- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Synthesis of Acridone Base Phenolic Compounds for Antibacterial Activity
Afreen Begum*, Syed Imam Pasha and Syeda Saniya Fatima
Department of Pharmaceutical Chemistry, Sultan-Ul-Uloom College of Pharmacy, India
Submission: October 10, 2019; Published: November 14, 2019
*Corresponding author: Afreen Begum, Department of Pharmaceutical Chemistry, Sultan-Ul-Uloom College of Pharmacy, India
How to cite this article: Afreen Begum, Syed Imam Pasha, Syeda Saniya Fatima. Synthesis of Acridone Base Phenolic Compounds for Antibacterial Activity. Organic & Medicinal Chem IJ. 2019; 9(2): 555756 DOI: 10.19080/OMCIJ.2019.09.555756
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Abstract
In this work the synthesis of tricyclic ring having nitrogen at ten(10th) position with two benzene rings leads to the formation of derivatives of acridone which helps in synthesizing heterocycles of medicinal compounds in an arranged form to encourage the scope of these compounds. The compound-1 containing C6H5 at R position showed moderate anti-bacterial activity whereas, the compounds-2 and 3 consisting of 4-CH3C6H4 and 4-FC6H4 at R position also showed moderate activity but the compounds-4 and 5 containing 4-ClC6H4 and 4-NO2C6H4 showed very good antibacterial activity and is nearby comparable with the standard sample of Ciprofloxacin.
Keywords: Ciprofloxacin Tricyclic Ring Antibacterial Activity
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Introduction
Acridone is an organic compound based on the acridine skeleton, with a carbonyl group at the 9 position. It may be synthesized by the self-condensation of N-phenyl anthranilic acid [1]. Carl Gräbe and Heinrich Caro separated acridine in 1870 from coal tar. Acridine 1 is characterized as a straight tricyclic ring having nitrogen at ten (10th) position and two benzene rings are joined symmetrically at cleared out left hand side and right-hand side keeping pyridine ring within the center. Pyridine, quinoline and acridine are comparative compounds which have no benzene ring, one benzene ring and two benzene rings separately and all are gently essential in nature. Acridone 2 is characterized as a tricyclic ring having nitrogen at ten (10th) positions and keto bunch at nine (9th) positions. Discoveries of writing overview proposes different organic exercises related with acridone subsidiaries incited us to synthesize heterocycles of medicinal intrigued in arrange to encourage expound the scope of these classes of compounds [2] (Compounds 1-3).
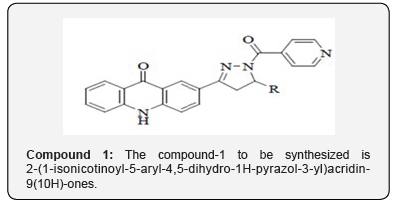
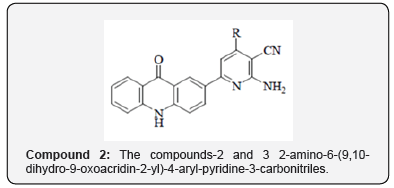
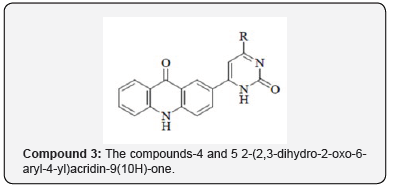
The chemistry of acridone and its subordinates has been considered for over a century due to their differing natural exercises as portrayed in Portion. Moreover, pyrazoline subsidiaries draw an uncommon consideration for their wide range of biological activities at the side their significance and utility as intermediates in planning variety of heterocyclic compounds. Keeping in intellect, different biomedical applications and with a see to assist evaluate the pharmacological profile of this course of compounds ,a novel arrangement of 2-(1-isonicotinoyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl) acridin-9 (10H) -ones (Compound-I) have been synthe sized. The amalgamation of 2-(1-isonicotinoyl-5-aryl-4,5-dihydro- 1H-pyrazol-3- yl) acridin-9 (10H)-ones (Compound-I) was accomplished by multistep synthesis. In step one, the response of o-chlorobenzoic corrosive with p-amino acetophenone in presence of K2CO3 and Cu powder in DMF managed 2-(4-acetylphenylamino) benzoic acid, which on cyclization in nearness of PPA donate 2-acetylacridin-9 (10H)-one. 2- acetylacridin-9 (10H)-one, on response with aryl aldehydes in nearness of potassium hydroxide in ethanol managed 2-(3-aryl acryloyl) acridin-9 (10H)-one (chalcones), which on response with isoniazid beneath microwave light in nearness of glacial acetic corrosive in DMF managed 2-(1-isonicotinoyl-5-aryl-4,5-dihydro-1H-pyrazol-3-yl) acridin-9 (10H)-ones (Compound-I).The items were characterized by FTIR, 1H NMR, 13C NMR, mass spectra and natural investigations. The newly synthesized compounds are subjected to antimicrobial organic movement screening viz., antibacterial and antifungal activity [3].
Comparative Review Literature
I. Salimon J. et al. 71 synthesized 1, 3, 4 oxadiazole derivatives 77 of acridone and screened them for antifungal and antibacterial activity. The SAR study revealed that all the synthesized compounds have a significant biological activity against the antibacterial (Staphylococcus aureus, Streptococcus viridans and Escherichia coli) [4].
II. Giridhar A. et al. 72 synthesized the 9(10H) acridone derivatives 78 (Figure 4). These compounds were evaluated for their antibacterial activity against Staphylococcus aureus, Bacillus subtilis and Escherichia coli. Among these compounds, 9-acridone- N-acetic acid, 9-acridone-N-2-propionic acid, 2-methoxy-9- acridone-N-acetic acid showed good antibacterial activity [5].
III. Singh P. et al.74 synthesized a few novel acridone subordinates and assessed utilizing Candida albicans as antifungal operator and explore them for their impact on convergence or efflux of Rhodamine 6G (R6G) in CA14 cells. Comes about show that the compound 81 hinder the Ca14 cells.11Different acridone subordinates have amazing antiviral movement. Acridone-10 yl acidic corrosive (Neovir) 82 is found to be successful as antiviral medicate [6].
Anti-Bacterial Activity
All the compounds synthesized within the display examination were screened for their against- bacterial movement by Container plate Strategy. Antibacterial exercises were tried on supplement medium against, Staphylococcus aureus, and Escherchia coli which are agent sorts of gram positive and gram-negative living beings separately. The antibacterial movement of the compounds was evaluated by disc-diffusion strategy.
The sterilized media was cooled to 450C with delicate shaking to bring approximately uniform cooling and after that immunized with 18-24 hrs ancient culture beneath aseptic conditions, blended well by delicate shaking [7]. This was poured into sterile Petridishes (appropriately labelled) and permitted the medium to set. After hardening all the Petri dishes were exchanged to laminar stream unit. At that point the circles which were already arranged were carefully kept on the set media by utilizing sterilized forceps. These Petri dishes were kept because it is for one-hour dissemination at room temperature and after that for brooding at 370C for 24 hours in a hatchery. The degree breadth of restraint after 24 hours was measured as the zone of restraint in millimeter [8].
Impact
This study determines the antibacterial activity of acridone derivatives to help the ongoing studies regarding acridone. Even very simple acridone derivatives find their use in bio-analytical science. Organic exercises related with acridone subsidiaries motivated us to synthesize heterocycles of medicinal intrigued in an arranged form to encourage the scope of these compounds. The compound-1containing C6H5 at R position showed moderate anti- bacterial activity whereas the compounds-2 and 3 consisting of 4-CH3C6?H4 and 4-FC6H4 at R position also showed moderate activity but the compounds-4 and 5 4-ClC6H4 and 4-NO2C6H4 showed very good antibacterial activity and is nearby comparable with the standard sample of Ciprofloxacin. The compounds showing great antibacterial activities can contribute in cost effecting parameters.
Significance
i. The prepared compounds are having potent anti-bacterial activity which is comparable with market product i.e. ciprofloxacin.
ii. So, the prepared derivatives can be used as topical drug dosage form to prevent and treat microbial infections.
iii. The formed derivative was obtained by changing the R’ position with an aldehyde by reflux method to obtain the desired potent derivative (Table 1).
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Methodology
Reaction Scheme for Compound -1
It is a multistep synthesis in which 5mg chlorobenzoic acid with p-amino acetophenone in presence of 2 mg of K2CO3 and 6 mg Cu powder in 4 mg DMF (Dimethylformamide) managed to give 2-(4-acetylphenylamino) benzoic acid, which on cyclization in nearness of 4.5 mg Phenylpropanolamine donate 2-acetylacridin-9(10H)-one. 2- acetylacridin-9(10H)-one, on response with aryl aldehydes in nearness of 2.5 mg potassium hydroxide in 5.2 ml ethanol managed 2-(3-aryl acryloyl)acridin-9(10H)-one (chalcones), which on response with 6mg isoniazid beneath microwave light in nearness of 3 ml glacial acetic corrosive in DMF managed 2-(1-isonicotinoyl-5-aryl-4,5-dihydro-1H-pyrazol-3- yl) acridin-9(10H)-ones (Compound I) (Compound 4).
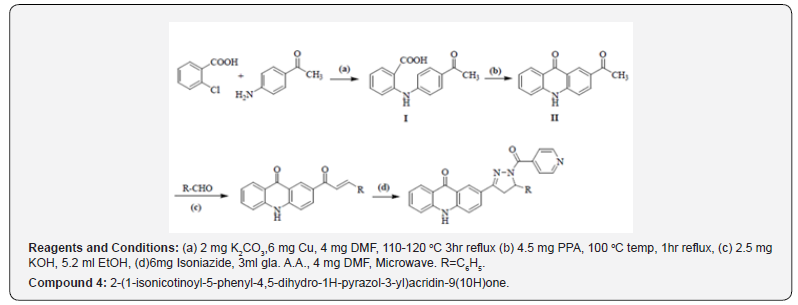
Reaction Scheme for Compound - 2 and 3
It is a multistep synthesis in which 5mg chlorobenzoic acid with p-amino acetophenone in presence of 2.5mg K2CO3 and 6.2mg Cu powder in 4.2mg DMF managed to give 2-(4-acetylphenylamino) benzoic acid, which on cyclization in nearness of 4.5mg PPA donate 2-acetylacridin-9(10H)-one. 2- acetylacridin-9(10H)-one, on response with aryl aldehydes in nearness of 3mg potassium hydroxide in 5.2ml ethanol managed 2-(3-aryl acryloyl)acridin- 9(10H)-one (chalcones), which on response with 6.2mg isoniazid beneath microwave light in nearness of 3ml glacial acetic corrosive in DMF managed to give 2-amino-6-(9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl-pyridine-3-carbonitriles (Compound 5).
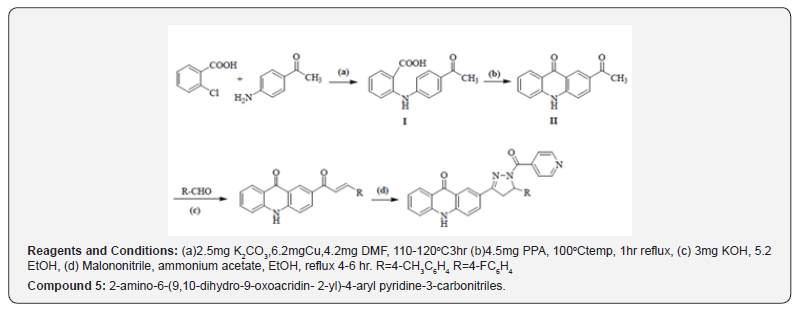
Reaction Scheme for Compound - 4 and 5
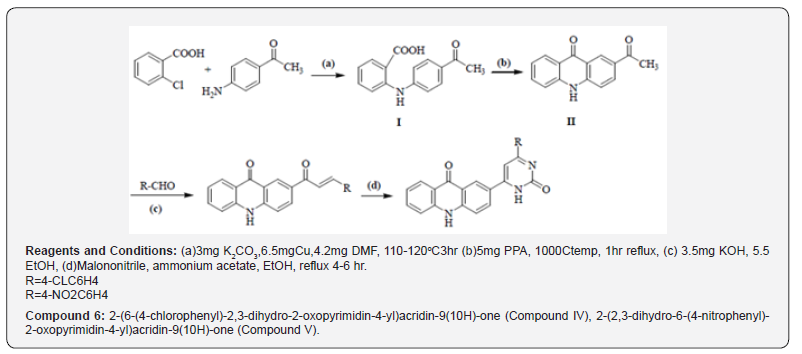
It is a multistep synthesis in which 5.5ml chlorobenzoic acid with p-amino acetophenone in presence of 3mg K2CO3 and 6.5mg Cu powder in4.5mg DMF managed to give 2-(4-acetylphenylamino) benzoic acid, which on cyclization in nearness of 5 PPA donate 2-acetylacridin-9(10H)-one. 2- acetylacridin-9(10H)-one, on response with aryl aldehydes in nearness of 3.5mg potassium hydroxide in 5.5ml ethanol managed 2-(3-aryl acryloyl)acridin- 9(10H)-one (chalcones), which on response with 6.5mg isoniazid beneath microwave light in nearness of 3ml glacial acetic corrosive in DMF managed to give 2-(2,3-dihydro-2-oxo-6-aryl-4-yl) acridin-9(10H)-one (Compound 6).
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Result and Discussion
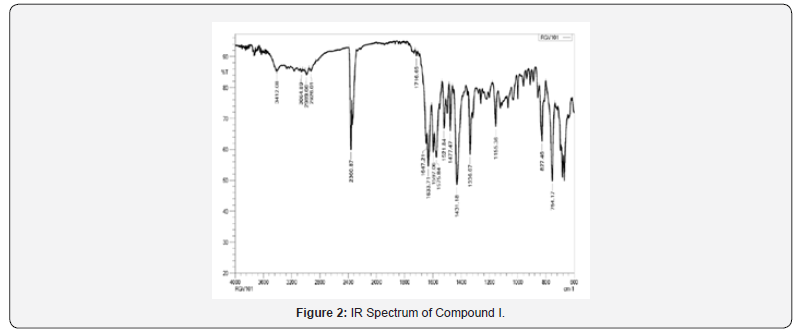
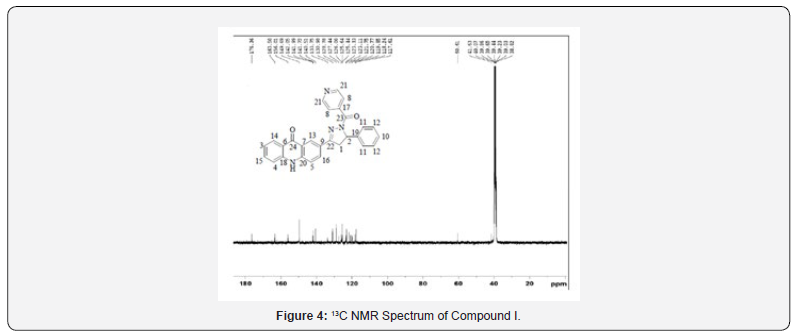
(Compound 7) 149.69, 156.01, 163.50, 176.36; MS: m/z 444; Butt-centric. Calcd for C28H20N4O2 : C, 75.66; H, 4.54; N, 12.60%; Found: C, 75.78; H, 4.64; N, 12.73%. Abdicate: 73%; mp 190 ºC; IR (cm-1): 3412 (-NH extending), 3064 (C-H extending of aromatic ring), 2989, 2926 (C-H extending of CH2 and CH), 1716 (C=O extending of carbonyl bunch), 1633 (C=N extending), 1597, 1575,1521, 1477 (C=C extending of aromatic ring), 1431, 1336 (C-H bowing of CH2 and CH), 827 (C-H bowing of four adjacent hydrogen iota of mono-substituted pyridine ring), 754 (C-H bowing of five adjacent hydrogen molecule of mono-substituted fragrant ring); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 3.628-3.755 (dd, 1H, Ha), 3.839-3.892(dd, 1H, Hb), 5.808 (s, 1H, Hc), 7.268-7.367 (bd, 6H, Hde), 7.522 (bs, 2H, Hfg), 7.773 (bs, 3H, Hhii’), 7.891-7.908 (d, 2H, Hj, J = 6.8 Hz), 8.065-8.082 (d, 2H, Hk, J = 6.8 Hz), 8.197 (s, 1H, Hl), 8.613 (s, 2H, Hmm’), 12.026 (s, 1H, Hn); 13C NMR (DMSO-d6, 400 MHz) δ ppm: 41.63, 60.61, 117.61, 118.24, 119.85, 120.77, 121.75, 123.11, 123.33, 125.44, 125.64, 126.00, 127.44, 128.78, 130.98, 133.75, 140.51, 141.72, 141.99, 142.05.
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Spectral Discussion
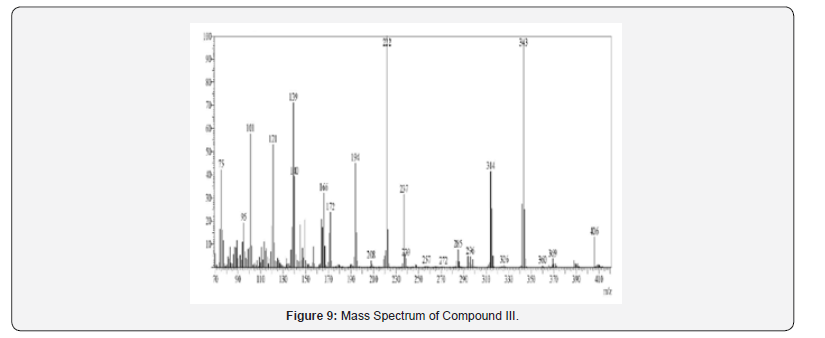
Mass Spectral Study
Mass spectra were recorded on Shimadzu MS-QP-2010 show utilizing Direct Injection Test procedure. Orderly fracture design and atomic particle peak was watched in understanding with atomic weight of compound.
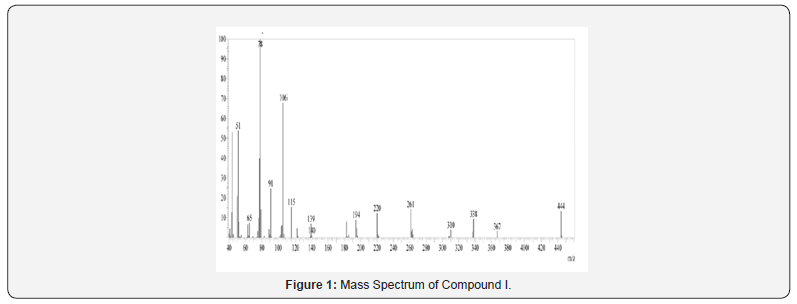
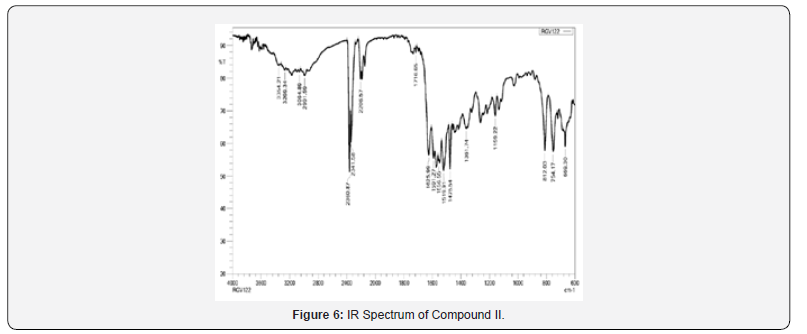
IR spectra were recorded on IRAffinity-1S FTIR Spectrophotometer- Shimadzu using ‘neat’ test. Different utilitarian bunches show in particle were distinguished by characteristic recurrence gotten for them. For Compound I, confirmatory bands for -NH and carbonyl bunches were watched at 3412-3200 cm-1 and 1716 cm- 1 separately. Another characteristic C=N extending band of pyrazoline ring was observed at close 1630 cm 1, which recommended arrangement of wanted functional group Compound I.
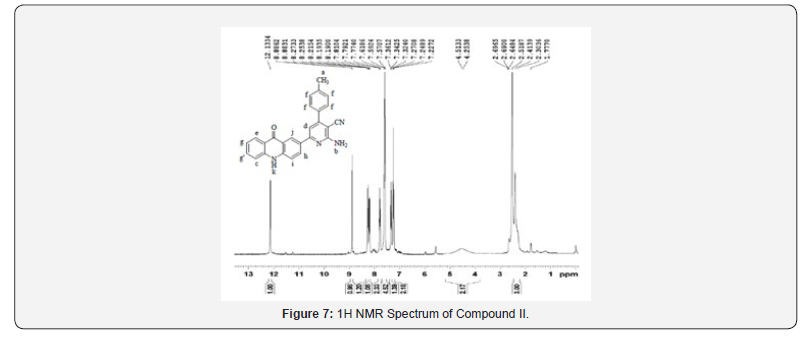
1H NMR Spectral Study
1H NMR spectra were recorded in DMSO-d6 arrangement on a Bruker Ac 400 MHz spectrometer utilizing TMS as an inside standard. Number of protons and their chemical shifts were found to bolster the structure of the synthesized compounds. 1H NMR spectra affirmed the structures of Compound I on the premise of following signals: two twofold doublet within the locale of 3.628-4.029 δ ppm of pyrazoline ring, a singlet at 8.19-8.40 δ ppm, two doublet in locale of 7.89-8.08 δ ppm and singlet of -NH at close 12 δ ppm of acridone ring. The fragrant ring protons and J esteem were found to be in understanding with substitution design on phenyl ring.
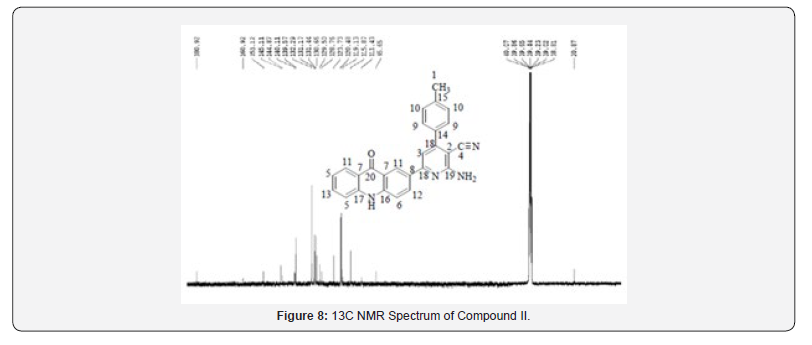
13C NMR Spectral Study
13C NMR spectra were recorded in DMSO-d6 arrangement on a Bruker Ac 400 MHz spectrometer. Number of carbons and their chemical shifts were found to back the structure of the synthesized compounds. 13C NMR spectra affirmed the structures of Compound I on the premise of taking after signals: flag for auxiliary and tertiary carbon of pyrazoline watched at close 40 δ ppm and 60 δ ppm respectively. Signal for carbonyl carbon of isoniazide and acridone was watched at 160-170 and 170-180 δ ppm respectively (Figures 1-4).

- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
A blend of intermediate III (0.01 mol), malononitrile (0.01 mol) and ammonium acetic acid derivation (0.015 mol) was refluxed in ethanol for 4-6 hr. The advance of the reaction was checked upon completion of the response, the isolated solid was collected by filtration, washed with cold methanol and crystallized from DMF: MeOH (1:1) to managed last product (Compound II).
For Compound 2
Yield: (Compound 8)78%; mp 254 ºC; IR (cm-1): 3354, 3269 (N-H extending of essential and secondary amines), 3064 (C-H extending of fragrant ring), 2991 (C-H extending of CH3), 2206 (C≡N extending), 1716 (C=O extending of carbonyl gather), 1625 (C=N stretching), 1591 (N-H twisting), 1556, 1519, 1475 (C=C extending of fragrant ring), 1361 (C-H twisting of -CH3), 812 (C-H bowing of p-di-substituted fragrant ring), 754 (C-H bowing of o-di-substituted fragrant ring); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 2.413 (s, 3H, Ha), 4.253-4.513 (d, 2H, Hb), 7.227-7.361 (m,3H, Hcde), 7.570-7.618 (m, 4H, Hf), 7.774-7.810 (t, 2H, Hgg’, J = 7.2 Hz), 8.193-8.215 (d, 1H, Hh, J = 8.8 Hz), 8.253-8.273 (d, 1H, Howdy, J = 8 Hz), 8.883-8.886 (s, 1H, Hj), 12.133 (s, 1H, Hk); 13C NMR (DMSO-d6, 400 MHz) δ ppm: 20.87, 95.65, 111.43, 115.87, 119.13, 120.48, 123.73, 128.76, 129.50, 130.66, 131.44, 131.77, 132.29, 139.57, 140.11, 144.87, 145.11, 153.12, 160.92, 180.92; MS: m/z 402; Butt-centric. Calcd for C26H18N4O : C, 77.59; H, 4.51; N, 13.92%; Found: C, 77.72; H, 4.64; N, 13.73%.
For Compound 3
Yield: (Compound 9)69%; mp 205 ºC; IR (cm-1): 3269, 3234 (N-H extending of essential and secondary amines), 3060 (C-H extending of fragrant ring), 2270 (C≡N stretching), 1716 (C=O extending of carbonyl bunch), 1627 (C=N extending), 1591 (N-H bending), 1558, 1512, 1475 (C=C extending of fragrant ring), 1272 (C-F stretching), 835 (C-H bowing of p-di-substituted fragrant ring), 756 (C-H bowing of o-disubstituted aromatic ring); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 4.229-4.291 (d, 2H, Ha), 7.221-7.342 (m, 3H, Hbcd), 7.550-7.613 (m, 4H, He), 7.752-7.788 (t, 2H, Hff’, J = 7.2 Hz), 8.172-8.193 (d, 1H, Hg, J = 8.4 Hz), 8.239-8.259 (d, 1H,Hh, J = 8 Hz), 8.869 (s, 1H, Hello there), 12.096 (s, 1H, Hj); 13C NMR (DMSO-d6, 400 MHz) δ ppm: 94.48, 110.48, 115.17, 118.37, 119.33, 122.50, 127.66, 128.44, 129.80, 130.52, 130.57, 131.89, 138.68, 139.11, 144.17, 144.68, 152.52, 163.12, 180.04; MS: m/z 406; Butt-centric. Calcd for C25H15FN4O: C, 73.88; H, 3.72; N, 13.79%; Found: C, 73.96; H, 3.90; N, 13.95%.

- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Spectral Discussion
Mass Spectral Study
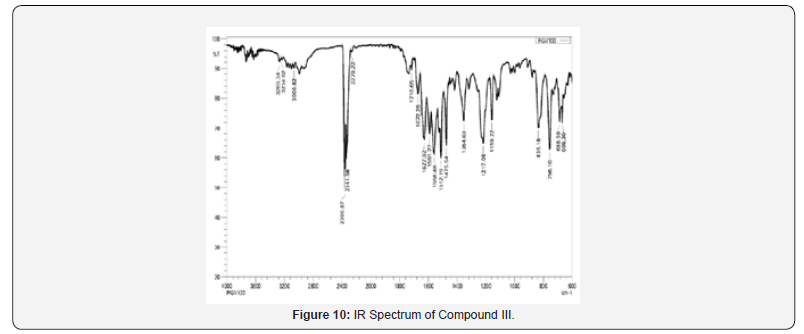
IR spectra were recorded on IRAffinity-1S FTIR Spectrophotometer- Shimadzu using ‘neat’ test. Different utilitarian bunches display in particle were recognized by characteristic recurrence gotten for them. For Compound II, confirmatory bands watched for essential amines at 3360-3200 cm-1 and auxiliary amines at 3280- 3100 cm-1. Another characteristic C≡N extending band ofnitriles were watched at 2300-2200 cm-1 and carbonyl bunches were watched at 1716 cm-1, which suggested formation of craved items Compound II.
1H NMR Spectral Study
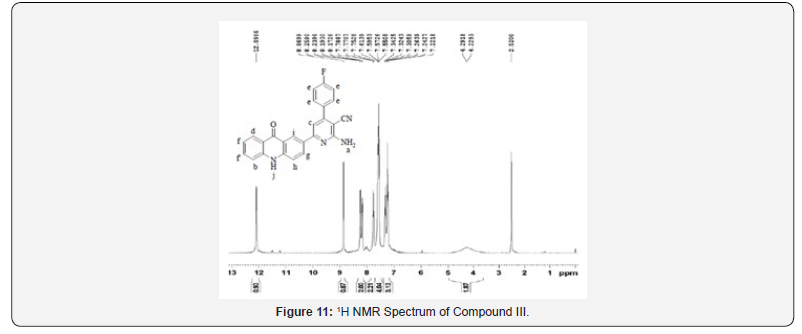
1H NMR spectra were recorded in DMSO-d6 arrangement on a Bruker Ac 400 MHz spectrometer utilizing TMS as an inside standard. Number of protons and their chemical shifts were found to bolster the structure of the synthesized compounds. 1H NMR spectra affirmed the structures of Compound II and III on the premise of following signals: a wide doublet of –NH2 at 4.2-4.5 δ ppm of pyridine ring, a singlet at close 8.86 δ ppm, two doublet in locale of 8.17-8.25 δ ppm and singlet of -NH at near 12 δ ppm of acridone ring. The fragrant ring protons and J esteem were found to be in understanding with substitution design on phenyl ring.
13C NMR Spectral Study
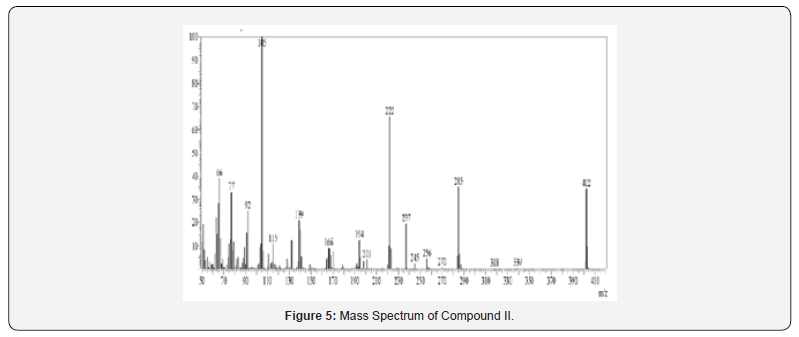
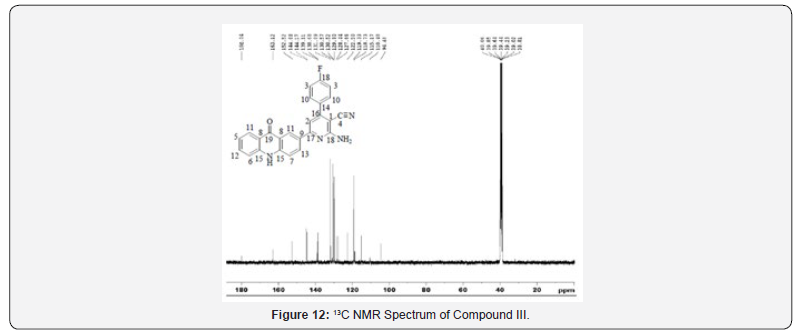
13C NMR spectra were recorded in DMSO-d6 arrangement on a Bruker Ac 400 MHz spectrometer. Number of carbons and their chemical shifts were found to back the structure of the synthesized compounds. 13C NMR spectra affirmed the structures of Compound I on the premise of taking after signals: flag for nitrile carbon at near 120 δ ppm. Flag for carbonyl carbon of acridone was watched at 170-190 δ ppm (Figures 5-12).
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
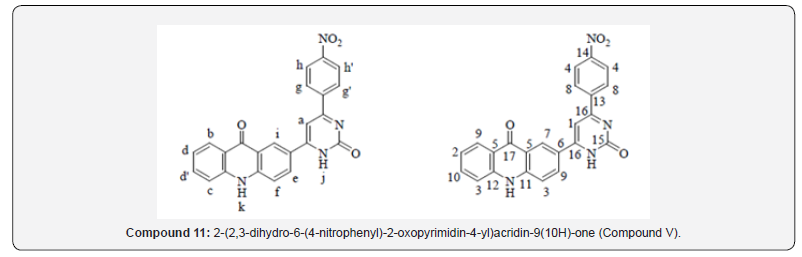
General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
For Compound 4
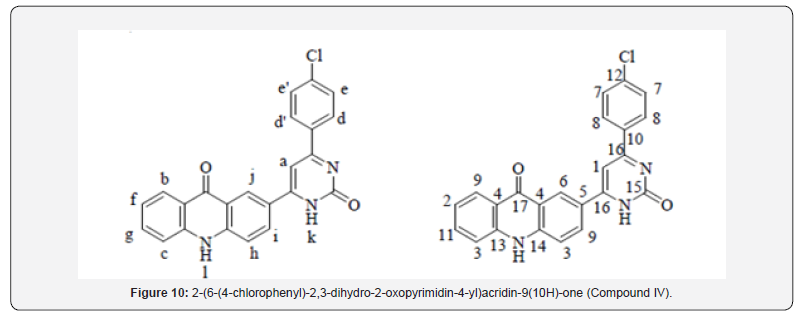
A blend of middle of the road III-1 to III-20 (0.01 mol), urea (0.015 mol) and KOH (0.5 g) was refluxed in ethanol (20 mL) for 4-6 hr. The advance of the response was monitored upon completion of the response, response blend was poured into pulverized ice, and the isolated item was sifted, dried, and recrystallized from DMF: MeOH (1:1) to managed last product (Compound IV and V) (Compound 10).
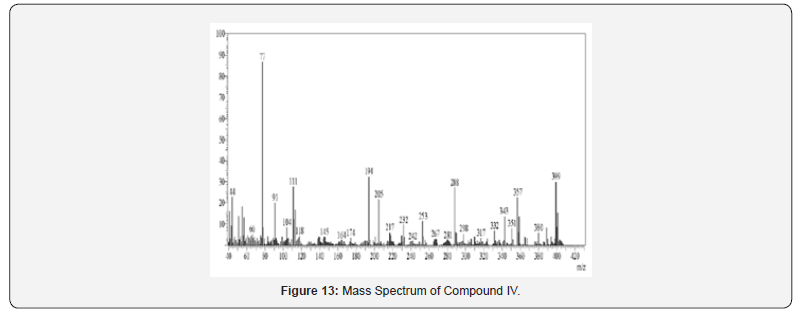
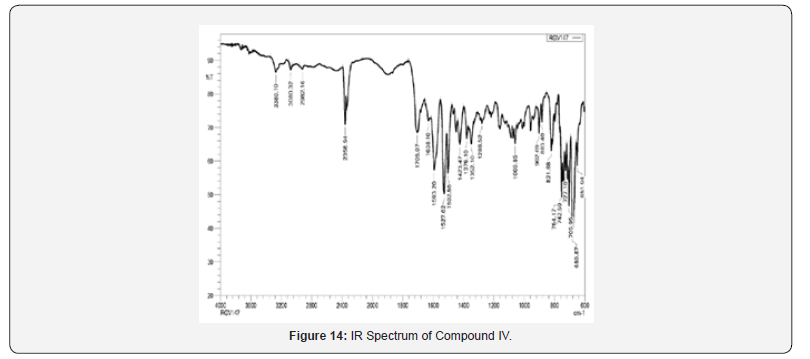
Yield: 69%; mp>300 ºC; IR (cm-1): 3380 (-NH extending), 30802, 2982 (C-H stretching of fragrant ring), 1705 (C=O extending of carbonyl gather), 1638 (C=N stretching), 1593, 1527, 1423 (C=C extending of fragrant ring), 821 (C-H twisting of p-di-substituted fragrant ring), 754 (C-H bowing of o-di-substituted fragrant ring), 680 (C-Clstrtetching); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 7.383-7.463 (m, 3H, Habc), 7.579-7.595 (d, 4H, Hdd’ee’, J = 6.4 Hz), 7.759-7.933 (m, 4H, Hfghi), 8.079 (s, 1H, Hj), 9.069 (s, 1H, Hk), 12.130 (s, 1H, Hl); 13C NMR (DMSO-d6, 400 MHz) δ ppm: 106.10,116.25, 118.26, 122.46, 124.38, 126.49, 129.26, 129.98, 130.38, 130.65, 133.26, 136.41, 142.22, 142.65, 160.48, 165.44, 180.43; MS: m/z 399; Butt-centric. Calcd for C23H14ClN3O2: C, 69.09; H, 3.53; N, 10.51%; Found: C, 69.28; H, 3.65; N, 10.73%.
For Compound 5
Yield: (Compound 11)65%; mp 278 ºC; IR (cm-1): 3341 (-NH extending), 3022, 2970 (C-H stretching of fragrant ring), 1717 (C=O extending of carbonyl gather), 1628 (C=N stretching), 1587, 1487, 1433 (C=C extending of fragrant ring), 1506 (C-NO2 extending), 1338 (C-NO2 bowing), 812 (C-H bowing of p-di-substituted fragrant ring), 756 (C-H twisting of o-di-substituted fragrant ring); 1H NMR (DMSO-d6, 400 MHz) δ ppm: 7.580-7.759 (t, 3H, Habc), 7.941-7.989 (t, 2H, Hdd’, J = 9.6 Hz), 8.281-8.300 (d, 1H, He, J = 7.6 Hz), 8.483-8.507 (d, 1H, Hf, J = 9.6 Hz), 8.887-9.150 (m, 4H, Hgg’hh’), 9.252 (s, 1H, Hello there), 9.341 (s, 1H, Hj), 12.136 (s,1H, Hk); 13C NMR (DMSO-d6, 400 MHz) δ ppm: 105.20, 114.17, 118.10, 123.87, 124.68, 126.25, 128.16, 129.48, 129.77, 139.02, 146.10, 146.41, 148.72, 154.25, 159.31, 165.20, 180.12; MS: m/z 410; Butt-centric. Calcd for C23H14N4O4: C, 67.31; H, 3.44; N, 13.65%; Found: C, 67.48; H, 3.60; N, 13.73%.
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Spectral discussion
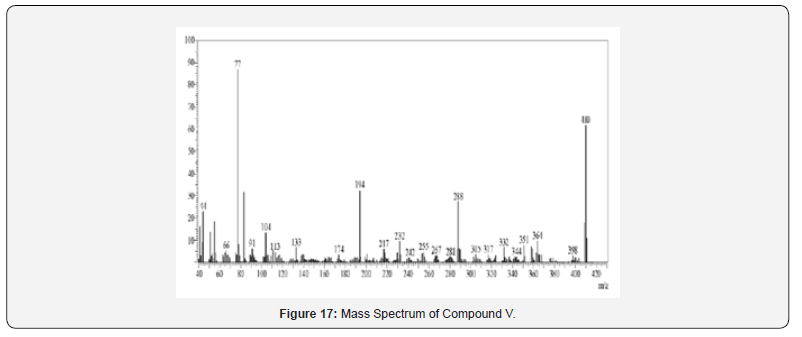
Mass Spectral Study
Mass spectra were recorded on Shimadzu GC-MS-QP-2010 show utilizing Direct Injection Test method. Orderly fracture design and atomic particle peak was watched in understanding with atomic weight of compound.
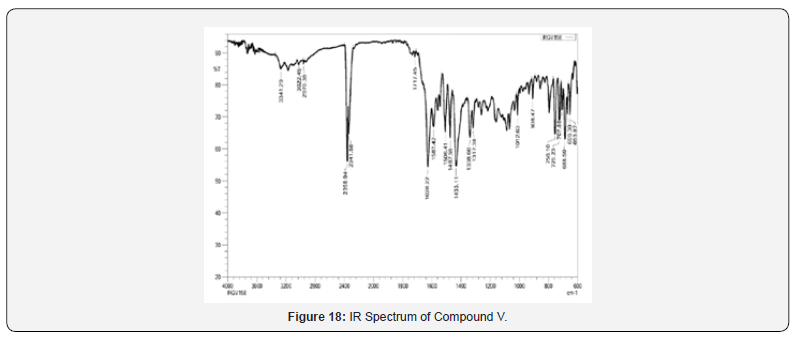
IR Spectral Study
IR spectra were recorded on IRAffinity-1S FTIR Spectrophotometer- Shimadzu using ‘neat’ test. Different useful bunches display in atom were distinguished by characteristic recurrence gotten for them. For Compound IV and V, confirmatory bands for –NH extending watched at 3380-3241 cm-1, corroborative groups forcarbonyl of acridones watched at 1717-1705 cm-1, corroborative groups for C=N stretching watched at 1638-1628 cm-1, which proposed arrangement of desired products Compound IV and V.
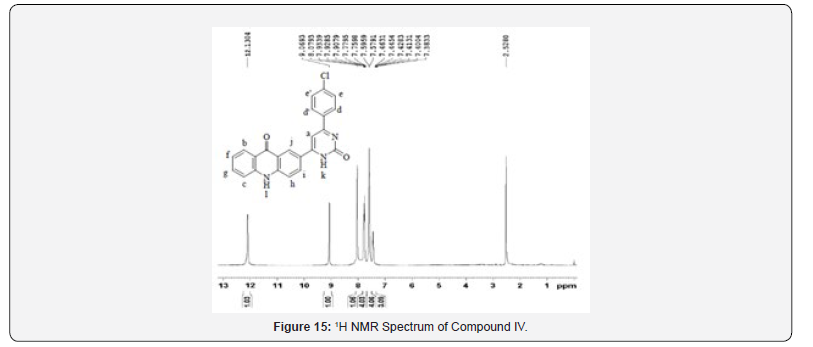
1H NMR Spectral Study
1H NMR spectra were recorded in DMSO-d6 arrangement on a Bruker Ac 400 MHz spectrometer utilizing TMS as an inner standard. Number of protons and their chemical shifts were found to back the structure of the synthesized compounds. 1H NMR spectra affirmed the structures of Compound IV and V on the premise of following signals: a singlet for –NH of pyrimidone ring at 9.063- 9.341 δ ppm of, a singlet for –NH of acridones ring at close 12 δ ppm. The fragrant ring protons and J value agreed with substitution design on phenyl ring.
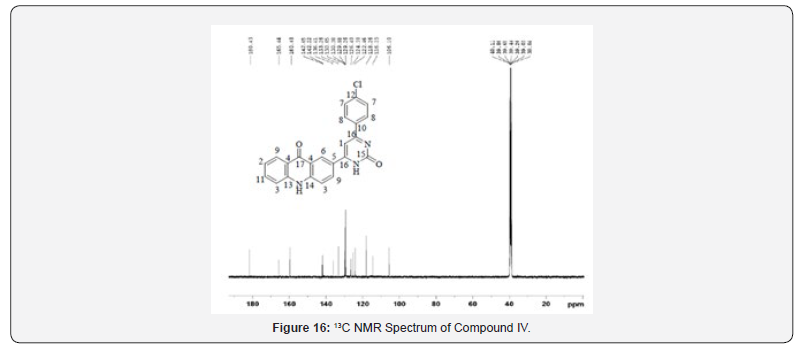
13C NMR Spectral Study
13C NMR spectra were recorded in DMSO-d6 arrangement on a Bruker Ac 400 MHz spectrometer. Number of carbons and their chemical shifts were found to bolster the structure of the synthesized compounds. 13C NMR spectra affirmed the structures of Compound IV and V on the premise of taking after signals: flag for carbonyl carbon of pyrimidone ring was watched at close 160 δ ppm. Flag for carbonyl carbon of acridone was watched at 170- 190 δ ppm. The fragrant ring carbon were found to be in understanding with substitution design on phenyl ring (Figures 13-20).
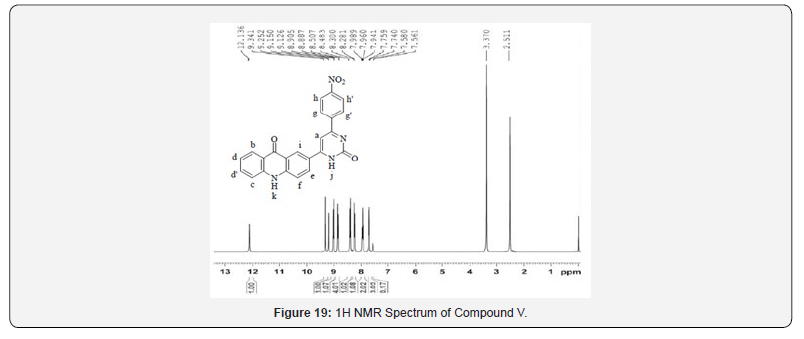
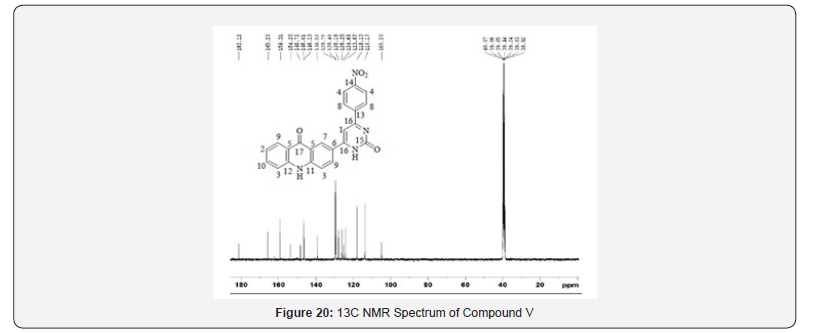
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Anti-Bacterial Activity
All the compounds synthesized within the display examination were screened for their against- bacterial movement by Container plate Strategy. Antibacterial exercises were tried on supplement medium against, Staphylococcus aureus, and Escherchia coli which are agent sorts of gram positive and gram-negative living beings separately. The antibacterial movement of the compounds was evaluated by disc-diffusion strategy [9].
Preparation of Discs
Plates of 6-7 mm in distance across were punched from No: 1 Whattmann channel paper with sterile stopper borer of same estimate. These plates were sterilized by keeping in broiler at 1400C for 60 minutes. At that point standard and test arrangements were included to each plate and plates were air-dried.
Method of Testing
The sterilized media was cooled to 450C with delicate shaking to bring approximately uniform cooling and after that immunized with 18-24 hrs ancient culture beneath aseptic conditionsblended well by delicate shaking. This was poured into sterile Petri dishes (appropriately labelled) and permitted the medium to set. After hardening all the Petri dishes were exchanged to laminar stream unit. At that point the circles which were already arranged were carefully kept on the set media by utilizing sterilized forceps. These Petri dishes were kept because it is for one-hour dissemination at room temperature and after that for brooding at 370C for 24 hours in a hatchery. The degree breadth of restraint after 24 hours was measured as the zone of restraint in millimeters [10] (Table 2).
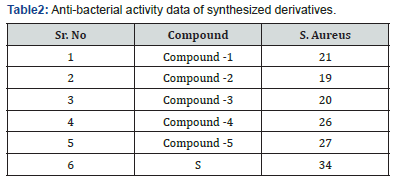
Zone of Inhibition of Synthesized Compounds Against Bacteria
Note: 0-15 mm poor activity, 15-25 mm moderate activity, 25 above good. Standard(S) = Ciprofloxacin (Figure 21)
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
Summary and Conclusion
Acridone is an organic compound based on the acridine skeleton, with a carbonyl group at the 9 position. The synthesized compounds are characterized as a straight tricyclic ring having nitrogen at ten (10th) position and two benzene rings are joined symmetrically at both hand sides keeping pyridine ring within the center. Organic exercises related with acridone subsidiaries incited us to synthesize heterocycles of medicinal intrigued in an arrange to encourage the scope of these compounds. The compound- 1 containing C6H5 at R position showed moderate anti-bacterial activity whereas, .The compounds-2 and 3 consisting of 4-CH3C6H4 and 4-FC6H4 at R position also showed moderate activity but The compounds-4 and 5 4-ClC6H4 and 4-NO2C6H4 showed very good antibacterial activity and is nearby comparable with the standard sample of Ciprofloxacin.
- Research Article
- Abstract
- Introduction
- Methodology
- Result and Discussion
- Spectral Discussion
- General Procedure for the Synthesis of 2-amino-6- (9,10-dihydro-9-oxoacridin- 2-yl)-4-aryl pyridine-3- carbonitriles (Compound II)
- Spectral Discussion
- General Procedure for the Synthesis of 2-(2,3-dihydro-2-oxo-6-aryl-4-yl)acridin-9(10H)-one (Compound IV and V)
- Spectral discussion
- Anti-Bacterial Activity
- Summary and Conclusion
- References
References
- Hopper DW, Crombie AL, Clemens JJ, Kwon S (2009) Progress in Heterocyclic Chemistry. In Gordon W G, John A J(Eds) 1st Edition, Elsevier p. 21.
- Manlove A, Groziak MP (2009) Progress in Heterocyclic Chemistry. In Gordon W G, John A J(Eds), 1st Elsevier 21.
- Ju Y, Varma RS (2005) Aqueous N-Heterocyclization of Primary Amines and Hydrazines with Dihalides: Microwave-Assisted Syntheses of N-Azacycloalkanes, Isoindole, Pyrazole, Pyrazolidine, and Phthalazine Derivatives. J Org Chem 71: 135.
- Ju Y, Kumar D, Varma R S (2006) Revisiting Nucleophilic Substitution Reactions: Microwave-Assisted Synthesis of Azides, Thiocyanates, and Sulfones in an Aqueous Medium. J Org Chem 71: 6697.
- Lokhande P, Waghamare B, Sakate S (2005) Regioselective one-pot synthesis of 3,5-diarylpyrazoles. Indian journal of chemistry section 44: 2338.
- Reddy GJ, Manjula D, Rao KS, Khalilullah M, Latha D (2005) Indian journal of chemistry section B 44: 2412.
- Zificsak C A, Hlasta D J (2004) Tetrahedron 60: 8991.
- Haino, T, Tanaka M, Ideta K, Kubo K, Mori A, et al. (2004) Tetrahedron Letters 45: 2277.
- García Valverde M, Torroba T (2005) Sulfur-Nitrogen Heterocycles. Molecules 10: 318.
- Katritzky AR, Rees CW (1984) Comprehensive Heterocyclic Chemistry (Ed.) Pergamon Press, Oxford, 2 UK.






























