Synthesis and Characterization of New Biologically Active Pyrrolo[2,3-b]Pyridine Scaffolds
Farid M Sroor*
Organometallic and Organometalloid Chemistry Department, National Research Centre, Egypt
Submission: August 04, 2019; Published: September 16, 2019
*Corresponding author: Organometallic and Organometalloid Chemistry Department, National Research Centre, 12622 Cairo, Egypt
How to cite this article: Farid M Sroor. Synthesis and Characterization of New Biologically Active Pyrrolo[2,3-b]Pyridine Scaffolds. Organic & Medicinal Chem IJ. 2019; 9(1): 555752 DOI: 10.19080/OMCIJ.2019.09.555752
Abstract
The 4-amino-1-(2,4-dichlorophenyl)-3-(3,4-dimethoxyphenyl)-6-phenyl-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile, 8 and 4-amino-6-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-(3,4-dimethoxyphenyl)-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile, 9 were prepared and fully characterized by reaction of the new 2-amino-1-(2,4-dichlorophenyl)-4-(3,4-dimethoxyphenyl)-1H-pyrrole-3-carbonitrile 4 with 2-arylidenemalononitriles 6 and 7 in ethanol and the presence of piperidine (1 mL) to afford 8 and 9 in excellent yield 87% and 91%, respectively.
Keywords: Heterocyclic Synthesis Pyrroles Pyrrolopyridine Benzylidinemalononitrile
Introduction
Heterocyclic system with pyrrolopyridine nucleus is an important class of organic chemistry that covers several pharmacologically active compounds which can be synthesized in the laboratory as well as obtained from the natural sources [1]. The pyrrolopryridines are present in the molecular structure of various biologically active compounds such as vemurafenib, pexidartinib, Plexxikon, Genentech famitinib, peficitinib, Antalarmin, etc. [2]. They are displaying interesting chemical reactions and important biological actions such as antibacterial [3], antimycobacterial [4], anti-inflammatory [5], antifungal [6], antiparkinson’s [7], antitumor [8], antiproliferative [9], antiviral [10], muscarinic antagonist [11], antimicrobial [12], anticancer [13-15]. In light of the above all, it was the target to design and synthesize the expected biologically active structures of pyrrolo[2,3-b]pyridines 8 and 9 by combine the new 2-amino-1-(2,4-dichlorophenyl)-4-(3,4-dimethoxyphenyl)-1H-pyrrole-3-carbonitrile 4 and 2 arylidenemalononitriles 6 and 7.
An essential component of the search for new leads in drug designing program is the synthesis of molecules, which are novel still resembling known biologically active molecules by virtue of the presence of some critical structural features. Moreover, the nature and position of the substituents are important factors toward significantly affect the biological actions [16,17]. Phenolic and poly-phenolic as well as the EDGs such as OCH3 is the key factors to expose the activity of the molecule [18-20].
Hence, I synthesized the title compounds with a range of alkoxy substituents such as OCH3 groups at 3,4- positions of thephenyl group on the side of the pyrrole ring; besides, an electron-withdrawing Cl groups were also introduced on the phenyl group which bonded with the pyrrole nitrogen atom at positions 2,4- to improve the activity. Keeping in view the applications of the fused heterocycles like pyrrolopyridine derivatives and in continuation of the previous work [21-25]. I herein report the synthesis and characterization of new 4-amino-1-(2,4-dichlorophenyl)-3-(3,4-dimethoxyphenyl)-6-phenyl-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile 8 and 4-amino-6-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-3-(3,4-dimethoxyphenyl)-1H-pyrrolo[2,3-b]pyridine-5-carbonitrile 9.
Experimental
As illustrated in Scheme 1, the phenacyl bromide 2 was prepared by reaction of 3,4-dimethoxy acetophenone with N-brmosuccinimide in acetonitrile and the presence of p-toluenesufonic acid as a catalyst as reported in literature [26]. The compound 2 reacted with 2,4-dichloroaniline in ethanol and NaHCO3 at 70 °C to give the new α-aminoketone 3. The 2-aminopyrrolo-3-carbonitrile 4 was synthesized by reaction of 3 with malononitrile in NaOEt/EtOH. The 2-arylidenemalononitriles 6 and 7 were prepared by condensation of benzaldehyde or p-cholorobenzaldehyde with malononitrile in the presence of NaOH/EtOH.
The target compounds 8 and 9 were prepared by cyclocondensation of the 2-aminopyrrolo-3-carbonitrile 4 with the 2-arylidenemalononitriles 6 and 7, respectively, in the presence of drops of piperidine and refluxing ethanol followed by treatingthe products with crushed ice/dilute HCl. The compounds 8 and 9 have been crystallized from ethanol in excellent yield 87% and 91%, respectively. The structures of all new compounds prepared in this paper have been confirmed by their spectral data (Scheme 1).

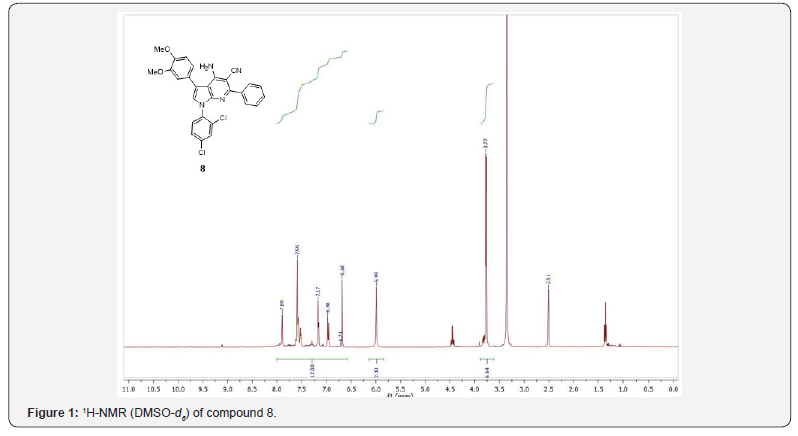
The IR spectra displayed the presence of NH2, CN, and C=N absorption bands in the 3322–3463, 2172 and 1569 cm-1 regions, respectively, as well as the presence of C=O bands in the 1705 cm-1 for compounds 8 and 9. The 1H-NMR spectrum in DMSO-d6 of compound 8 showed the NH2 protons appears at 5.99 ppm and the expected aromatic signals and the pyrrole CH appears in the rang 6.60 – 7.92 ppm, while the protons of methyl groups appears at 3.77 pm, as shown in figure 1.
Conclusion
Pyrrolopyridine and its derivatives have become important compounds due to their applications in medicinal and natural products chemistry. Pyrrolopyridine synthesis by reacting of 2-amino-pyrrole-3-carbonitrile with 2-arylidenemalononitriles as one of the key components became very demanding since it enables the generation of diverse range of libraries of pyrrolopyridine derivatives. As a part of this initiative, herein there is two of fused pyrrolo[2,3-b]pyridine derivatives have prepared as expected biologically active compounds. The synthesized compounds with alkoxy substituents such as OCH3 groups at 3,4- positions of the phenyl group on the side of the pyrrole ring, besides, an electronwithdrawing Cl groups were also introduced on the phenyl group which bonded with the pyrrole nitrogen atom at positions 2,4- to improve the activity.
References
- Merour J Y, Joseph B (2001) Synthesis and Reactivity of 7-Azaindoles (1H-Pyrrolo(2,3-b)pyridine). Current Org Chem 5(5): 471 – 506.
- Flaherty KT, Puzanov I, Kim KB, Antoni Ribas , Grant A McArthur, et al. (2010) Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med 363:809-819.
- Steven DP, Christine MB, Barbara DF, Raul MG, Dennis JH, et al. (2006) Antibacterial activity of pyrrolopyridine-substituted oxazolidinones: synthesis and in vitro SAR of various C-5 acetamide replacements. Bioorg Med Chem Lett 16: 4537–4542.
- Abhijit DK, Colin C, Baojie W, Scott F, Lise-Lotte G (2011) Synthesis and antimycobacterial activities of non-purine analogs of 6-aryl-9-benzylpurines: Imidazopyridines, pyrrolopyridines, benzimidazoles, and indoles. Bioorg Med Chem 19: 3483–3491.
- Fatahala SS, Hasabelnaby Sh, Goudah A, Mahmoud GH, Abd El Hameed RH (2017) Pyrrole and Fused Pyrrole Compounds with Bioactivity against Inflammatory Mediators. Molecules 22: 461 – 479.
- Han WB, Zhang AH, Deng XZ, Lei X, Tan RX (2016) Curindolizine, an Anti-Inflammatory Agent Assembled via Michael Addition of Pyrrole Alkaloids Inside Fungal Cells. Org Lett 18: 1816–1819.
- Goodfellow VS, Loweth CJ, Ravula SB, et al (2013) Discovery, synthesis, and characterization of an orally bioavailable, brain penetrant inhibitor of mixed lineage kinase 3. J Med Chem 56: 8032-8048.
- Cincinelli R, Musso L, Merlini L, et al. (2014) 7-Azaindole-1-carboxamides as a new class of PARP-1 inhibitors. Bioorg Med Chem 22: 1089-103.
- Narva S, Chitti S. Bala BR, et al (2016) Synthesis and biological evaluation of pyrrolo[2,3-b]pyridine analogues as antiproliferative agents and their interaction with calf thymus DNA. Eur J Med Chem 114:220-231.
- Schering Corp, (2009) Preparation of 2,3-substituted azaindole derivatives for treating viral infections.
- Blass B. (2015) Pyrrolopyridine or Pyrazolopyridine Derivatives. ACS Med Chem Lett 6(7): 726–728.
- Mohamed MS, Rashad AE, Zaki MEA, Fatahala SS (2005) Synthesis and antimicrobial screening of some fused heterocyclic pyrroles. Acta Pharm 55: 237–249.
- Hong S, Lee S, Kim B, et al (2010) Discovery of new azaindole-based PI3Kα inhibitors: Apoptotic and antiangiogenic effect on cancer cells. Bioorg Med Chem Lett 20: 7212-7215.
- El Gamal MI, Oh C H (2012) Design and synthesis of an anticancer diarylurea derivative with multiple-kinase inhibitory effect. Bull Korean Chem Soc 33:1571-1576.
- Hong S, Kim J, Seo JH, et al (2012) Design, synthesis, and evaluation of 3,5- disubstituted 7-azaindoles as Trk inhibitors with anticancer and antiangiogenic activities. J Med Chem 55: 5337-5349.
- Ahmad Sh, Alam O, Naim MJ, Shaquiquzzaman M, et al. (2018) Pyrrole: An insight into recent pharmacological advances with structure activity relationship. Eur J of Med Chem 157:527 – 561.
- Karthikeyan SV, Perumal S, Shetty KA, Yogeeswari, P, Sriram D (2009) A microwave-assisted facile regioselective Fischer indole synthesis and antitubercular evaluation of novel 2-aryl-3,4-dihydro-2H-thieno[3,2-b]indoles. Bioorg Med Chem Lett 19: 3006.
- Bandgar BP, Gawande SS, Bodade RG, Gawande NM, Khobragade CN (2009) Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg Med Chem 17: 8168.
- Lakshmi NV, Thirumurugan P, Noorulla KM, Perumal PT (2010) InCl3 mediated one-pot multicomponent synthesis, anti-microbial, antioxidant and anticancer evaluation of 3-pyranyl indole derivatives. Bioorg Med Chem Lett 20: 5054.
- Jung HA, Jin SE, Choi RJ, Kim DH, Kim YS, et al. (2010) Life Sci 87:420.
- Khatab TK, El-Bayouki KAM, Basyouni WM, Sroor FMA (2013) An Efficient Synthesis of Biopertinent Dihydropyrimidine (thi) one Derivatives via Three-component One-pot Synthesis Catalyzed by Tetrachlorosilane. Egypt J Chem 56:291–305.
- Sroor FM, Khatab TK, Basyouni WM, El-Bayouki KAM (2019) Synthesis and molecular docking studies of some new thiosemicarbazone derivatives as HCV polymerase inhibitors. Synth comm 49: 1444–1456.
- Sroor FM, Hrib CG, Hilfert L, Busse S, Edelmann FT (2015) Synthesis and catalytic activity of homoleptic lanthanide-tris(cyclopropylethinyl)amidinates. New J Chem 39:7595 – 7601.
- Sroor FM, Hrib CG, Hilfert L, Jones PG, Edelmann FT (2015) anthanide(III)-bis(cyclopropylethinylamidinates): Synthesis, structure, and catalytic activity. J Organomet Chem 785:1–10.
- Sroor FM, Hrib CG, Hilfert L, Hartenstein L, Roesky Pw, et al. (2015) Synthesis and structural characterization of new bis(alkynylamidinato)lanthanide(III)-amides. J Organomet Chem 799-800: 160–165.
- Lee JC, Bae UH, Chang S K (2003) Efficient α-Halogenation of Carbonyl Compounds by N-Bromosuccinimide and N-Chlorosuccinimde. Bull Korean Chem Soc 24(4):407–408.






























