Synthesis and Cytotoxicity Evaluation of Some New 4-(4oxo-4H-Quinazoline-3yl)- Thiobenzoic Acid-S-(1-H-Benzimidazole-2-Yl) Ester Derivates
Shirin Banitalebi Dehkordi1, Farshid Hassanzadeh1*, Marzieh Rahmani Khajouei2, Hojat Sadeghi1 and Nasim Dana3
1 Department of Medicinal Chemistry, Isfahan University of Medical Sciences, Iran
2Isfahan Pharmaceutical Sciences Research Center, Isfahan, Iran
3Physiology Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
Submission: August 16, 2019; Published: September 11, 2019
*Corresponding author: Farshid Hassanzadeh, Department of Medicinal Chemistry, School of Pharmacy and Pharmaceutical Science, Isfahan University of Medical Sciences, Isfahan, Iran
How to cite this article: Shirin Banitalebi Dehkordi, Farshid Hassanzadeh, Marzieh Rahmani Khajouei, Hojat Sadeghi, Nasim Dana. Synthesis and Cytotoxicity Evaluation of Some New 4-(4oxo-4H-Quinazoline-3yl)- Thiobenzoic Acid-S-(1-H-Benzimidazole-2-Yl) Ester Derivates. Organic & Medicinal Chem IJ. 2019; 8(5): 555750. DOI: 10.19080/OMCIJ.2019.08.555750
Abstract
Quinazolines are a group of heterocyclic compounds that have different biological activities such as cytotoxicity, anti-bacterial, anti-fungal. benzimidazoles on the other hand are also a group of heterocyclic compounds with anti-tumor, anti-virus, anti-fungal and anti-inflammatory effects. Due to significant cytotoxic effects of both quinazoline and benzimidazole derivatives , in this work a group of quinazolinone-benzimidazole hybrids were prepared. The structures of synthesized compounds were confirmed by IR and 1H-NMR. Cytotoxic activity of the compounds was evaluated at 1, 10, and 100 μM concentrations against MCF-7 and HT-29 cell lines using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay. The results show that compound 9d has highest cytotoxic activity against both MCF-7 and HT-29 cell lines.
Keywords: Quinazoline Imidazole Cytotoxic
Introduction
Cancer is the leading cause of death in the developed world [1]. Despite discovery of numerous drugs for treating cancer and lots of progresses, due to the toxicity and drug resistance associated with the common treatments of cancer continued efforts to discover new anticancer drugs is still a very important issue among medicinal chemists and the efforts to develop new leading compounds is of great importance [2].
In order to discover new target drugs, a new approach; combining two or more pharmacophores can be effective ,as there’s one single molecule with different mechanism of actions that can also eliminate resistance problems and may reduce the side-effects [3]. Quinazolines are a group of heterocyclic compounds and have a broad spectrum of biological effects including sedative [4], anti-compulsive [4-6], anti-inflammatory [4,7], antitumor [4,8], antibacterial [4,9-11], antifungal[5,6], anti-Tuberclosis [6,8,11], anti-malaria [10], anti-virus [5,7], anti-HIV [4,9-11], and lowering of blood lipid [12-14]. iproqualone,Pazosin,Doxazosin,Febrifungine,Evodiamine,Lutonin A are some examples of synthetic and natural Quinazolines with good therapeutic effect [15].
Thymitaq and lapatinib are two examples of Quinazoline structured anticancer drugs. Lapatinib has tyrosin kinase(dual kinase) inhibition activity, it also inhibits EGFR(ErbB1) and HER2(ErbB2) by reversibly binding to tyrosine kinase [16-18]. Anticancer effect of quinazolinone derivatives are mainly attributed to their multi target activities including inhibition of topoisomerase I, EGFR tyrosine kinase and dihydrofolate reductase inhibition [19-21].
Benzimidazoles are an important class of heterocyclic compounds with a wide variety of effects such as anticancer,anti-inflammatory,antibiotics,antiviral,anti-fungal,anti-HIV,antihistamine, antioxidant and lowering of blood pressure [22]. Some well-known anti-fungal drugs such as clotrimazol, miconazole and ketoconazole have imidazole structures [23]. According to the biological activities and cytotoxic effects of both Quinazolines and Benzimidazoles, in this study we try to combine these two pharmacophores in one single molecule in order to reach new compounds with better cytotoxic effects and hopefully less side-effects [24].
Materials and Method Instrumentation
All initial materials, solvents and regents were prepared from commercial suppliers such as Merck (Germany) and Aldrich (USA). By using different solvents, the purity of purchased compounds was proved via thin layer chromatography (TLC). 1HNMR spectra of synthetized compounds were determined by (Bruker 400 MHz, Germany) spectrometer, and chemical shifts were shown as δ (ppm) with tetramethyl silane (TMS) as internal standard. Melting points were recorded by utilizing electro thermal melting point analyzer apparatus (IA 9000, UK) and are uncorrected. TheIR spectra were obtained on a Shimadzu 470 spectrophotometer (potassium bromide disks). All cell lines were bought from Pasteur Institute of Iran
Prepration of Compounds
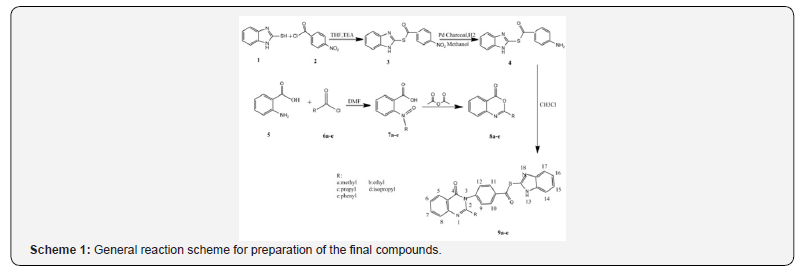
4-(4-Oxo-4H-quinazolin-3-yl)-thiobenzoic acid S-(1Hbenzoimidazol- 2-yl)ester derivates(9a-e) were prepared through two separate reactions one to produce the Benz imidazol 4 and the other Benz oxazinone derivates 8a-e,Respectively. In the first part,4-Nitro-thiobenzoic acid S-(1H-benzoimidazol-2-yl) ester( 3) (Scheme 1) was prepared through addition of 4-Nitro benzoyl chloride 2 to Benz imidazol 2-thiol 1 to give the Nitro derivate 3 which was reduced to S-1H-benzoimidazol-2-yl 4-aminobenzothioate 4 using palladium-Charcoal powder [25- 27]. In the second part a group of Benzoxazinone derivatives with different substituents at position 2 were synthesized from the reaction of 5 with different acyl chlorides [27]. Finally, the primary amine 4 was reacted with the benz oxazinones 8a-8e to produce the target compounds 9a-9e as presented in Scheme1.
Cell Culture Conditions
MCF7 (human breast adenocarcinoma cell line) cells were maintained at 37°C in a humidified atmosphere (90%) containing 5% CO2.MCF-7 cell line was cultured in Roswell Park Memorial Institute medium (RPMI) with 5% v/v fetal bovine serum, 100 U/ml penicillin, and 100 mg/mL streptomycin. The medium was changed every two to three days and sub- cultured when the cell population density reached to 70–80% confluence. Cells were seeded at an appropriate density according to each experimental design (), and HT-29 (human colon adenocarcinoma cell line) cells were maintained at 37°C in a humidified atmosphere (90%) containing 5% CO2. HT-29 cell line was cultured in Dulbecco’s modified Eagle’s medium (DMEM-F12) with 10% v/v fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The medium was changed every two to three days and sub-cultured when the cell population density reached to 70-80% confluence, Cells were seeded at an appropriate density according to each experimental design.
Cytotoxicity Assay
HT29 and MCF-7 cells were seeded on 96-well tissue culture plates (15 × 103 cells/well) and incubated overnight. Cells were treated with different concentrations of the derivatives for 48h. Then the medium was removed and the MTT substrate was prepared in a physiologically balanced solution (PBS), added to cells in culture, concentration of 0.5 mg/ml, and incubated for 1 to 4 h. The formazan crystals were solubilized in dimethyl sulfoxide (DMSO) and the quantity of formazan (presumably directly proportional to the number of viable cells) was measured by recording changes in absorbance at 570 nm using a plate reading spectrophotometer. Cell viability was calculated using following formula: IC50 values were calculated by plotting the cell viability against compound concentrations All statistical analyzes were performed with the SPSS Statistics 24.
Statistical Analysis
For analysis of data One-way analysis of variance (ANOVA) followed by LSD post hoc test were used. All results were expressed as mean ± SEM. P < 0.05 was considered statistically significant.
Results
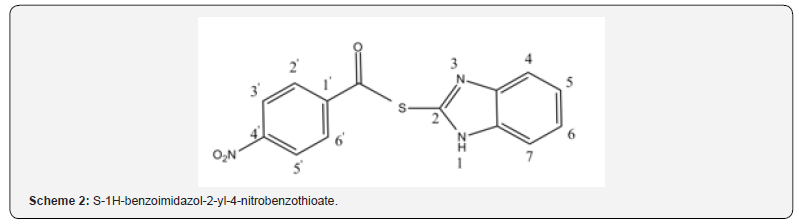
Procedure for the Preparation S-1H-benzoimidazol-2- yl-4-nitrobenzothioate [3]
The mixture of 4-nitro benzoylChloride (9.6g,50mmol) in Dimethylformamide(DMF)(5ml) was added to a mixture of 2-mercaptobenzimidazol(6g,40mmol) in DMF(5ml)and the rest of DMF was added dropwise, the mixture was stirred for 24 hours at room temperature. Water(200 ml) was added to the solution. The product was filtered and the separated cake was washed with water and dried to give the final product 3 as a yellow powder, Yield: 85% ,mp:207- 210˚C lit. mp 206-208˚C ref (14)1H NMR (400 MHZ: d6-DMSO: 8.298-8.371(2H, m, H-C3’,H- C4’),8.012(2H ,dd, J=6.8 Hz, j=2 Hz,H-C2’,H-C5’), 7.7402(1H,d,J=7.8 Hz,H-C6),7.310- 7.391(2H,m,H-C5H-C6),7.257(1H, dd, J=8.8 Hz, j=1.6 Hz,H-C7)( Scheme 2).
Procedure for the Preparation of S-1H-benzoimidazol- 2-yl 4-aminobenzothioate [4]
A suspension of S-1H-benzoimidazol-2-yl-4-nitrobenzothioate (1g, 3mmol) and palladium-charcoal powder(120mg,1.1mmol) in Anhydrous methanol(20ml,99.8% v/v) was shake for 30 minutes, the air was vacuumed and H2 gas was slowly added to the mixture. The solution was filtered after cooling to remove the catalyst. The solvent was then evaporated under reduced pressure to give the final product S-1H-benzoimidazol-2-yl 4-aminobenzothioate 4 as an orange-yellow powder, yield: 60%, mp: 290-295, lit. mp 292- 293 ref [14,15].
Procedure for the Preparation of Benz oxazinones (8a- 8e)
To a solution of anthranilic acid (1.37g, 10 mmol) in dimethylformamide (DMF) (5 ml) different acyl chlorides 6a-6e (15 mmole) were added drop wise and the resulting solutions were stirred for 3 h. The end of the reactions was determined by TLC. The mixtures were then poured into distilled water and stirred for additional 1 h. Finally, the precipitated products were collected by filtration and washed with water to furnish 7a-7e (16). Each compound of the previous step (7a-7e) (2.5 mmol) was added to acetic anhydride (2 mL) and refluxed at 140 °C until the starting materials 7a-7e were disappeared from TLC. At the end of the reaction the excess of acetic anhydride was removed from the reaction medium under reduced pressure. The resulting products were cooled to give solid mass. Finally, the products were washed with hexane to give Benz oxazinones (8a-8e).
Procedure for the Prepration of 4-(4oxo-4H-quinazoline- 3yl)-thiobenzoic acid-S-(1-H- benzimidazole-2-yl)ester derivates(9a-9e)
A mixture of compound 4 (0.6, 2mmol) and compounds 8a-8e (1mmol) were refluxed for a period of 4-6 hours in glacial aceticacid (10 ml). The reaction progression was investigated using TLC and at the end of the reaction, acetic acid was removed using rotary evaporator to give target compounds 9a-9e.
S - ( 1H-benzoimidazol - 2 - y l ) 4 - ( 2-methyl - 4 - oxoquinazolin-3(4H)-yl)benzothioate(9a)
Pale orange powder, yield: 55%, mp: 112-114°C. IR (KBr, cm-1) vmax =3114(NH), 3081(C- H, Ar), 2982(C-H), 1693(C=O), 1604(C=C) ).1HNMR (400 MHZ: d6-DMSO): 8.4505(1H, d, J8.4 Hz, H-C5),8.323(3H, t, J=6.8 Hz,H-C7,H-C10,H-C11), 8.171(3H, dd, J=6.8 Hz, j=2 Hz, H-C6, H-C9, H-C12), 7.964(1H, dd, J=8 Hz, j=1.6 Hz, H-C17), 7.558(1H, t, J= 8.4 Hz, H-C15), 7.105-7.146(3H, m, H-C8, H-C14, H-C16), 2.125(3H, s, H-C19).
S-(1H-benzoimidazol-2-yl)4-(2-ethyl-4-oxoquinazolin- 3(4H)-yl)benzothioate (9b)
Pale orange powder,yield: 50%, mp: 109-110°C. IR (KBr, cm-1) vmax =3115(NH), 3081(C- H, Ar), 2982(C-H),1693(C=O),1604(C=C). 1HNMR (400 MHZ: d6-DMSO): 8.610(1H, d, J=8.4 Hz, H-C4), 8.438(3H, d, J=8.8 Hz, H-C6, H-C10, H-C11) ,8.283(3H, d, J=8.8 Hz, H-C5, H-C9, H-C12), 8.083(1H, dd, J=8 Hz, j= 1.6 Hz, H-C17), 7.613(1H, t, J=7.6 Hz,H-C15), 7.175- 7.268(3H, m, H-C8, H-C14, H-C16), 2.496(2H, qr, J=7.6 Hz, H-C19), 1.235(3H, t, J=7.2 Hz, H-C20).
S - ( 1 H - b e n z o i m i d a z o l - 2 - y l ) 4 - ( 4 - o x o - 2 - propylquinazolin-3(4H)-yl)benzothioate (9c)
Pale orange powder, yield: 55%, mp: 115-117°C. IR (KBr, cm-1) vmax =3115(NH),3080(C-H, Ar), 2962(C-H), 1688(C=O), 1605(C=C). 1HNMR (400 MHZ: d6-DMSO): 8.5 46(1H, d, J=8.4 Hz, H-C4), 8.376(3H, dd, J=6.8 Hz, j=1.6 Hz, H-C7, H-C10, H-C11) ,8.22(3H, dd, J=6.8 Hz, j=2 Hz, H-C6,H-C9,H-C12), 8.019(1H, dd, J=8 Hz, j=1.6 Hz,H-C17), 7.594(1H, t, J=7.8 Hz, H-C15), 7.163- 7.178(3H, m,H-C6,H-C14,H-C16),2.402 (2H, t, J=7.2 Hz, H-C19), 1.645- 1.737(2H, m, H-C20), 0.979(3H, t, J=7.2 Hz,H-C21).
S-(1H-benzoimidazol-2-yl)4-(2-isopropyl -4- oxoquinazolin-3(4H)-yl)benzothioate (9d)
Pale orange powder, yield: 55%, mp: 120-123°C. IR (KBr, cm-1) vmax =3114(NH),3080(C- H,Ar),2972(CH), 1692(C=O),1606(C=C). 1HNMR (400 MHZ: d6-DMSO): 8.5065(1H, d, J=8.4 Hz, H-C5), 8.331(3H, d, J=8.8 Hz, H-C7, H-C10, H-C11), 8.1755(3H, d, J=8.4Hz, H-C6, H-C9,H-C12), 7.9805(1H, d, J=7.6 Hz, H-C17),7.494(1H, t,J=7.2 Hz,H-C15),7.063-7.137(3H, m, H- C8, H-C14, H-C16), 2.510(1H, s, H-C19), 1.1675(6H, d, J=6.8 Hz, H-C20, H-C21).
S - ( 1 H - b e n z o i m i d a z o l - 2 - y l ) 4 - ( 4 - o x o - 2 - phenylquinazolin-3(4H)-yl)benzothioate (9e)
Pale orange powder, yield: 38%, mp: 134-137°C. IR (KBr, cm-1) vmax=3114(NH), 3085(C- H,Ar),2983(CH), 1693(C=O),1560(C=C). 1HNMR (400 MHZ: d6-DMSO): 8.825(1H, d, J=8.4 Hz, H-C5), 8.44(3H, d, J=8.8 Hz, H-C7, H-C10, H-C11), 8.282(3H, d, J= 8.8 Hz, H-C6, H-C9, H-C12), 8.169(1H, dd, J=8 Hz, j=1.2 Hz,H-C17).

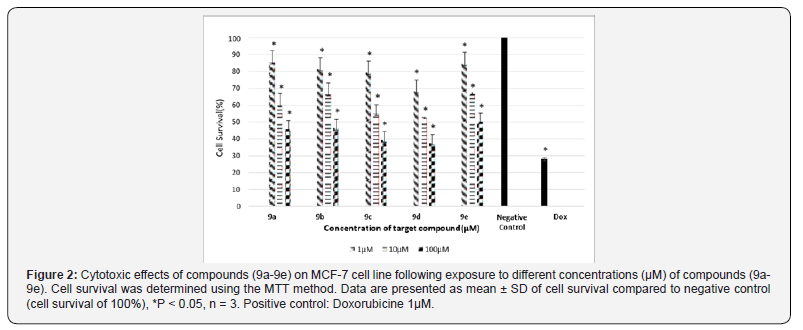
Cytotoxic Effect of Synthesized Compounds
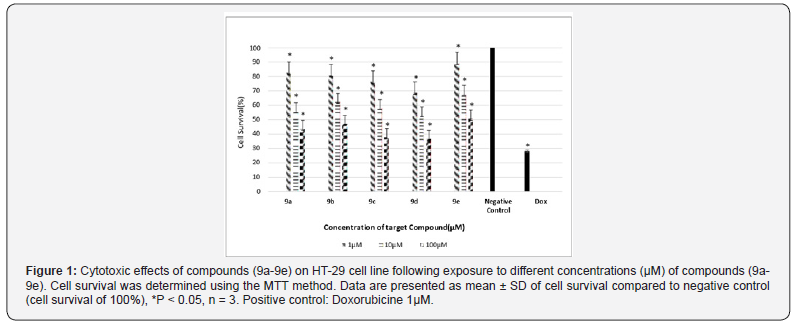
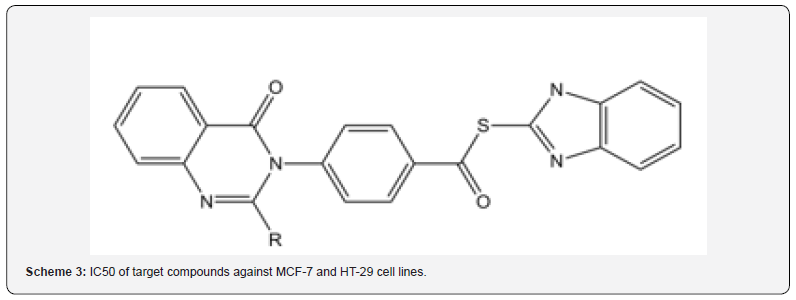
IC50 of target compounds against MCF-7 and HT-29 cell lines are listed in (Table 1) (Scheme 3) (Figures 1 & 2).
Discussion
In this study, we synthesized some new quinazoline derivatives with substitute containing benzimidazole at position 3. Amine bearing benzimidazole moiety [4] was synthesized via 2 steps. In the first step,4-Nitrobenzoyl chloride was added to 2-Mercaptobenzimidazol, the water was used in order to neutralize the acid. In the second step, reduction of the nitro group to amine was carried out using palladium on activated charcoal in anhydrous methanol.
Palladium on activated charcoal also referred as Pd/c is used as catalyst. The metal is supported on activated carbon in order to maximize its surface area and activity and is known to be used for Reductive amination as in our case [17] Benz oxazinones are highly reactive and should be used immediately after preparation. In this study, benz oxazinones were prepared in two steps. In the first part, anthranilic acid was treated with the acyl chlorides to prepare N-acyl anthranilic acids. These compounds could be prepared easily at room temperature via a nucleophilic substitution reaction
This could be because of the high electrophilicity of the carbonyl group next to a powerful electron withdrawing group (Cl) in acyl chloride. In addition, DMF could also facilitate this reaction by removal of hydrogen from the amino group [18]. Subsequent reflux of N-acyl anthranilic acids in acetic anhydride resulted in production of the corresponding benz oxazinones via a dehydrative cyclization mechanism. The excess amount of acetic anhydride was removed immediately to avoid its side reactions with primary amines utilizing in the next step to prevent the production of corresponding amide by product.
Finally, compounds 8a-8e were added to 4 to produce final hybrids via a nucleophilic substitution mechanism. Cytotoxiceffects of the synthesized hybrids were investigated on HT- 29 and MCF-7 by MTT assay. According to the results shown in Table 1, compound 9e bearing aromatic substituents on C2 of the quinazolinone ring showed lowest cytotoxic activity on both cell lines, i.e. IC50s 100 μM , while compounds 9a-9d containing aliphatic substituents on C2 were more active on both cell lines with IC50 values between 25-80 μM. It seems that the presence of electron donating substituents on C2 could be in favor of the activity for these compounds while electron withdrawing groups have opposite effects, perhaps because of un favored electronic effects on the site of action.
Conclusion
In summary, the novel derivatives of quinazolinone with substituted benzimidazole at position 3 were synthesized in several steps and their in vitro cytotoxic activities were evaluated against MCF-7 and HT-29 cell lines. The cytotoxic evaluation of synthesized derivatives on both MCF-7 and HT-29 cell lines represented that compound with phenyl substitute 9e had the lowest cytotoxic activity against both cell lines and compound 9d with isopropyl substitute had the highest potency
References
- Paul K, Sharma A, Luxami V (2014) Synthesis and in vitro antitumor evaluation of primary amine substituted quinazoline linked benzimidazole. Bioorganic & medicinal chemistry letters 24(2): 624-9.
- Kuemmerle J, Jiang S, Zhang H, Sirisoma N, Kasibhatla S, et al. (2008) Discovery of 1-benzoyl-3-cyanopyrrolo[1,2-a]quinolines as a new series of apoptosis inducers using a cell- and caspase-based high-throughput screening assay. Part 1: Structure–activity relationships of the 1- and 3-positions. Bioorg Med Chem Lett 18: 6259-6264.
- Solomon VR, Hu C, Lee H (2010) Design and synthesis of anti-breast cancer agents from 4- Piperazinylquinoline : a hybrid pharmacophore approach. Bioorg MedChem 18(4): 1563- 1572.
- Mhaske SB, Argade NP (2006) The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron 62:9787-9826.
- Gursoy A, Unal B, Karali N, Otuk G (2005) Synthesis,Characterization and Primary Antimicrobial Activity Evaluation of 3-Phenyl-6-methyl-4-(3H)-quinazolinone-2-yl- mercaptoaceticAcid Arylidenehydrazides. Turk J Chem 29: 233-245.
- Mourad AE, Aly AA, Farag HH, Beshr EA (2007) Microwave assited synthesis of triazoloquinazolinones and benzimidazoqinazolinones. Beilstein J Org Chem 3:1-5.
- Zhou Y, Murphy DE, Sun Z, Gregor VE (2004) Novel parallel synthesis of N-(4-oxo-2- substituted-4-Hquinazolin-3-yl)-substituted sulfonamides. Tetrahedron Lett 45: 8049- 8051.
- Dinakaran M, Selvam P, DeClercq E, Sridhar SK (2003) Synthesis, antiviral and cytotoxic activity of 6-bromo-2, 3-disubstituted-4 (3H)-quinazolinones. Biol & Pharm Bull 26: 1278-1282.
- Raghavendra NM, Thampi P, Gurubasavarajaswamy PM, Sriram D (2007) Synthesis and antimicrobial activities of some novel substituted 2-Imidazolyl-N-(4-oxoquinazolin- 3 (4H)-yl)-acetamides. Chem Pharm Bull 55:1615-1619.
- Laddha SS, Wadodkar SG, Meghal SK (2006) Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1: Synthesis, characterization and anti-inflammatory- antimicrobial activity of 6, 8-disubstituted 2-phenyl-3-[substituted-benzothiazo 2-yl]-4 (3H)-quinazolinones. Arkivoc 11: 1-20.
- Pandeya SN, Sriram D, Nath G, De Clercq E (1999) Synthesis, antibacterial, antifungal and anti- HIV evaluation of Schiff and Mannich bases of isatin derivatives with 3-amino-2- methylmercaptoquinazolin-4 (3H)-one. Pharm Acta Helvet 74:11-17.
- Ghabrial SS, Gaber HM (2003) Dipolar cycloaddition reactions with quinazolinones: a new route for the synthesis of several annelated pyrrolo and pyridazinoquinazoline derivatives. Molecules 8: 401-410.
- Refaie FM, Esmat AY, Gawad SMA, Ibrahim AM, Mohamed MA (2005) The antihyperlipidemic activities of 4 (3H) quinazolinone and two halogenated derivatives in rats. Lipids Health Dis 4: 22-33.
- Alagarsamy V, Chitra K, Saravanan G, Solomon VR, Sulthana MT, (2018) An overview of quinazolines: Pharmacological significance and recent developments. European journal of medicinal chemistry 151: 628-85.
- Hameed A, Al Rashida M, Uroos M, Ali SA, Arshia, et al. (2018) Quinazoline and quinazolinone as important medicinal scaffolds: a comparative patent review (2011-2016). Expert opinion on therapeutic patents 28(4): 281-97.
- Jafari E, Khajouei MR, Hassanzadeh F, Hakimelahi GH, Khodarahmi GA (2016) Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Research in Pharmaceutical Sciences 11(1):1-14.
- El Azab AS, Al Omar MA, Abdel Aziz AAM, Abdel Aziz NI, El Sayed MAA, et al. (2010) Design, synthesis and biological evaluation of novel quinazoline derivatives as potential antitumor agents: Molecular docking study. EurJMedChem 45(9): 4188-98.
- Al Omary FAM, Abou zeid LA, Nagi MN, Habib ESE, Abdel Aziz AAM, et al. (2010) Non-classical antifolates. Part 2: Synthesis, biological evaluation, and molecular modeling study of some new 2, 6-substituted-quinazolin-4-ones. Bioorg. Med. Chem 18(8): 2849-63.
- Cruz Lopez O, Conejo García A, Nunez MC, Kimatrai M, Garcia Rubino ME, et al. (2011) Novel substituted quinazolines for potent EGFR tyrosine kinase inhibitors. Curr Med Chem 18(7): 943-963.
- Taliani S, Pugliesi I, Barresi E, Salerno S, Marchand C, et al. (2013) Phenylpyrazolo[1,5- a]quinazolin-5(4H)-one: a suitable scaffold for the development of noncamptothecin topoisomerase I (Top1) inhibitors. J Med Chem 56(18): 7458-7462.
- Al Rashood ST, Aboldahab IA, Nagi MN, Abouzeid LA, Abdel Aziz AA, et al. (2006) Synthesis, dihydrofolate reductase inhibition, antitumor testing, and molecular modeling study of some new 4(3H)-quinazolinone Bioorg Med Chem 14(24): 8608-8621.
- Salahuddin, Shaharyar M, Mazumder A (2017) Benzimidazoles: A biologically active compounds. Arabian Journal of Chemistry 10:S157-S73.
- Fikry R M, Ismail N A , Said S A, Hafez M E (2015) Synthesis and Reaction of Some New Benzimidazole Derivatives. Eur Chem Bull 4: 428-431.
- Hosseinzadeh L, Aliabadi A, Kalantari M, Mostafavi A, Rahmani Khajouei M (2016) Synthesis and cytotoxicity evaluation of some new 6-nitro derivatives of thiazole-containing 4-(3H)- quinazolinone. Res Pharm Sci 11(3): 210-218.
- Shi L, Wu TT, Wang Z, Xue JY, Xu YG (2014) Discovery of N-(2-phenyl-1H- benzo[d]imidazol-5-yl)quinolin-4-amine derivatives as novel VEGFR-2 kinase inhibitors. European journal of medicinal chemistry 84: 698-707.
- Hosseinzadeh L, Aliabadi A, Rahnama M, Sadeghi HMM, Khajouei MR (2017) Synthesis and cytotoxic evaluation of some new 3-(2-(2-phenylthiazol-4-yl) ethyl)-quinazolin-4(3H) one derivatives with potential anticancer effects. Research in pharmaceutical sciences 12(4): 290-8.
- Garcia J, Sorrentino J, Diller EJ, Chapman D, Woydziak ZR (2016) A General Method for Nucleophilic Aromatic Substitution of Aryl Fluorides and Chlorides with Dimethylamine using Hydroxide-Assisted Decomposition of N,N-Dimethylforamide. Synthetic communications 46(5): 475-81.






























