Noscapine as Anticancer Agent & Its Role in Ovarian Cancer
Nidhi Singh1, Snigdha Singh1, Shubham Sewariya1, Aarushi Singh1, Garima Rathee1, Damini Sood1, Neeraj Kumar1, Ishita Chandra4, Sujata K Dass2, Vartika Tomar1 and Ramesh Chandra1,3*
1Department of Chemistry, University of Delhi, Delhi, India
2BL Kapur Hospital, New Delhi-110005, India
3Dr B R Ambedkar Center for Biomedical Research, University of Delhi, Delhi, India
4Department of Chemistry, Michigan State University, Michigan, India
Submission: November 29, 2019; Published: December 12, 2019
*Corresponding author: Ramesh Chandra, Drug Discovery & Development Laboratory, Department of Chemistry, University of Delhi and Dr B R Ambedkar Center for Biomedical Research, University of Delhi, Delhi- 110007, India
How to cite this article: Ramesh C, Nidhi S, Snigdha S, Shubham S, Aarushi S, et al. Noscapine as Anticancer Agent & Its Role in Ovarian Cancer. Organic & Medicinal Chem IJ. 2019; 9(2): 555757. DOI: 10.19080/OMCIJ.2019.09.555757
Abstract
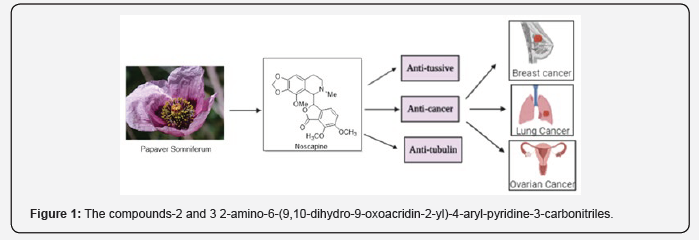
Cancer is a public health problem accounting for an estimated 9.6 million deaths worldwide, according to the latest WHO report, mainly affecting low-middle income countries. Noscapine (narcotize), a non-toxic benzylisoquinoline alkaloid derived from opium poppy, is commonly used as a cough suppressant in humans and exhibited various activities against a variety of cancers with unclear mechanism of action. Unlike other alkaloids obtained from opium poppy, noscapine is not sleep-inducing, hypnotic drug, rendering it as non-addictive drug and therefore used as antimitotic & antitussive drug around the world, can be orally consumed. Ovarian Cancer is one of the common causes of gynecologic cancer affecting women around the world. The main reason for treatment failure and mortality is the drug resistance against DDP (cisplatin), which has emerged the use of Noscapine against drug-resistant ovarian cancer cell line SKOV3/DDP by activation of apoptosis. Herein, we will describe noscapine chemically; its medicinal utility and pharmacological history behind the noscapine family of compounds then follow its journey for treating ovarian cancer (Figure 1).
Keywords: Noscapine; Mitosis Apoptosis Antitussive Metastasis Sipuleucel-T vaccine
Introduction
Cancer remains the second frightful disease after cardiovascular diseases causing millions of deaths worldwide. Cancer accounts for an estimated 10 million deaths globally in 2018, especially in low and middle-income countries according to the recent WHO report [1,2]. Both external factors, i.e. regular usage of tobac co products, alcohol, and unhealthy food & internal factors that include genetic mutation in genetic material of cells, hormonal disorders involve the major risk factor for cancer and accounted for approximately 20% cancer deaths [3,4]. It is characterized by uncontrolled cell proliferation of normal cells that divide uncontrollably, and an absence of cell death causes an abnormal cell clump, which we called tumors that grow and metastasize to other parts of the body and finally leads to death [5]. Several types of cancer are reported in human beings; among them breast cancer is top listed in females followed by lung cancer in males [6,7].
The main systemic treatment options currently used for metastatic cancers are chemotherapy, hormone therapy, immunotherapy, cancer vaccination, and biological therapies, while surgery & radiotherapy are primary treatment used for non-metastatic cancers [8]. These frontline treatments depend upon type of cancer, its stage, and location which are often accompanied by harsh side effects involving toxicity, limited bioavailability, quick clearance and restricted metastasis [9]. Chemotherapeutic agents involve drugs that could show promising results either alone or in combination with other cancer therapies [10].
These agents include topoisomerase inhibitors, doxorubicin, carboplatin, cisplatin, docetaxel and paclitaxel etc. [11], are highly efficient but these agents also have limitations like cost, side effects, and toxic. Classical drugs targeted directly DNA of the cell which proved ineffective, while contemporary drugs involve targeting at protein that possessed abnormal expression inside the cell, which was successful in certain malignancies [12]. It also often limited by cancer cell’s resistance to these drugs as they go through mutations and side effects on normal tissues and cells with fast proliferation rates, such as bone marrow, hair follicles.
Recently, new FDA approved targeted therapies involve blocking of specific cancer protein to cause cancer cell death due to apoptosis, specific delivery to cancer drugs to cancer cells, blocking of transduction pathways, thereof minimizing side effects [13]. Several vaccines have been approved by FDA for the treatment of cancer, including hepatitis B vaccine and human papillomavirus (HPV), lately Sipuleucel-T (Provenge) has been approved by FDA in U.S. to treat prostate cancer which can no longer be treated by hormone therapy [14]. The use of various types of nanoparticles (NPs) has gained attraction recently in delivering anticancer drugs. Nanocarriers increase the therapeutic efficacy of drugs inside the tumor cell; also, they improve their specificity [15].
Lately, the emphasis on natural products was done in search of a novel treatment of this deadly disease cancer. Moreover, the cytotoxic effects of a few members of Papaveraceae family have been considered in medicines made in India, China & Iran to cure chronic cough, diarrhea, and gastrointestinal problems [16]. Noscapine (Narcotine), discovered in 1817, a phthalide isoquinoline alkaloid is a natural product derived from the opium poppy, Papaver somniferum. Unlike other alkaloids obtained from opium, noscapine is not sedative, non-narcotic, and non-analgesic. Noscapine is initially marketed as a safe; antitussive (cough suppressant) agent in early 1960’s and had a low toxic profile [17]. Later it was found to possess anticancer activity due to its action on tubulin; it binds with tubulin and slows down tumor growth [18]. In past years, many potential anticancer drug targets have been identified for itseffective treatment. The current review describes Noscapine and its analogs as promising anticancer targets.
Noscapine, a Biologically Active Natural Product
Noscapine is a widely used antitussive medication and now used as promising anticancer medicine, which can be administered orally. Noscapine has been found to inhibit progression of breast cancer, ovarian cancer, lung cancer, and prostate cancer, both in vitro & in vivo with no toxicity to other parts of human body such as heart, kidney, liver, bone marrow [19]. Based on literature, we observed that Noscapine has chemical similarity with colchicine, and hence it binds in a similar way to tubulin that leads to change in conformation affecting microtubule assembly and finally arrests mitosis.
Noscapine can also be used in combination with other anti- tumor drugs; for example, its combination with doxorubicin, an anthracycline drug against triple-negative breast cancer. They demonstrated that Noscapine inhibited growth of MDA-MB-231 (IC50=36 mM) and MDA-MB-468 (IC50=42 mM) cells with Confidence Interval (CI) values (0.59) that suggest strong synergistic interaction of Noscapine and Doxorubicin with increase in apoptotic cells significantly [20]. Similarly, Noscapine combination with gemcitabine is used in the treatment of non-small lung cancer [21]. Noscapine is water-soluble; it readily crosses the bloodbrain barrier. Also, its oral administration property potentiates the anti-cancer activity of doxorubicin as well as gemcitabine in a synergistic manner through anti-angiogenic apoptotic pathways.
In 2016, Isobolographic method has been utilized to decipher the interaction between Noscapine and Cisplatin against A549 and H460 lung cancer cells in vitro and also in vivo in murine xenograft model. The results demonstrated synergistic effect of Noscapine and Cisplatin together with reduced tumor volume by 78% as compared with 38% by Cisplatin or 35% by Noscapine alone in murine xenograft lung cancer model [22]. The mechanistic interaction of Noscapine and Lysozyme has been recently studied by Damini [23]. The study investigated their conformational changes and helped in understanding biophysical properties on interaction of Lysozyme with Noscapine.
Noscapine Analogs as Promising Anticancer Agents
Over the past years, many analogs of Noscapine have been synthesized and tested for anti-cancer activity, which is found superior to the parent Noscapine. These derivatives were chemically synthesized by modifying the parent Noscapine, whereas keeping the parent scaffold intact [24]. There are three generations of noscapinoids; first-generation noscapinoids were chemically synthesized by modifying isoquinoline and benzofuranone rings of Noscapine [25]. This also includes 9-halogenated (chloro, bromo, iodo-noscapine), 9-amino, 9-nitro, and 9-azido analogs.
9-bromonoscapine was found to have higher tubulin binding activity than Noscapine with improved effect on tubulin polymerization. Besides 9-bromonoscapine, in vitro cytotoxicity on U-87human glioblastoma cell lines by MTT assay were evaluated for 9-chloro, 9-iodonoscapine [26]. At 50 μM concentration, 9-bromo, 9-chloro, 9-iodonoscapine killed 51%, 88%, and 57% cells respectively after 72h, whereas Noscapine killed only 40% of the cell. The results reveal 9-chloonoscapine as more potent anticancer agent than Noscapine and 9-Bromonoscapine. However, at 1 μM concentration, both 9-chloro and 9-bromo derivatives showed similar activity results (Figure 2).
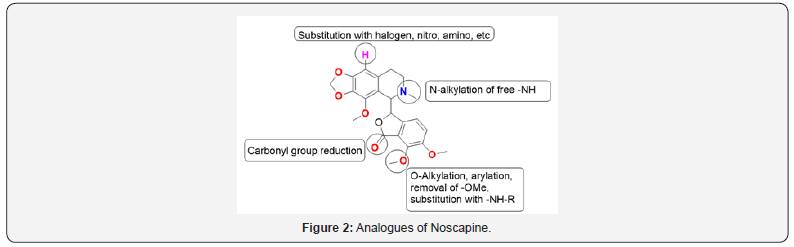
9-nitro-nos proved useful in mainly those cells that show multidrug resistance, for ex- lymphoma and an ovarian cancer cell. Computational studies showed that 9′-aminonoscapine analog would bind to tubulin at the site, which overlapped with the colchicine-binding site and could possess improved antitumor activity when compared to Noscapine. Amino noscapine effectively reduced the intrinsic fluorescence of tubulin in comparison to Noscapine. In halogenated noscapinoid, chlorinated derivative shows good results against ovarian cancer cells. Second generation noscapinoids represent the O-alkylated and O-acylated by modifying benzofuranone ring of Noscapine.
The 7-acetyl derivative of Noscapine was prepared to analyze the influence of the polarizable carbonyl group on activity in contrast to the inert 7-OMe of parent compound noscapine. 7-substituted Noscapine analog with acetyl group showed better activity as compared to Noscapine in A549, MCF7, PC3 cell lines [27]. Third generation noscapinoids were produced by alteration in substituents coupled to the nitrogen of the isoquinoline ring. The substitution of the N-methyl group with longer alkyl chain derivative was seemed to be less stable. All these derivatives are more potent in inhibiting the proliferation of Human Cancer cells [28].
Noscapine Mechanism of Action
Although the molecular mechanism of anticancer activity of Noscapine is not yet clear, however, several experiments indicate that Noscapine induces apoptosis tumor cells. The induction of apoptosis is verified by the increase in activity of caspase -2, -3, -6, -8 and -9, nucleation of chromatin, DNA fragmentation, and detection of phosphatidyl serine on the outer layer of the cell membrane. Hence this could be used as treatment of hematological malignancies [29]. Newcomb [30] have done a similar study on human glioma cells and demonstrated that Noscapine is an inhibitor of the Hypoxia-inducible factor-1 (HIF-1) pathways in human glioma cell lines and umbilical vein endothelial cells. Also, Noscapine activates JNK signaling pathway, inactivate ERK signaling pathwayand phosphorylation of the Bcl-2, an antiapoptotic protein while inducing apoptosis. In glioma cell lines, sometimes there is the release of mitochondrial protein AIF along with PARP and cytochrome C cleavage. While in others, AIF released without PARP and cytochrome C cleavage.
Noscapine molecular mechanism of action on tubulin reveals that it binds to tubulin. The evidence revealed by concentration- dependent quenching of the tryptophan fluorescence of tubulin [18]. Also, in the literature survey, it is found that Noscapine has altered the dynamic instability of microtubules by increasing the attenuated state [31], in this way it arrests the cancer cells. Noscapine reduces the catastrophic frequency and increases the rescue frequency. Therefore, Noscapine suppresses the overall dynamicity of microtubule by 60%. The binding sites of Noscapine and its derivative have been investigated in silico [32] Findings predict that the binding site may lie at the a/b-tubulin interface near the colchicine’s binding site.
For the verification, Fluorescence experiment was performed, and the results were interpreted that Noscapine has no interference with binding sites of Colchicine. Alisaraie et al. [33] used molecular docking and molecular dynamic (MD) simulations to study the binding site of Noscapine in silico. The result indicated that the binding sites of Noscapine were found at the intradomain region of the a- and b-tubulin. The same studies were performed on nitrated and brominated Noscapine, and they experimentally measured the dissociation constants proved them a better option [34]. The result could have been improved by studying the noscapine binding to tubulin in a dynamic mode and by including water molecules.
There are two GTP-binding sites; one is an exchangeable site (E-site), and other is nonexchangeable site (N-site) in the -and b-tubulin subunits. GTP molecule was added to the system in the N and E-site. In the a-tubulin subunit, the water molecule surrounding the binding sites hold the Noscapine and forced it toward thecentral region of the intradomain interface. The dynamic nature of the surrounding environment of Noscapine and the above event were found to force it toward GTP at the N-site, as the distance between the centers of mass of GTP and Noscapine decreased around 20 ns of the MD simulations. Distance variation between the center of mass of GTP and the important elements of the N-site effect the structure element of tubulin [35]. The binding of Noscapine revealed that the stability of tubulin elements of the E-site components has increased considerably and the dynamical motions of parts of tubulin have reduced. These elements interfere with the noscapine longitudinal interaction with microtubule, and as a result, positive effects on microtubule polymerization observed.
Noscapine activity as an anticancer agent is mediated by inhibiting NF-kB activation pathway. It also abrogated all the inducible expression of proteins, which are regulated by transcription factors NF-kB, including angiogenesis, survival, proliferation, and invasion. It suppresses the proliferation of human leukemia and myeloma cells by suppressing the NF-Kb signaling pathway. For the inhibition of activity of NF-kB reporter, it must suppress phosphorylation and nuclear translocation of p65; that also inhibited the activity of the NF-Kb-containing cyclooxygenase-2-promoter. One of the important mechanisms of anticancer agents involves antiangiogenic activity; hence, Noscapine also possesses antiangiogenic activity. There are two mechanisms of action involved by which it could show antiangiogenic activity. Firstly, by decreasing HIF-1 expression in hypoxic tumor cells and upregulating the target genes like VEGF [36].
While in others, it inhibits the endothelial cells from forming blood vessels in response to VEGF stimulation. Noscapine being a low toxic agent acted well as an anticancer agent in several animal models of cancer and inhibited the HIF-1 pathway. Considering these properties, it should be considered as antiangiogenic chemotherapeutic agent for glioma [37].
Biological Aspects of Noscapine
Antitussive Activity
Since the 1960s, Noscapine has been widely used as antitussive (cough-suppressing effect) throughout the world with high safety. Noscapine is a drug with a low-toxicity profile, and hence it is orally administered drug either in tablet form or syrup with immediate reduction of cough reflex without affecting respiration. It is still available as medication in most European and Asian countries [38,39].
Anti-Tubulin Activity
Microtubules structures are involved in cell division; they are highly dynamic cytoskeletal fibers composed of tubulin subunits, i.e. α-tubulin and β-tubulin heterodimers arranged in the form of thin filamentous tubes. Noscapine shows its effect by slight suppression of both the growth and shortening of microtubules. To check whether Noscapine affects tubulin polymer ratio in cells, cell extracts containing cytoskeletal polymeric tubulin were incubatedwith different concentrations of Noscapine, i.e. 1, 10, 100 μM. The polymeric tubulin % in cells treated with Noscapine was determined using Western Blot method and was found to be 58, 59 and 59%, respectively [40].
Naik et al. indicated the binding site for Noscapine ligands using docking studies either close to or overlapping with the colchicines binding site [41]. Based on computational studies, Noscapine binding pocket of tubulin was found to be hydrophobic. The di-substituted brominated derivatives of noscapine, 9-Br-7- OH-Nos, 9-Br-7-OCONHEt-Nos, 9-Br-7-OCONHBn-Nos, and 9-Br- 7-OAc-Nos were recently reported by Ram C. Mishra et al. [42], and their chemotherapeutic efficacy on PC-3 and MDA-MB-231 cells were investigated. It was found that these derivatives have higher tubulin binding activity than Noscapine and affect tubulin polymerization.
Anticancer Activity
Non-Small Cell Lung Cancer
Noscapine enhances the antitumor activity of gemcitabine in an addictive to synergistic manner against Non-small cell lung cancer (NSCLC) through apoptotic and antiangiogenic pathways [43]. The combination index value (<0.59) was indicative of synergistic behavior between noscapine and gemcitabine, thus suggesting the potential benefit for the use of combination treatment for treatment of NSCLC. The Noscapine and Gemcitabine combination treatment decreased cancer volume by maximum percentage as compared to single-agent treatment
Similarly, the efficacy of Noscapine and Cisplatin combination was examined in vitro in H460 and A549 lung cancer cells and in vivo in murine xenograft lung cancer model [44]. The combination index value (<0.6) demonstrated the synergistic effects of noscapine and cisplatin, which resulted in tremendous increase in percentage of NSCLC cell death, increase expression of p21, p53, cleaved PARP, Bax, and decreased expression of Akt, cyclin D1, Bcl2, PARP. Such findings also suggested the potential benefit for the use of Noscapine and Cisplatin combination therapy for treatment of small lung cancer cells
Triple-Negative Breast Cancer
Noscapine significantly increased the antitumor activity of Doxorubicin in an additive to synergistic manner against triple- negative breast cancer cells (TNBC) through inactivation of anti-angiogenic and NF-KB pathways [45]. The Noscapine and Doxorubicin combination treatment caused increase in the volume of apoptotic cells effectively. The Flow cytometry and cytotoxicity analysis of the Docetaxel in Noscapine pretreated MDAMB- 231 cells displayed 3.0-fold increase in cell death and about 30% increase in no of late apoptotic cells [46].
Noscapine when used in combination with docetaxel, activated p38 and JNK pathways. The noscapine exposure would significantly upregulate the p38 phosphorylation. Docetaxel showeddown regulation in the expression of surviving, pAKT, and bcl-2 in noscapine pre-treated cells. The anti-migration effect of Docetaxel was significantly increased by noscapine pre-sensitization. The anti-fibrotic and chemo-sensitization effect of noscapine significantly enhanced anti-tumor effect of Docetaxel against TNBC. Moreover, Noscapine in combination with Docetaxel also has the potential to overcome multidrug resistance of TNBC even at low dose [47].
In-vitro and in-vivo results suggested that the Noscapine in combination with Docetaxel formulations inhibited the proliferation of both wild type and the drug-resistant TNBC cells. The potential of microtubule compounds to inhibit TNBC growth can be exploited to overcome drug resistance of TNBC cells. The killing of Docetaxel treated drug-resistant cells was more prominently observed in Noscapine pre-treated cells than without Noscapine pre- sensitization. Also, Br-TMB-Nos targeted tubulin via S-phase arrest instead of G2/M arrest [48]. Far-UV CD spectra suggested that the helical stability of tubulin was disrupted by the presence of Br-TMB-Nos.
The noscapinoid promoted the binding of colchicine to tubulin, altered the tubulin’s surface configuration as well as slightly decrease polymer mass of microtubule. The Br-TMB-Nos was tested for three cell lines (HeLa, PANC-1, MDA-MB-231), out of which it suppressed clonogenicity of MDA-MB-231 cell line and, displayed most inhibition of this cell viability. The presence of this drug did not affect DNA and cellular microtubule.
Ovarian Cancer
There are two types of microtubule affecting agents, one those which bundle and polymerize microtubules and the otherthose which decrease the polymeric chain or depolymerize microtubules [49]. However, the major issues such as low aqueous solubility, toxicity, and drug resistance, severely hampered the clinical success of microtubule affecting agents [50]. Although the patients show good initial response to such agents but mostly patients relapse and did not respond to the same agents at the later stage. Simple and successful chemotherapy become complex and difficult due to multidrug resistance, which is most common problem in chemical biology research nowadays.
There are numerous factors that contribute to drug resistance such as upregulation of bcl-xL and bcl-2, overexpression of MDR1 and increased DNA repair [51]. Toxicity poses another major challenge to successful chemotherapy. This is because the antimicrotubule agents perform other functions such as axonal transport and cytoplasmic organization, in addition to their role in the chromosome’s movement during mitosis. The agents, such as taxane and vinica alkaloids, are associated with several toxicities such as alopecia, peripheral neuropathy, and gastrointestinal toxicities.
Such aspect of toxicity is due to the lack of specificity for dividing cells. Considerable efforts have been made in last few decades to discover new and effective antimicrotubule agents having same mechanism of action to the preexisting drugs (such as paclitaxel, docetaxel, and vinica alkaloids) [52-54] but with better pharmaceutical features. Based on the systematic screening of structurally similar antimicrotubular agents, opium alkaloid noscapine was identified as the microtubule-targeting agent [55]. Noscapine effectively inhibited the proliferation and induced apoptosis in both paclitaxel-resistant and paclitaxel-sensitive human ovarian carcinoma cells (Figure 3).
This result is in good agreement with the assumption that noscapine binds to tubulin at the site different from the paclitaxel binding site. The noscapine exhibited non-inhibitory effect on the tubulin-binding by paclitaxel. Unlike other antimicrotubule agents, Noscapine does not inhibit or promote polymerization. In other words, even the high concentration of noscapine does not alter total polymer mass of the tubulin [56]. Noscapine arrest mitosis by causing changes to steady-state dynamics of microtubule assembly and this was done by increasing the period spend by microtubule in attenuated phase. This feature of noscapine ensures that noscapine would not affect other cellular events like axonal transport and cytoplasmic distribution.
Besides, the high dose of noscapine it did not affected the neurons, the post-mitotic cells. Noscapine causes little or no toxicity to small intestine, heart, bone marrow, kidney, spleen, and didn’t inhibit oral immune response in mice [57]. The antimicrotubule agent causes apoptotic cell death as the result of alterationof normal physiological balance in microtubule dynamics. The noscapine causes JNK activation among all three human ovarian carcinoma cell lines, one-1A9 and other two tubulin mutant cell lines i.e., 1A9PTX22 and 1A9PTX10, while the paclitaxel induces JNK activation only in one parental ovarian cell line-1A9. These findings suggest that the mutations neither hinder the interaction of noscapine with tubulin nor the downstream effects that leads to apoptosis.
9-nitro-nos effectively inhibited cell proliferation of ovarian cancer cells especially the cell lines that showed multidrug resistance. 9-nitro-nos arrested progression of cell cycle at G2/M phase, followed by apoptosis. Nitro analog of Noscapine was found to be more effective against paclitaxel resistant variant 1A9/PTX22 than over parental ovarian cancer cell line-1A9 [49]. The IC50 value obtained for the drug-resistant variant was lower than that of parental ovarian cancer cells. 9-nitro-nos have binding pocket different from paclitaxel binding site. Hence, 9-nitro-nos displayed great sensitivity to drug-resistant cells.
The combination of chemotherapy and cytoreductive surgery has increased the survival time span of patients and decreased the mortality rate due to ovarian cancer. Some cases have become resistant to DDP-centered chemotherapy; majority of these cases account for gynecologic cancer deaths [58].
The survival rate of patients who are treated with DDP is seriously reduced due to cancer reoccurrence, development of drug resistance and toxic side effects of DDP therapy [59]. The drug resistance induced by DDP in ovarian cancer cell lines might be related to DAN repair dysfunction, abnormal cell cycle progression, drug metabolism disorder, and inhibition of apoptosis. DDP resistance could be stimulated in cancer cells by decreasing the p53 and Bax expression and enhancing expression of Bcl-2 and XIAP [60-61]. New combination regimens have become targets to overcome chemo resistance and to improve response rate of patients. Small molecule compounds such as noscapine and its nitro derivative, 9-nitro-nos effectively inhibited proliferation of paclitaxel resistant ovarian cancer cells [49,50].
This result proposes the effective use of noscapine to sensitize the chemo resistant ovarian cancer cell to DDP. Out of several factors that contribute to drug resistance, α subunit of HIF-1 has attracted great attention. Although the exact role of HIF-1 in cancer development is still controversial, but the various reports regarding its role suggested that HIF-1 allows the proliferation and survival of tumorous cells through its angiogenic properties and their transactivation during cancer progression. Several oncogenes, Hypoxia, and growth factors regulate HIF-1α and the inhibition of this protein complex by small molecule provides an effective therapeutic goal.
Various non-specific inhibitors of HIF-1α such as geldanamycin [62], 2ME2 [63], topotecan [64,65], 103D5R [66], rapamycin, bevacizumab [65] have recently shown effective anticancer activity. Under hypoxia-mimicking conditions, noscapine efficientlydownregulated HIF-1α levels and significantly suppressed transcriptional activity of HIF-1. The downregulation of MDR1 and HIF-1α by noscapine was associated with DDP induced apoptosis [67]. Wenjing Su et al. reported that the noscapine decreases the transcriptional activity of HIF-1 and HIF-1α protein levels in C13K cells [67].
HIF-1α degradation was stimulated by noscapine through proteasome pathway and its degradation abolished MDR1 overexpression. Such effect forms the basis for chemosensitization by small-molecule compounds such as Noscapine. Under hypoxic conditions, noscapine inhibited the proliferation of C13K cells in concentration and time-dependent manner. The noscapine can reverse the chemo resistance of C13 K cells induced by hypoxia at very low concentration when given along with DDP. The antitussive agent Noscapine inhibited HIF-1 and HIF-1α regulated gene products and as a result, shows the potential to modulate the chemosensitivity of ovarian cancer cells to DDP
Noscapine inhibited the proliferation of both DDP-sensitive SKOV3 and DDP-resistant ovarian cancer cell line SKVO3/DDP [68]. Low concentration of noscapine in combination with DDP effectively increased the toxicity of drug DDP to SKOV3/DDP cells line, enhanced cell apoptosis, and altered cell morphology. The anticancer activity of noscapine might be due to increased cytotoxicity caused by the coordinated effect of noscapine and DDP. The combined treatment with noscapine and DDP decreased the percentage of SKOV3/DDP cells in S phase and increased their percentage in G2/M phase. Both in-vivo and in-vitro experiments showed that noscapine enhanced DDP-mediated apoptosis, decreased the mRNA and protein level of XIAP, NF-jB, surviving and increased the mRNA expression of caspase-3. Hence the combination treatment of noscapine and DDP promoted apoptosis in DDP-resistant ovarian cancer cell SKVO3/DDP by controlling the expression of caspase-3, surviving, XIAP, and NF-jB.
Conclusion
Noscapine has poor absorption, limited water solubility, and short biological half-life; all such properties restrict its development as a prominent oral anticancer drug [69,70]. Novel water- soluble analogs of Noscapine such as 9-bromonoscapine and 9-aminonoscapine contained positively charged quaternary ammonium group and negatively charged sulfonate group. In addition to antitussive activity, Noscapine also has the great potential to treat wide range of cancers. Noscapine showed synergistic effect with another anti-tumor treatment. Although high dose of noscapine produces side effects such as abdominal discomfort and nausea, its usual dose does not produce any noticeable untoward effect [71].
The noscapine and its analog subtly modulate the microtubule dynamics rather than affecting monomer-polymer ratio. The potential ability of noscapine and its derivatives has been enhanced via nanoscale-based delivery system, such as noscapine loaded magnetic nanoparticle, Human serum albumin nanoparticle, andenveloped gelatin nanoparticle [72-75]. More research should be carried out on these compounds to explore new modifications increasing efficacy of drug delivery system and to identify more effective combination of noscapine or its analog with targeted agents to design clinical trials and preclinical studies.
Acknowledgement
Prof. Ramesh Chandra and Aarushi Singh are thankful to DSTSERB (EEQ/2016/000489) for providing financial assistance for conducting research. Prof. Ramesh Chandra would like to acknowledge University of Delhi for providing support and necessary facilities to carry out research work. Nidhi Singh is grateful to CSIR-SRF for providing the fellowship. Snigdha Singh is thankful to ICMR (45/66/2018-PHA/BMS/OL) for providing Senior research fellowship.
References
- https://www.who.int/news-room/fact-sheets/detail/cancer
- B Kumar, S Singh, I Skvortsova, V Kumar (2017) Promising Targets in Anti-cancer Drug Development: Recent Updates. Curr Med Chem 24: 4729-4752.
- J Ferlay, I Soerjomataram, R Dikshit, S Eser, C Mathers, et al. (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136: E359-86.
- M Kharb, R K Jat, R Gupta (2012) A review on medicinal plants used as a source of anticancer agents. Int J Drug Res Technol 2: 177-83.
- E P Herrero, A F Medarde (2015) Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur J Pharma Biopharma 93: 52-79.
- Z Zhou, M Tang, Y Liu, Z Zhang, R Lu, et al. (2017) Apigenin inhibits cell proliferation, migration, and invasion by targeting Akt in the A549 human lung cancer cell line. Anti-Cancer Drugs 28: 446-56.
- A G Waks, E P Winer (2019) Breast Cancer Treatment: A Review. JAMA 321: 288-300.
- J Iqbal et al (2017) Plant-derived anticancer agents: A green anticancer approach. Asian Pac J Trop Biomed 7: 1129-1150.
- S Mukherjee, C R Patra (2016) Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale 8: 12444-70.
- B A Weaver (2014) How taxol/paclitaxel kills cancer cells. Mol Biol Cell 25: 2677-81.
- J Daniel, Maeva Montaleytang, Sounderya Nagarajan, Sébastien Picard, Guillaume Clermont et al. (2019) Hydrophilic Fluorescent Nanoprodrug of Paclitaxel for Glioblastoma Chemotherapy. ACS Omega 4: 18342−18354.
- M Toloudi (2014) Recent Developments in Cancer Treatment: A Review. Pharmaceut Reg Affairs 1-4.
- T N Aung, Z Qu, R D Kortschak, D L Adelson (2017) Understanding the effectiveness of natural compound mixtures in cancer through their molecular mode of action. Int J Mol Sci 18: 656.
- R E Hollingsworth, K Jansen (2019) Turning the corner on therapeutic cancer vaccines. npj Vaccines 4.
- Lee MS, Dees EC, Wang AZ (2017) Nanoparticle-Delivered Chemotherapy: Old Drugs in New Packages. Oncology 31(3): 198-208.
- Afzali, Padideh Ghaeli, Mahnaz Khanavi, Maliheh Parsa, Hamed Montazeri et al. (2015) Non-addictive opium alkaloids selectively induce apoptosis in cancer cells compared to normal cells DARU. J Pharmaceutical Sciences 23: 16.
- C A Winter, L Flataker (1961) Toxicity studies on noscapine. Toxicol Appl Pharmacol 3: 96-106.
- K Ye, Y Ke, N Keshava, J Shanks, J A Kapp, et al. (1998) Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proc Natl Acad Sci 95: 1601-1606.
- M Tripathi, P L Reddy, D S Rawat (2014) Noscapine and Its Analogues as Anti-Cancer Agents. Chem Bio Inter 4: 1-22.
- M B Chougule, A R Patel, T Jackson, M Singh (2011) Antitumor Activity of Noscapine in Combination with Doxorubicin in Triple Negativ. Breast Cancer PLoS ONE 6: e17733.
- T Jackson, M B Chougule, N Ichite, R RPatlolla, M Singh (2008) Antitumor activity of noscapinein human non-small cell lung cancer xenograft model. Cancer Chemotherapy Pharma 63: 117-126.
- M Chougule, A R Patel, P Sachdeva, T Jackson, M Singh (2011) Anticancer activity of Noscapine, an opioid alkaloid in combination with Cisplatin in human non-small cell lung cancer. Lung Cancer 71: 271-282.
- D Sood, N Kumar, A Singh, V Tomar, S K Dass, et al. (2019) Deciphering the Binding Mechanism of Noscapine with Lysozyme: Biophysical and Chemoinformatic Approaches. ACS Omega 4: 16233-16241.
- P E Ghaly, Abou El Magd RM, Churchill CD, Tuszynski JA, West FG et al. (2016) A new antiproliferative noscapine analogue: chemical synthesis and biological evaluation. Oncotarget 7: 40518-30.
- A DeBono, B Capuano, P J Scammells (2015) Progress Toward the Development of Noscapine and Derivatives as Anticancer Agents. J Med Chem 58: 5699-5727.
- A K Verma, S Bansal, J Singh, R K Tiwari, V K Sankar, et al. (2006) Bio org Med Chem14: 6733-6736.
- R C Mishra, P Karna, S R Gundala, V Pannu, et al. (2011) Second Generation Benzofuranone Ring Substituted Noscapine Analogs: Synthesis and Biological Evaluation. Biochem Pharmacol 82: 110-121.
- J V Langermann, H Lorenz, O Boehm, A Flemming, A Bernsdorf, M Kockerling, et al. (2010) (3R*,5′S*)-6,7-Dimethoxy-3-(4′-methoxy-6′-methyl-5′,6′,7′,8′-tetrahydro-1,3-dioxolo[4,5-g]isoquinolin-5′-yl)isobenzofuran-1(3H)-one(Racemic A-Noscapine). Acta Crystallogr 66: 570.
- N Heidari, B Goliaei, P Rahimi-Moghaddam, N Rahbar-Roshandel, M Mahmoudian (2007) Apoptotic Pathway induced by noscapine in human myelogenous leukemia cells. Anti-Cancer Drugs 28: 1139-1147.
- E W Newcomb, Y Lukyanova, I Simirnova, T Schnee, D Zagzag (2008) Noscapine induces apoptosis in human glioma cells by an AIF dependent pathway. Anti-Cancer Drugs 19: 553-555.
- J Zhou, D Panda, J W Landen, L Wilson, H C Joshi (2002) Minor alteration of microtubule dynamics causes loss of tension across kinetochore pairs and activates the spindle checkpoint. J Biol Chem 277: 17200-17208.
- A P K Naik, B P Chatterji, S N Vangapandu, R Aneja, R Chandra, et al. (2011) Rational design, synthesis and biological evaluations of amino-noscapine: a high affinity tubulin-binding noscapinoid. J Comput Aided Mol Des 25: 443-454. & P K Naik, S Santoshi, A Rai, H C Joshi (2011) Molecular modelling and competition binding study of Br-noscapine and colchicine provide insight into noscapinoid-tubulin binding site. J Mol Graph Model 29: 947-955.
- L Alisaraie, J A Tuszynski (2011) Determination of noscapine’s localization and interaction with the tubulin-alpha/beta heterodimer. Chem Biol Drug Des 78: 535-46.
- J Zhou, K Gupta, S Aggarwal, R Aneja, R Chandra, et al. (2003) Brominated derivatives of noscapine are potent microtubule-interfering agents that perturb mitosis and inhibit cell proliferation. Mol Pharmacol 63: 799-807. & R Aneja, S N Vangapandu, M Lopus, R Chandra, P Dulal, et al. (2007) Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. MolPharmacol 69: 1801-1809.
- M Mahmoudian, P Rahimi Moghaddam (2009) The anti-cancer activity of noscapine: a review Recent Pat. Anti-Cancer Drug Discovery 4: 92-97.
- B Sung, K S Ahn, B B Aggarwal (2010) Noscapine, a Benzylisoquinoline Alkaloid, Sensitizes Leukemic Cells to Chemotherapeutic Agents and Cytokines by Modulating the NF-κB Signaling Pathway. Cancer Res 70: 3259-3268.
- E W Newcomb, Y Lukyanov, T Schnee, M A Ali, L Lan, et al. (2006) Noscapine inhibits hypoxia-mediated HIF-1· expression and angiogenesis in vitro: A novel function for an old drug. Int J Oncol 28: 1121-1130.
- R E Loder (1969) Safe reduction of the cough reflex with Noscapine. Anaesthesia 24: 3.
- X Chen, TT T Dang, P J Facchini (2015) Noscapine comes of age. Phytochemistry 111: 7-13.
- J Zhou, D Panda, J W. Landen, L Wilson, H C Joshi (2002) Minor Alteration of Microtubule Dynamics Causes Loss of Tension across Kinetochore Pairs and Activates the Spindle Checkpoint. J Biol Chem 277: 17200-17208.
- P K Naik, S Santoshi, A Rai, H C Joshi (2011) Molecular Modelling and Competition Binding Study of Br-Noscapine and Colchicine Provide Insight into Noscapinoid−Tubulin Binding Site. J Mol Graphics Modell 29: 947-955.
- R C Mishra et al. (2015) Design, synthesis and biological evaluation of di-substituted noscapine analogs as potent and microtubule-targeted anticancer agents. Bioorg Med Chem Lett 25: 2133–2140.
- MB Chougule, A Patel, P Sachdeva, T Jackson, M Singh (2011) Enhanced anticancer activity of gemcitabine in combination with noscapine via antiangiogenic and apoptotic pathway against non-small cell lung cancer. PloS one 6: 27394.
- M Chougule, A R Patel, P Sachdeva, T Jackson, M Singh (2011) Anticancer activity of Noscapine, an opioid alkaloid in combination with Cisplatin in human non-small cell lung cancer. Lung cancer 71: 271-282.
- MB Chougule, AR Patel, T Jackson, M Singh (2011) Antitumor activity of Noscapine in combination with Doxorubicin in triple negative breast cancer. PloS one 6: 17733
- R Doddapaneni, K Patel, N Chowdhury, M Singh (2016) Noscapine chemosensitization enhances docetaxel anticancer activity and nanocarrier uptake in triple negative breast cancer. Experimental cell research 346: 65-73.
- R Doddapaneni, K Patel, N Chowdhury, M Singh (2017) Reversal of drug-resistance by noscapine chemo-sensitization in docetaxel resistant triple negative breast cancer. Scientific reports. 7: 15824.
- T Mahaddalkar, N Manchukonda, S Choudhary, S Cheriyamundath, N Mohanpuria, et al. (2016) Subtle Alterations in Microtubule Assembly Dynamics by Br‐TMB‐Noscapine Strongly Suppress Triple‐Negative Breast Cancer Cell Viability Without Mitotic Arrest. ChemistrySelect 1: 4313-4319.
- R Aneja, S N Vangapandu, M Lopus, R Chandra, D Panda, et al. (2006) Development of a novel nitro-derivative of noscapine for the potential treatment of drug-resistant ovarian cancer and T-cell lymphoma. Molecular pharmacology 69: 1801-1809.
- J Zhou, K Gupta, J Yao, K Ye, D Panda, et al. (2002) Paclitaxel-resistant human ovarian cancer cells undergo c-Jun NH2-terminal kinase-mediated apoptosis in response to noscapine. J Biological Chemistry 277: 39777-39785.
- D Weng, X Song, H Xing, X Ma, X Xia, et al. (2009) Implication of the Akt2/survivinpathway as a critical target in paclitaxel treatment in human ovarian cancer cells. Cancer Lett 273: 257–265.
- Eric K Rowinsky (1997) The development and clinical utility of the taxane class of antimicrotubule chemotherapy agents. Annual review of medicine 48: 353-374.
- J Crown, M O'Leary (2000) The taxanes: an update. The Lancet 355: 1176-1178.
- J H Sips, JH Beijnen, A Bult, WJ Nooijen (1992) Pharmacology, bio-analysis and pharmacokinetics of the vinca alkaloids and semi-synthetic derivatives. Anticancer research 12: 1699-1715.
- Y Ke, KYe, HE Grossniklaus, DR Archer, HC Joshi, et al. (2000) Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses Cancer Immunology. Immunotherapy 49: 217-225.
- J Zhou, D Panda, J W. Landen, L Wilson, H C Joshi (2002) Minor Alteration of Microtubule Dynamics Causes Loss of Tension across Kinetochore Pairs and Activates the Spindle Checkpoint. J Biol Chem 277: 17200-17208.
- Y Ke, K Ye, H E Grossniklaus, D R Archer, H C Joshi, et al. (2000) Noscapine inhibits tumor growth with little toxicity to normal tissues or inhibition of immune responses. Cancer Immunology, Immunotherapy 49: 217-225.
- Y Kikuchi, T Kita, M Takano, K Kudoh, K Yamamoto (2005) Treatment options in the management of ovarian cancer. Expert opinion on pharmacotherapy 6: 743-754.
- M Harries, M Gore (2002) Part I: chemotherapy for epithelial ovarian cancer–treatment at first diagnosis. The lancet oncology 3: 529-536.
- SW Lowe, AW Lin (2000) Apoptosis in cancer. Carcinogenesis 21: 485-495.
- L Goyal (2001) Cell death inhibition: keeping caspases in check Cell 104: 805-808.
- NJ Mabjeesh, DE Post, MT Willard, B Kaur, EG Van Meir, et al. (2002) Geldanamycin induces degradation of hypoxia-inducible factor 1α protein via the proteosome pathway in prostate cancer cells. Cancer research 62: 2478-2482.
- NJ Mabjeesh, D Escuin, TM LaVallee, VS Pribluda, GM Swartz, et al. (2003) 2ME2 inhibits tumor growth and angiogenesis by disrupting microtubules and dysregulating HIF. Cancer cell 3: 363-375.
- A Rapisarda, B Uranchimeg, O Sordet, Y Pommier, RH Shoemaker, et al. (2004) Topoisomerase I-mediated inhibition of hypoxia-inducible factor 1: mechanism and therapeutic implications. Cancer research 64: 1475-1482.
- L Seeber, RP Zweemer, RH Verheijen, PJ van Diest (2010) Hypoxia-inducible factor-1 as a therapeutic target in endometrial cancer management. Obstetrics and gynecology international 65(2): 605-12.
- C Tan, RG de Noronha, AJ Roecker, B Pyrzynska, F Khwaja, et al. (2005) Identification of a novel small-molecule inhibitor of the hypoxia-inducible factor 1. pathway Cancer research 65: 605-612.
- W Su, LHuang, Q Ao, Q Zhang, X Tian, et al. (2011) Noscapine sensitizes chemoresistant ovarian cancer cells to cisplatin through inhibition of HIF-1α. Cancer lett 305: 94-99.
- W Shen, B Liang, JYin, X Li, J Cheng (2015) Noscapine increases the sensitivity of drug-resistant ovarian cancer cell line SKOV3/DDP to cisplatin by regulating cell cycle and activating apoptotic pathways. Cell biochem Biophysics 72: 203-213.
- H Singh, P Singh, K Kumari, A Chandra, S K Dass, et al. (2013) A review on noscapine, and its impact on heme metabolism. Current drug metabolism 14: 351-360.
- M Henary, L Narayana, S Ahad, SR Gundala, R Mukkavilli, et al. (2014) Novel third-generation water-soluble noscapine analogs as superior microtubule-interfering agents with enhanced antiproliferative activity Biochemical pharmacology 92: 192-205.
- Institute MR Noscapine a safe cough suppressant with newly discovered effects in treating cancer and stroke 2007
- R Chandra, J Madan, P Singh, A Chandra, P Kumar, et al. (2012) Implications of nanoscale-based drug delivery systems in delivery and targeting tubulin binding agent, noscapine in cancer cells. Current drug metabolism 13: 1476-1483.
- J Madan, N Dhiman, S Sardana, R Aneja, R Chandra, et al. (2011) Long-circulating poly (ethylene glycol)-grafted gelatin nanoparticles customized for intracellular delivery of noscapine: preparation, in-vitro characterization, structure elucidation, pharmacokinetics, and cytotoxicity analyses. Anti-cancer drugs 22: 543-555.
- S Sebak, M Mirzaei, M Malhotra, A Kulamarva, S Prakash (2010) Human serum albumin nanoparticles as an efficient noscapine drug delivery system for potential use in breast cancer: preparation and in vitro analysis. Int J nanomedicine 5: 525.
- MO Abdalla, R Aneja, D Dean, V Rangari, A Russell, et al. (2010) Synthesis and characterization of noscapine loaded magnetic polymeric nanoparticles. Journal of magnetism and magnetic materials 322: 190-196.






























