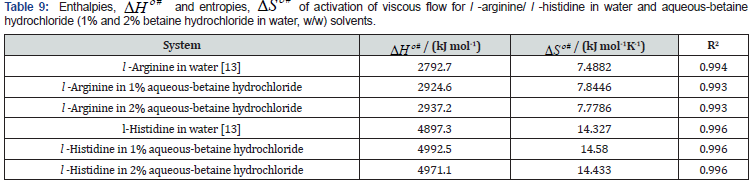
The calculated values of activation parameters ΔSο # and ΔHο # from the slopes and intercepts of linear fit of ΔGο # versus T and the results are recorded in table 9.
The parameter ΔHο # gives evidence about the structure of the solute species, whereas ΔSο # provides signal apropos solutesolvent interactions [43,44]. The positive values for both enthalpies of activation ΔHο # and entropies of activation ΔSο # of viscous flow demonstrates that the association process is endothermic in nature and more energy consuming. Positive ΔHο # reveal that formation of activated species for viscous flow becomes more difficult. Moreover, l-arginine-betaine hydrochloride is more ordered than l-histidine-betaine hydrochloride system as pointed out by ΔSο # values.
The sign of ΔSο # reproduces the field-effect on various molecular processes ensuing in the liquid. It is interesting to note that for the amino acids under study, the enthalpies of activation ΔHο # and entropies of activation ΔSο # of viscous flow are positive signifying that the formation of the transition state is linked with solute-solvent bond making in transition state, as a result the formation of activated complex becomes easier. This remark submits the bigger size of the ion or the larger structural intricacy appears to disrupt an otherwise condensed and homogeneous hydration shell around the amino acid molecules. The results can be also viewed in terms of the geometrical fit of solute species in an ordered solvent [15].
The values of ΔSο # are found to be positive and show a pronounced decrease as the concentration of drug increases in the solution, implying that the system is more structured during the viscous flow than it was in the initial state, henceforward, indicating the presence of significant solute-solvent interactions in the systems under investigation. Thus, the solute-solvent interactions are thus favourable and the solute molecules assumes an extended random conformation. These results are in the accordance of the conclusion drawn from earlier parameters.
Conclusion
The present article reported the densities, ρ, ultrasonic speed, u and viscosities, η results of solutions of l-arginine/l-histidine in aqueous-betaine hydrochloride solvents. Based on these experimentally measured data, the nature of interactions has been assessed by calculating various volumetric, thermo-acoustical and viscometric parameters, viz., Vο φ , ,tr Vο φ , s, Kο φ , s, ,tr Kο φ , hydrsation number, H n , Falkenhagen coefficient, A, Jones-Dole coefficient, B, # 1 Δμο , # 2 ο μ Δ and dB/dT as functions of concentration and temperature. From the analysis of results, it may be concluded that there exist strong interactions between solute and solvent molecules in these systems, which increase with increase in betaine hydrochloride concentration. These amino acids act as structure makers in aqueous betaine hydrochloride solvents. If these results hold up to further investigation, interesting perceptions about hydrophilic hydration may result. The present thermodynamic study, i.e., thermodynamic and physico-chemical behaviour of the investigated systems plays a very significant role in medicinal and pharmaceutical chemistry.
Acknowledgement
The author JG is thankful to University Grants Commission, Government of India for the award of Senior Research Fellowship.
References
- R Gaba, A Pal, D Sharma, J Kaur (2017) Solvation behavior of glycine and glycyl dipeptide in aqueous 1-butyl-3-methylimidazolium bromide ionic liquid solutions at different temperatures. J Mol Liq 233: 38-44.
- A Kumar, R Rani, B Saini, R K Bamezai (2017) Volumetric, compressibility, taste behavior and viscometric studies of methionine with some saccharides in aqueous medium at different temperatures. J Solut Chem 46: 931-956.
- H Kumar, M Singla, R Jindal (2015) Studies of interionic interactions of L-serine/L-threonine in aqueous trilithium citrate solutions using density and speeds of sound measurements at different temperatures. J Mol Liq 208: 170-182.
- A Sarkar, B Sinha (2017) Volumetric, acoustic and transport properties of metformin hydrochloride drug in aqueous D-glucose solutions at T = (298.15-318.15) K. J Solut Chem 46: 424-445.
- J E Kinsella, N Melachouris (1976) Functional properties of proteins in foods: a survey. CRC Critical Reviews in Food Science and Nutrition 7: 219-280.
- C Xiao Ming, T C W Mak (1990) Crystal structure of bis (pyridine betaine) hydrochloride monohydrate. J Mol Str 221: 265-269.
- M S Fischer, D H Templeton, A Zalkin (1970) Solid state structure and chemistry of the choline halides and their analogues. Redetermination of the betaine hydrochloride structure [(CH3)3NCH2COOH]+ Cl−. Acta Cryst 26B: 1392-1397.
- S Ryshetti, A Gupta, S J Tangeda, R L Gardas (2014) Acoustic and volumetric properties of betaine hydrochloride drug in aqueous D (+)-glucose and sucrose solutions. J Chem Thermodyn 77: 123-130.
- J Gupta, A K Nain (2018) Study of solute-solute and solute-solvent interactions of streptomycin sulphate in aqueous-l-asparagine/l-glutamine solutions at different temperatures by using physicochemical methods. J Mol Liq 249: 666-676.
- J Gupta, A K Nain (2019) Physicochemical study of solute-solute and solute-solvent interactions of homologous series of α-amino acids in aqueous-isoniazid solutions at temperatures from 293.15 to 318.15. K. J Mol Liq 278: 262-278.
- J Gupta, A K Nain (2019) Effect of concentration and temperature on apparent molar properties of homologous α-amino acids in aqueous-semicarbazide hydrochloride solutions: A quest on the concept of kosmotropic/chaotropic behaviour of amino acids. J Chem Thermodyn 135: 9-26.
- J Gupta, A K Nain (2019) Molecular interactions of gentamicin sulphate in aqueous-l-asparagine/l-glutamine solutions at different temperatures: Volumetric, acoustic and viscometric properties. J Mol Liq 293: 111547.
- J Gupta, A K Nain (2020) Correlation between physicochemical properties and non-covalent interactions involving l-arginine and l-histidine and semicarbazide hydrochloride at temperatures from 293.15 to 318.15. K. J Chem Thermodyn 144: 106067.
- D O Masson (1929) XXVIII. Solute molecular volumes in relation to solvation and ionization. Phil Mag 8: 218-223.
- M Iqbal, R Verrall (1989) Apparent molar volume and adiabatic compressibility studies of aqueous solutions of some drug compounds at 25 °C . Can J Chem 67: 727-735.
- T Banipal, H Singh, P Banipal (2010) Volumetric and viscometric properties of some sulpha drugs in aqueous solutions of sodium chloride at T = (288.15 to 318.15). K. J Chem Eng Data 55: 3872-3881.
- D P Kharakoz (1991) Volumetric properties of proteins and their analogues indiluted water solutions. 2. Partial adiabatic compressibilities of amino acids at 15-70. C. J Phys Chem 95: 5634-5642.
- R K Wadi, P Ramasami (1997) Partial molal volumes and adiabatic compressibilities of transfer of glycine and DL-alanine from water to aqueous sodium sulfate at 288.15, 298.15 and 308.15 K. J Chem Soc Faraday Trans 93: 243-247.
- N Sawhney, M Kumar, Sandarve, P Sharma, A K Sharma, M Sharma (2019) Structure-making behaviour of L-arginine in aqueous solution of drug ketorolac tromethamine: volumetric, compressibility and viscometric studies. Phys Chem Liq 57: 184-203.
- H Rodriguez, A Soto, A Arce, M K Khoshkbarchi (2003) Apparent molar volume, isentropic compressibility, refractive index, and viscosity of DL- alanine in aqueous NaCl solutions. J Solut Chem 32: 53-63.
- A Soto, A Arce, M K Khoshkbarchi (2003) Thermodynamics of diglycine and triglycine in aqueous NaCl solutions: Apparent molar volume, isentropic compressibility, and refractive index. J Solut Chem 33: 11-21.
- P Bernal, S Brown, M Mera, Y Bouchibti (2019) Partial molar volumes and isentropic compressions of sugar alcohols in aqueous solutions from 15 °C to 40 °C at atmospheric pressure. Food Chem 280: 164-174.
- F J Millero (1972) In: R A Horne (Ed), Structure transport process in water aqueous solutions, John Wiley, New York, pp. 519-564 (chapter 13).
- J Wawer, J Krakowiak (2018) Structural changes of water caused by non-electrolytes: Volumetric and compressibility approach for urea-like analogues. J Mol Liq 259: 112-123.
- S Naz, M A Jamal, M K Khosa, B Naseem, M Muneer (2018) Effect of temperature on thermo-acoustical parameters of arginine in colloidal solutions. J Mol Liq 269: 476-484.
- H L Friedman, C V Krishnan (1973) In: F Franks (Ed) Water: A Comprehensive Treatise, Plenum Press, New York (Chapter 1).
- S Fang, H Xie, H Chen, L Wang, S Tian (2017) Solute-solvent interactions of amino acid L-phenylalanine in aqueous 1-butyl-2, 3-dimethylimidazolium bromine ionic liquid solutions. J Chem Thermodyn 113: 144-150.
- H R Rafiee, F Frouzesh (2016) Volumetric properties for glycine and L-serine in aqueous solutions of 1-Ethyl-3-methylimidazolium hydrogen sulfate ([Emim][HSO4]) at T= (293.15-313.15) K and ambient pressure. J Chem Thermodyn 102: 398-405.
- H Kumar, I Behal (2016) Thermodynamics of (solute +solute) and (solute + solvent) interactions of homologous series of amino acids with thiamine hydrochloride in aqueous medium at T = (305.15, 310.15, 315.15) K: A volumetric and acoustic approach. J Chem Thermodyn 102: 48-62.
- S Chauhan, K Singh, M S Chauhan, A Umar, C N Sundaresan (2016) Intermolecular interactions of L-glutamine and L-histidine in aqueous solutions of metformin hydrochloride: Thermo-acoustic and optical properties. J Mol Liq 214: 390-399.
- M C Roy, M N Roy (2017) Study to explore diverse interactions of amino acids and vitamin molecule in aqueous environment. Phys Chem Liq 55: 334-346.
- G Jones, M Dole (1929) The viscosity of aqueous solutions of strong electrolytes with special reference to barium chloride. J Am Chem Soc 51: 2950-2964.
- H Falkenhagen, M Dole (1929) The internal friction of electrolytic solutions and its interpretation according to debye theory. Z Phys 30: 611-616.
- H Falkenhagen, E L Vernon (1932) The viscosities of strong electrolytes solution according to electrostatic theory. Z Phys 33: 140-145.
- N V Sastry, P H Valand, P M Macwan (2012) Effect of hydrophilic additives on volumetric and viscosity properties of amino acids in aqueous solutions at T (283 to 333.15) K. J Chem Thermodyn 49: 14-23.
- C S Solanki, S Tripathy, M Tripathy, U N Dash (2010) Studies on the solute solvent interaction of nimesulide in aqueous solutions of hydrotropic agents at different temperatures. E-J Chem 7(S1): S223-S230.
- H J V Tyrell, M Kennerley (1968) Viscosity B-coefficients between 5° and 20° for glycolamide, glycine, and N-methylated glycines in aqueous solution. J Chem Soc A 2724-2728.
- T S Sarma, J C Ahluwalia (1973) Experimental studies on the structures of aqueous solutions of hydrophobic solutes. Chem Soc Rev 2: 203-232.
- F J Millero, A L Surdo, C Shin (1978) Apparent molal volumes and adiabatic compressibilities of aqueous amino acids at 25.degree.C. J Phys Chem 82: 784-792.
- E Berlin, M J Pallansch (1968) Densities of several proteins and L-amino acids in the dry state. J Phys Chem 72: 1887-1889.
- H Peng, A V Nguyen (2018) A link between viscosity and cation-anion contact pairs: Adventure on the concept of structure-making/breaking for concentrated salt solutions. J Mol Liq 263: 109-117.
- D Feakins, F M Canning, W E Waghorne, K G Lawrence (1993) Relative viscosities and quasi-thermodynamics of solutions of tert-butyl alcohol in the methanol + water system: a different view of the alkyl-water interaction. J Chem Soc Faraday Trans 89: 3381-3388.
- D Feakins, D J Freemantle, K G Lawrence (1974) Transition state treatment of the relative viscosity of electrolytic solutions. Applications to aqueous, non-aqueous and methanol + water systems. J Chem Soc Faraday Trans I 70: 795-806.
- S Glasstone, K J Laidler, H Eyring (1941) The Theory of Rate Processes, McGraw-Hill, New York, pp. 477.
- O Y Samoilov (1957) A new approach to the study of hydration of ions in aqueous solutions. Discuss Faraday Soc 24: 141-146.
- M Kaminsky (1957) Ion-solvent interaction and the viscosity of strong electrolyte solutions. Discuss Faraday Soc 24: 171-179.






























