In Silico Approach Towards the Prediction of Drug-Likeness, In Vitro Microbial Investigation and Formation of Dihydropyrrolone Conjugates
Keyur M Pandya1* Bhavesh P Dave2, P S Desai1
1Department of Chemistry, Arts, Science, and Commerce College, Surat, Veer Narmad South Gujrat University, Gujrat, India
2Department of Chemistry, M.B. Patel Science College, Sardar Patel University, Anand, Gujarat, India
Submission: July 22, 2019; Published: August 30, 2019
*Corresponding author: K M Pandya, Deptartment of Chemistry, Arts, Science and Commerce College, Veer Narmad South Gujarat University, Surat, Gujarat, India Organic
How to cite this article: Keyur M Pandya, Bhavesh P Dave, P S Desai. In Silico Approach Towards the Prediction of Drug-Likeness, In Vitro Microbial Investigation and Formation of Dihydropyrrolone Conjugates. Organic & Medicinal Chem IJ. 2019; 8(5): 555748. DOI: 10.19080/OMCIJ.2019.08.555748
Abstract
Benzotriazole and 2-thioxo-1,3,4- Oxadiazole are very important moieties clubbed with other heterocyclic compounds to get better biological activity. After knowing importance and medicinal value of these heterocyclic compounds, we have synthesized a library of 2-substituted-1-(2-(5-((5-5-benzoyl-1H-benzo[d][1,2,3]triazole-1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-5-oxo-2,5-dihydro-1H-pyrrole-3-carboxylic acids from 1H-benzo[d][1,2,3] triazole-5-yl)(phenyl)methanone. Newly synthesized compounds were characterized by 1H NMR, 13C NMR, elemental analysis and mass spectral studies. All the compounds were investigated for them in silico ADME prediction properties, in vitro antibacterial activity against four bacterial strains, antifungal activity against two fungal strains and antimycobacterial activity against H37Rv . All the compounds showed good to moderate activity against the bacterial strain. Among all compounds, 6b and 6f were found to be better antimycobacterial agents compared to standard drug Ciprofloxacin and Pyrazinamide whereas 6a, 6b and 6e were found to be excellent antifungal and antibacterial agent compared standard drugs Clotrimazole and Ciprofloxacin. The synthesized compounds also studied for their in-silico properties and showed good drug-likeness properties.
Keywords: Antibacterial Antifungal Antituberculosis Benzotriazole 1,3,4-Oxadiazoles Dihydropyrrolone
Introduction
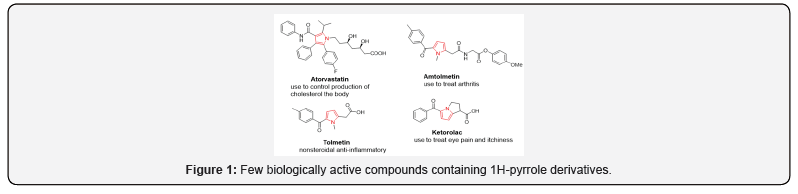
Heterocyclic molecules are cyclic molecules whose ring contains one or more heteroatoms besides carbon atom. The most common heteroatoms are Nitrogen, Oxygen and Sulphur. The heterocyclic compounds containing three to six carbons in the ring are numerous, but those containing five or six atoms are very important in the pharmaceutical world. Pyrroles demonstrate such flexibility (Figure 1) and they are valuable substructure in a collection of pharmaceuticals, involving products potent against HIV [1,2], influenza [3], cytomegalovirus [4], anticancer agents [5,6] and compounds effective against microbiological infections [7-9] e.g. bacterial and fungal. In addition, pyrroles are known as building blocks in the synthesis of alkaloids [10-13] and intermediates like 2,2’- bipyrroles, pyrroles and pigments [14-19]. Structural alteration of the pyrrole ring to create more bioactive molecules has drawn the attention of many researchers. Over the years much effort has been directed towards the development of new strategies for 2-arylpyrrole and oligopyrrole synthesis [20–27].
Continuing interest in the development of the synthetic methodology of dihydropyrrole synthesis provides the impetus to initiate a project designed to develop a new and more expedient route to the formation of pyrrole rings. Dihydropyrroles may be employed as important synthetic intermediates for synthesis of pyrroles. Substituted dihydropyrroles are a vital class of five membered nitrogen-containing heterocycles as they are present in numerous bioactive compounds and pharmaceuticals, such as sibiromycin, [28] anthramycin, [29] serotonin reuptake inhibitor, [30] and thienamycin [31]. Furthermore, dihydropyrroles can be used as versatile synthetic intermediates for the synthesis of natural products [32]. Therefore, considerable efforts have been devoted to the synthesis of these heterocyclic motifs, and numerous synthetic methods have been established.
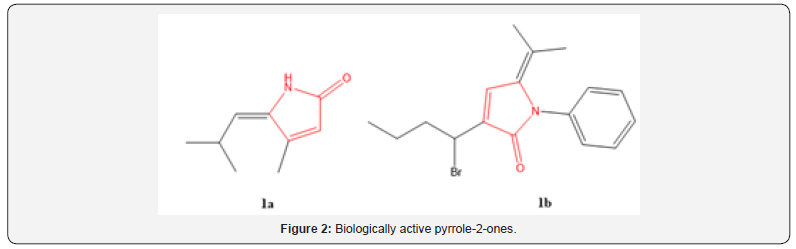
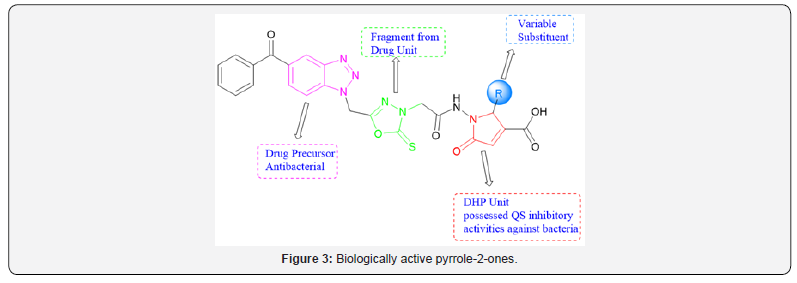
Among them, the commonly used approaches are cyclization reactions. Related dihydropyrrol-2-one (DHP) analogues, which bear a nitrogen atom in place of the oxygen in the heterocyclic ring, are more hydrolytically stable under physiological conditions [33]. DHP is a common moiety in several classes of biologically active molecules such as pulchellalactam (1a-b, Figure 2), jatropham and rolipram [34–36]. Novel dihydropyrrolone derivatives have been developed in our laboratory (Figure 3) and their bioactivities as well as in silico properties are disclosed in this article.
Experimental
Materials and Methods
All the reactants were of reagent grade, and purchased from Sigma Aldrich, and used without further purification. All solvents were used without further drying or purification and were of ACS grade purchased from local suppliers. TLC plates (Silica Gel) were purchased from Sigma-Aldrich. Melting points were determined in open capillary tubes on a Stuart SMP 10 melting point apparatus and are uncorrected. Nuclear Magnetic Spectroscopy (NMR) spectra were produced using the Varian 300 MHz spectrophotometer. The instrument was maintained at 25o C operating at 300 MHz for 1H NMR, and 75 MHz for 13C NMR. The deuterated solvent (DMSO- d6) used for each respective spectrum is referenced to the appropriate literature peak shift.
1H-benzo[d][1,2,3]triazol-1-yl)acetohydrazide. 2 [37]
To the solution of reactant 1 (1eq.) in absolute ethanol (50ml), methyl chloroacetate (1eq.), hydrazine monohydrate and anhydrous K2CO3 (1.2eq.) were added and the reaction mixture was heated under reflux for 16 hours. The potassium salt was filtered off and the excess of ethanol was removed. The residue solidified on cooling to give the desired product 2.
General procedure for the synthesis of phenyl(1-((5- thioxo-4,5-dihydro-1,3,4-oxadiazol-2-yl)methyl)-1Hbenzo[ d][1,2,3]triazol-5-yl)methanone. 3
A mixture of compound 2 (1 eq.) was added in methanol (100 mL), potassium hydroxide (2 eq.) and heated with CSCl2 (2 eq.) and refluxed for about 18 hours at 65 ºC. The separated solid was filtered, dried in vacuum and purified over a column of silica gel, eluted with C6H6: CHCl3 (2:8 v/v) mixture to give a final product which was crystallized with CHCl3.
General procedure for the synthesis of 2-(5-((5-benzoyl- 1H-benzo[d][1,2,3] triazol-1-yl)methyl)-2-thioxo-1,3,4- oxadiazol-3(2H)-yl)acetohydrazide. 4 [38,39]
To the solution of reactant 3 (1eq.) in absolute ethanol (60 mL); methyl chloroacetate (1eq.), hydrazine monohydrate and anhydrous K2CO3 (0.01 mol) were added and the reaction mixturewas heated under reflux for 16 hours. The potassium salt was filtered off and the excess ethanol was removed. The residue solidified on cooling to give the product 4.
General procedure for the synthesis of (E)-N’- (substituted methylene)-2-(5-((5-benzoyl-1H-benzo[d] [1,2,3]triazol-1-yl)methyl)-2-thioxo-1,3,4-oxadiazol- 3(2H)-yl)acetohydrazid.5a-h [40]
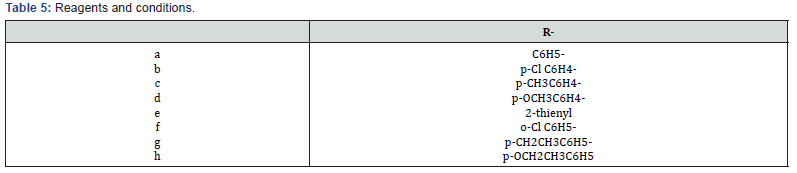
A mixture of compound 4 (0.01 mol) and benzaldehyde (0.01 mol) was made soluble in the absolute ethanol (100 mL) and was refluxed for about 4-8 hours at 79ºC with a catalytic amount of glacial acetic acid (1-3 drops) on a water bath. The product 5a thus obtained was separated, collected, dried and recrystallized from ethanol. Similarly, other compounds 5b-h were prepared using the same procedure and different aromatic aldehydes (as listed in scheme 1).
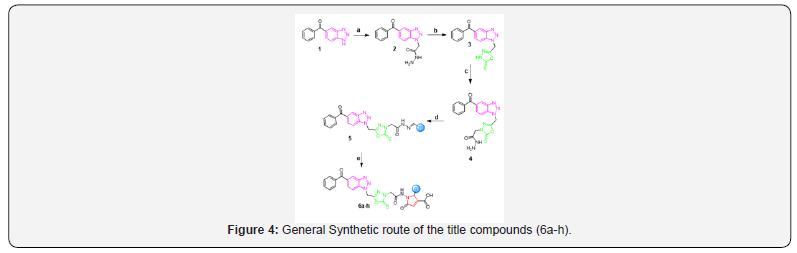
General procedure for the synthesis of 2-substituted- 1-(2-(5-((5-benzoyl-1H-benzo[d] [1,2,3] triazol-1-yl)methyl)-2-thioxo-1,3,4-oxadiazol- 3(2H)-yl)acetamido)-5-oxo-2,5-dihydro-1H-pyrrole-3- carboxylic acid. 6a-h [41]
A mixture of reactant 5a (0.135 mol) and maleic anhydride (0.15 mol, 15 g) was dissolved in anhydrous toluene (150 mL) and stirred under nitrogen during 24 hours at 35ºC. The mixture was cooled to room temperature and the solid was separated by filtration and purified by extraction with ethyl acetate after addition of sodium carbonate. The aqueous layer was treated with phosphoric acid. The white solid product separated by filtration was recrystallized in 45% ethanol to obtain white crystals of compound 6a. Similarly, other compounds 6b-h were prepared using the same procedure and different reactants 5b-h. (Figure 4) (Table 1)
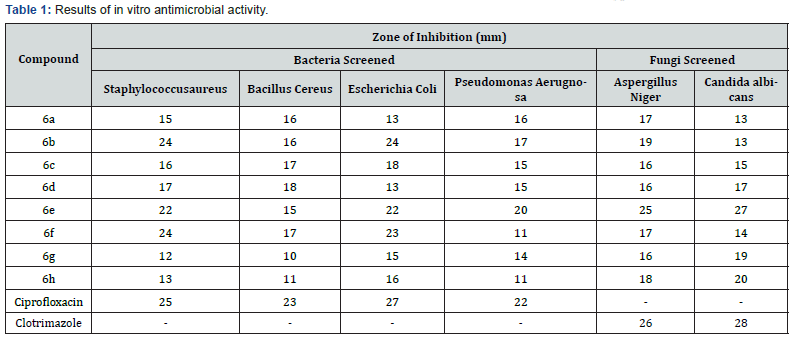
Boldfaced values indicate the active compounds; ‘–‘ indicates not tested
Scheme 1: General Synthetic Route of the Title Compounds (6a-H)
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol-1-yl)methyl)- 2-thioxo-1,3,4-oxadiazol-3(2H)-yl)acetamido)-5-oxo-2- phenyl-2,5-dihydro-1H-pyrrole-3-carboxylic acid (6a)
Yield: 61 %; white solid, mp 174 – 175 °C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.44 – 8.40 (m, 1H), 8.12 – 8.06 (m, 1H), 7.89 (dd, J = 10.2, 2.3 Hz, 1H), 7.84 – 7.78 (m, 2H), 7.63 – 7.56 (m, 1H), 7.54 – 7.47 (m, 2H), 7.43 (s, 5H), 6.69 (d, J = 1.7 Hz, 1H), 5.56 (d, J = 1.7 Hz, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.52 – 4.40 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H).13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 143.3, 142.6, 140.6, 137.6, 133.6, 133.3, 133.1, 131.9, 129.7, 129.1, 128.9, 128.8, 128.5, 118.1, 117.4, 110.1, 60.6, 48.0, 44.8; ESIMS: m/z calculated for C29H21N7O6S (M+H)+ 596.13 found 596.11, Anal. Calc. for C29H21N7O6S: C, 58.48; H, 3.55; N, 16.46%; found: C, 58.47; H, 3.53; N, 16.45%
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol- 1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-5-oxo-2-(p-tolyl)-2,5-dihydro-1H-pyrrole- 3-carboxylic acid (6b)
Yield: 67 %; white solid, mp 182 – 183 °C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.46 – 8.39 (m, 1H), 8.14 – 8.05 (m, 1H), 7.89 (dd, J = 10.2, 2.3 Hz, 1H), 7.86 – 7.76 (m, 2H), 7.54 – 7.45 (m, 1H), 7.22 (dd, J = 7.9, 0.9 Hz, 2H), 6.98 (dd, J = 8.0, 0.6 Hz, 2H), 6.69 (d, J = 1.7 Hz, 1H), 5.56 (d, J = 1.6 Hz, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.53 – 4.39 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H), 2.30 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 143.3, 142.6, 140.6, 137.6, 136.8, 134.3, 133.6, 133.3, 131.9, 129.7, 129.5, 128.9, 128.5, 126.9, 118.1, 117.4, 110.1, 60.6, 48.1, 44.8, 18.9.; ESIMS: m/z calculated for C30H23N7O6S (M+H) + 611.14 found 611.12 Anal. Calc. C30H23N7O6S: C, 59.11; H, 3.80; N, 16.04%; found: C, 59.09; H, 3.80; N, 16.03%
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol- 1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-2-(4-methoxyphenyl)-5-oxo-2,5-dihydro- 1H-pyrrole-3-carboxylic acid (6c)
Yield: 54.7 %; white solid, mp 181 – 182 °C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.45 – 8.39 (m, 1H), 8.14 – 8.04 (m, 1H), 7.89 (dd, J = 10.2, 2.3 Hz, 1H), 7.86 – 7.76 (m, 2H), 7.65 – 7.53 (m, 1H), 7.53 – 7.44 (m, 2H), 7.31 – 7.21 (m, 2H), 6.82 – 6.72 (m, 2H), 6.69 (d, J = 1.8 Hz, 1H), 5.60 – 5.53 (m, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.52 – 4.39 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H), 3.81 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 158.3, 143.3, 142.6, 140.6, 137.6, 133.6, 133.3, 131.9, 130.6, 129.7, 128.9, 128.5, 125.0, 118.1, 117.4, 114.2, 110.1, 60.6, 55.4, 48.1, 44.8.; ESIMS: m/z calculated for C30H23N7O7S (M+H) + 627.14 found 627.12 Anal. Calc. for C30H23N7O7S: C, 57.60; H, 3.71; N, 15.67%; found: C, 57.59; H, 3.71; N, 15.66%
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol- 1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-2-(4-chlorophenyl)-5-oxo-2,5-dihydro-1Hpyrrole- 3-carboxylic acid (6d)
Yield: 57.4%; white solid, mp 176 – 177 °C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.45 – 8.39 (m, 1H), 8.14 – 8.04 (m, 1H), 7.89 (dd, J = 10.2, 2.3 Hz, 1H), 7.86 – 7.76 (m, 2H), 7.65 – 7.53 (m, 1H), 7.53 – 7.44 (m, 2H), 7.23 – 7.13 (m, 2H), 7.13 – 7.06 (m, 2H), 6.69 (d, J = 1.8 Hz, 1H), 5.60 – 5.52 (m, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.52 – 4.39 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H). 13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 143.3, 142.6, 140.6, 137.6, 133.6, 133.3, 133.3, 132.1, 131.9, 129.7, 129.3, 128.9, 128.5, 127.8, 118.1, 117.4, 110.1, 60.6, 48.1, 44.8.; ESIMS: m/z calculated for C29H22ClN7O6S2 (M+H) + 630.09 found 630.07 Anal. Calc. for C29H22ClN7O6S2: C, 55.29; H, 3.20; N, 15.56%; found: C, 55.29; H, 3.19; N, 15.54%.
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol- 1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-5-oxo-2-(thiophen-2-yl)-2,5-dihydro-1Hpyrrole- 3-carboxylic acid (6e)
Yield: 56.1 %; white solid, mp 180 – 181°C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.45 – 8.39 (m, 1H), 8.14 – 8.04 (m, 1H), 7.89 (dd, J = 10.2, 2.3 Hz, 1H), 7.86 – 7.76 (m, 2H), 7.65 – 7.53 (m, 1H), 7.53 – 7.44 (m, 2H), 7.34 (dd, J = 5.3, 1.9 Hz, 1H), 6.98 – 6.84 (m, 3H), 5.56 (dd, J = 1.7, 0.5 Hz, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.52 – 4.39 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H). 13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 172.2, 168.7, 165.6, 143.3, 142.6, 137.9, 137.6, 135.3, 133.6, 133.3, 131.9, 131.3, 129.7, 128.9, 128.5, 126.2, 125.7, 124.8, 118.1, 110.1, 66.6, 48.1, 44.8.; ESIMS: m/z calculated for C27H19N7O6S2 (M+H) + 602.08 found 602.06 Anal. Calc. for C27H19N7O6S2: C, 53.90; H, 3.18; N, 16.30%; found: C, 53.89; H, 3.17; N, 16.28%
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol- 1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-2-(2-chlorophenyl)-5-oxo-2,5-dihydro-1Hpyrrole- 3-carboxylic acid (6f)
Yield: 64.8 %; white solid, mp 197 – 198 °C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.45 – 8.39 (m, 1H), 8.14 – 8.05 (m, 1H), 7.93 – 7.78 (m, 3H), 7.65 – 7.55 (m, 2H), 7.54 – 7.46 (m, 2H), 7.28 – 7.13 (m, 3H), 6.69 (d, J = 1.8 Hz, 1H), 5.56 (dd, J = 1.8, 0.6 Hz, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.53 – 4.40 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H).13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 143.3, 142.6, 140.9, 137.6, 134.2, 133.6, 133.3, 132.9, 131.9, 131.8, 131.2, 130.1, 129.7, 128.9, 128.5, 127.8, 118.1, 117.8, 110.1, 59.5, 48.1, 44.8.; ESIMS: m/z calculated for C29H20Cl- N7O6S (M+H) + 630.09 found 630.07 Anal. Calc. for C29H20ClN7O6S: C, 55.29; H, 3.20; N, 15.56%; found: C, 55.29; H, 3.19; N, 15.55%.
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol-1-yl) methyl)-2-thioxo-1,3,4-oxadiazol 3(2H)-yl)acetamido)- 2-(4-ethylphenyl)-5-oxo-2,5-dihydro-1H-pyrrole-3- carboxylic acid (6g)
Yield: 68.4 %; white solid, mp 204 – 206 °C; 1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.45 – 8.39 (m, 1H), 8.13 – 8.06 (m, 1H), 7.93 – 7.77 (m, 3H), 7.64 – 7.45 (m, 3H), 7.14 (dt, J = 8.2, 1.0 Hz, 2H), 7.07 – 6.99 (m, 2H), 6.69 (d, J = 1.8 Hz, 1H), 5.59 – 5.54 (m, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.52 – 4.40 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H), 2.74 (dddd, J = 12.3, 6.1, 5.1, 4.1 Hz, 1H), 2.50 (dqt, J = 12.3, 5.1, 1.0 Hz, 1H), 1.21 (t, J = 5.1 Hz, 3H).13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 143.5, 143.3, 142.6, 140.6, 137.6, 134.7, 133.6, 133.3, 131.9, 129.7, 128.9, 128.9, 128.5, 126.8, 118.1, 117.4, 110.1, 60.6, 48.1, 44.8, 28.4, 15.3.; ESIMS: m/z calculated for C31H25N7O6S (M+H) + 624.16 found 624.16 Anal. Calc. for C31H25N7O6S: C, 59.70; H, 4.04; N, 15.72%; found: C, 59.69; H, 4.04; N, 15.71%.
1-(2-(5-((5-benzoyl-1H-benzo[d][1,2,3]triazol- 1-yl)methyl)-2-thioxo-1,3,4-oxadiazol-3(2H)-yl) acetamido)-2-(4-ethoxyphenyl)-5-oxo-2,5-dihydro-1Hpyrrole- 3-carboxylic acid (6h)[42]
Yield: 64.7 %; white solid, mp 198 – 200 °C;1H NMR (300 MHz, DMSO-d6) δ (ppm) 10.30 (s, 1H), 8.45 – 8.40 (m, 1H), 8.14 – 8.03 (m, 1H), 7.94 – 7.79 (m, 3H), 7.66 – 7.45 (m, 2H), 7.30 – 7.22 (m, 2H), 6.89 – 6.81 (m, 2H), 6.69 (d, J = 1.8 Hz, 1H), 5.56 (d, J = 1.5 Hz, 1H), 4.69 (d, J = 17.6 Hz, 1H), 4.53 – 4.39 (m, 2H), 4.23 (d, J = 13.6 Hz, 1H), 4.12 – 3.92 (m, 2H), 1.33 (t, J = 4.6 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ (ppm) 197.9, 181.4, 169.0, 168.7, 167.4, 158.6, 143.3, 142.6, 140.6, 137.6, 133.6, 133.3, 131.9, 130.8, 129.7, 128.9, 128.5, 128.2, 118.0, 117.4, 115.4, 110.1, 63.5, 60.6, 48.1, 44.8, 14. 8.; ESIMS: m/z calculated for C31H25N7O7S (M+H) + 640.15 found 640.13 Anal. Calc. for C31H25N7O7S: C, 58.21; H, 3.94; N, 15.33%; found: C, 58.20; H, 3.93; N, 15.33%.
Results and Discussion
Biology
In vitro Antimicrobial Activity
One mg of each molecule was dissolved in 1mL of DMSO then made up to 10mL with sterile water to obtain a concentration of 100 μg/mL. The microorganisms were maintained on nutrient agar media. The agar media was incubated with the different tested bacteria. After 24 to 48hrs of incubation at ~37ºC, dimethyl sulfoxide showed no inhibition zones. The diameters of the of the inhibition zones of the tested compounds were measured. This method was utilized to prove the minimum inhibitory concentration (MIC). The outcomes are outlined in (Table 2).
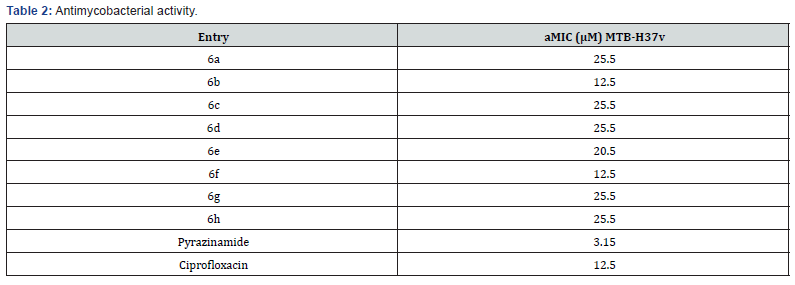
The Synthesized compounds under study were screened for their antimicrobial activity by cup plate method [43]. The bacteria screened were Staphylococcus, Bacillus, Escherichia coli and Pseudomonas aeruginosa and the fungi screened were Aspergillus niger and Candida albicans. Ciprofloxacin and Clotrimazole were used as standards for antibacterial studies and antifungal studies respectively. The details are given in Table 2. To analyze the impact of the nature of (R-)Substitution on the antimicrobial activity, derivatives incorporating phenyl, p-chloro phenyl, p-methyl phenyl, p-methoxy phenyl, o-chlorophenyl, p-ethyl phenyl, p- ethoxy phenyl and 2-thienyl were synthesized. The synthesized compounds containing chloro and 2-thienyl group has shown excellent antimicrobial activity whereas the rest of the series shown good to moderate antimicrobial activity against bacterial and fungal strain compared to standards.
Antibacterial and Antifungal Activity Results
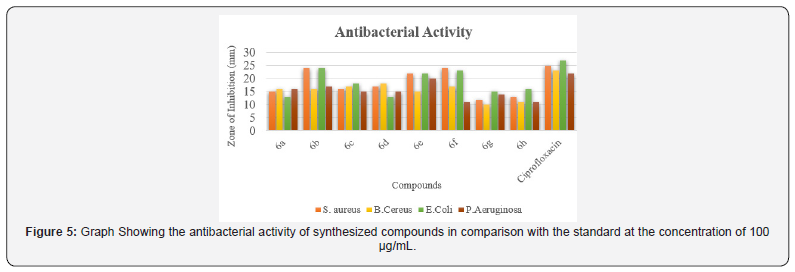
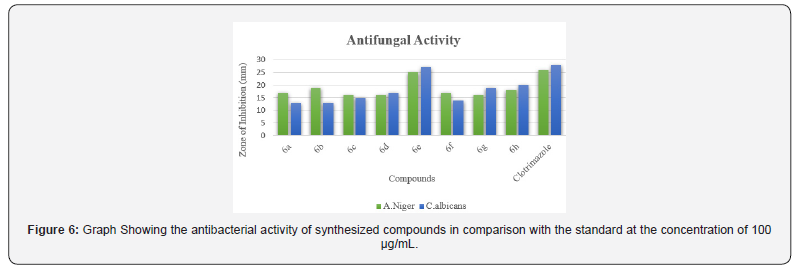
The Synthesized compounds under study were screened for their antimicrobial activity by cup plate method. The bacteria screened were Staphylococcus, Bacillus, Escherichia coli and Pseudomonas aeruginosa and the fungi screened were Aspergillus niger and Candida albicans. Ciprofloxacin and Clotrimazole were used as standards for antibacterial studies and antifungal studies respectively. The details are given in Table 2. To analyze the impact of the nature of (Ar-)Substitution on the antimicrobial activity, derivatives incorporating phenyl, p-chloro phenyl, p-methyl phenyl,p-methoxy phenyl, o-chlorophenyl, p-ethyl phenyl, p- ethoxy phenyl and 2-thienyl were synthesized. The synthesized compounds containing chloro and 2-thienyl group has shown excellent antimicrobial activity whereas the rest of the series shown good to moderate antimicrobial activity against bacterial and fungal strain compared to standards (Figures 5 & 6).
In vitro Antimycobacterial Assay
The antimycobacterial activity was examined against H37Rv employing Micro plate alamar blue assay [45] procedure. This method is nontoxic, employs a thermally stable reagent. This method is well explained as follows, 200μl of ultrapure water was added to whole outer perimeter wells of 96 sterile well plates to reduce drying up of the medium in the test wells while incubation. The 96 plates taken 100μl of the Middlebrook 7H9 broth and consecutive dilution of the compounds was generated directly on the plate. The end drug concentrations confirmed were 100 to 0.190 μg/mL. Plates were capped and sealed with parafilm and incubated at 37 ºC for 5 days. 25μL of freshly made 1:1 mixture of alamar blue reagent and 10% tween 85 was then added to the plate and incubated for about 24 hours. A blue color in the well was elucidated as no bacterial growth and a pink color was scored as growth. The potency is reported as MIC (minimum inhibitory concentration).
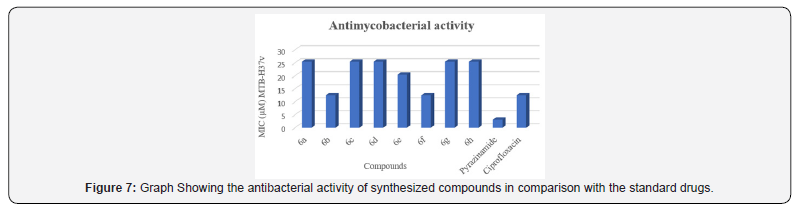
Antimycobacterial Activity Result
All novel developed molecules 6a-h were evaluated to in vitro antimycobacterial activity against MTB H37v employing the MABA method [29]. Ciprofloxacin and pyrazinamide were used as reference drugs. The outcomes of the in vitro antimycobacterial potency are outlined in (Table 3) as MIC. Among all the synthesized molecules 6b and 6f demonstrated a similar mycobacterial inhibitory potency at a MIC of 12.5 μM compared to standard drug Ciprofloxacin (Figure 7).
In silico ADME Studies
The aqueous solubility of a molecule undoubtably change its absorption and distribution tendency. Generally, a low solubility goes along with a poor absorption and therefore the common intention is to filter out less soluble molecules. Our predicted logS number is measured in mol/liter unit. From the literature it is evident that more than 85% of the drugs in the market have a (predicted) logS number greater than -4. As an essential merit of matter, lipophilicity is a characteristic used by scientists to estimate and interpret the transit and effect of chemicals in physiological systems. LogP values are crucial to many companies and areas of research in evaluating how to transport chemical substances to sites.
LogP is employed in the pharmaceutical or biotech companies to explain the action of drug candidates in the body. Drug molecules are usually screened confering to logP, some other benchmark also help to guide drug selection and optimization.
This is due to lipophilicity is a major determining key factor in a compound’s absorption, distribution in the body, penetration across membranes and biological barriers, metabolism and excretion (ADME properties). A drug targeting CNS (central nervous system) should admirably have a logP value around 2 for oral andintestinal absorption the ideal value is 1.35-1.8, while a drug intended for sub-lingual absorption may have a logP value >5. LogP help predict the likely transit of a molecule around the body, it also very useful in formulation, dosing, drug clearance, and toxicity.
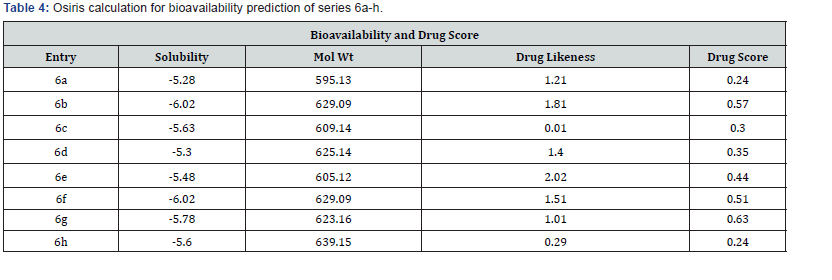
Though it is not the only deciding factor in these arguments, it plays a crucial role in helping scientists/researchers limit the liabilities of new drug molecules. There are many approaches around that assess a compound’s drug likeness partially based on topological descriptors, fingerprints of MDL structure keys or other properties as cLogP and molecular weights. The distribution of drug likeness values calculated from https://www.organic-chemistry. org/prog/. Positive values of these synthesized compound show that likely to be a good drug candidate in the drug development process.
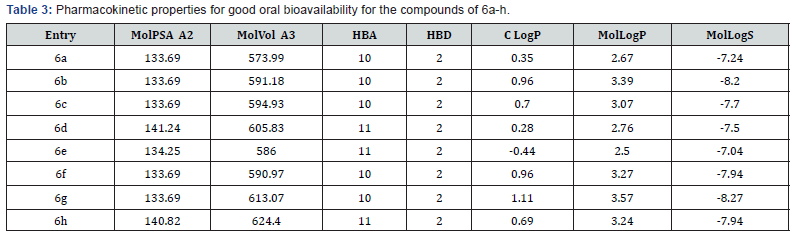
Lipinski’s rule of five also known as the Pfizer’s rule of five or simply the Rule of five (RO5) is a rule of thumb to evaluate drug-likeness or determine if a chemical compound with a certain pharmacological or biological activity has properties that would make it a likely orally active drug in humans. The rule describes molecular properties important for a drug’s pharmacokinetics in the human body, including their absorption, distribution, metabolism, and excretion (“ADME”) Components of the Lipinski’s rule listed in (Tables 4 & 5).
a.EtOH, ClCH2COOCH3, NH2NH2. H2O, Anhy. K2CO3, reflux, 16hrs.
b.MeOH, KOH, CSCl2, reflux, 12hrs.
c.EtOH, ClCH2COOCH3, NH2NH2. H2O, Anhy. K2CO3, reflux, 16hrs.
d.Aromatic aldehyde, EtOH, glacial acetic acid, reflux, 4-5 hrs.
e.Maleic anhydride, anhydrous toluene, under N2, 24hr, 35 ºC, ~54-68%.
Result of In silico ADME studies
These properties are calculated and discussed based on Lipinski’s rule and its component. The compounds 6a-h fulfill Lipinski’s rule and show good drug-likeness score which is positive values (Table 5). Milog P of these compounds was found below 5 that means these shows good permeability across the cell membrane. The logS values are greater than -4 which predict that these molecules have good aqueous solubility. TPSA below 160 Ǻ2, n violations =1 or <0 it means compound easily bind to the receptor, molecular mass >500, No. hydrogen bond donors ≤ 5 (The sum of OHs and NHs), No. hydrogen bond acceptor ≤ 10 ( The sum of Os and Ns).
Conclusion
A series of novel heterocyclic compounds incorporating Pyrrole and benzotriazole- 1,3,4-oxadiazole moieties were synthesized and characterized by 1H NMR, 13C NMR, mass spectroscopy and elemental analysis. The titled compounds were evaluated for their in vitro antimicrobial activity against six bacteria including two gram-positive, two gram-negative and two fungal strains. All the compounds show good to moderate activity against the bacterial strain. Among all compounds, 6b and 6f were found to be better antimycobacterial agents and 6a, 6b and 6e were found to be excellent antifungal and antibacterial agent compared to rest of the series. The synthesized compounds also studied for their in-silico properties and showed good drug-likeness properties.
Acknowledgement
The authors would like to thank Arts, Science and Commerce College, Surat for the providing laboratory facilities and M B Patel Science College, Anand for antimicrobial study.
Conflicts of Interest
The author declares no conflicts of interest.
Statement of Human and Animal Right
This article does not contain any studies with human and animal subjects performed by any of the authors
References
- Barreca M L, Rao A, De Luca L, Zappala M, Gurnari C, et al. (2004) Efficient 3D database screening for novel HIV-1 IN inhibitors. J chem Inform Comp Sci 44(4): 1450–1455.
- Pandya K M, Desai P S (2018) Synthesis and biological study of some schiff bases and their thiazolidinone derivatives. World J Pharm Res 7(10): 465-474.
- Kolocouris N, Kolocouris A, Foscolos G B, Fytas G, Neyts J, et al. (1996) Synthesis and antiviral activity evaluation of some new aminoadamantane derivatives. J Med Chem 39: 3307–3318.
- Pandya K M, Desai P S, Patel N B, Dave B P (2018) Synthesis and antimicrobial study of novel heterocycles containing azetidinone, benzotriazole and 1,3,4-oxadiazole moieties. Chem Biol Interface 8(5): 314-322.
- Koz’minykh V O, Igidov N M, Zykova S S, Kolla V E, Shuklina N S et al. (2002) Synthesis and pharmacological activity of 2-hydroxy-1,5-diaryl-4-pivaloyl-2,5-dihydro-2-pyrrolones. Pharm Chem J 36(4): 188–191.
- Pandya K M (2018) Synthesis and Cytotoxicity of Azaheterocyclic Compounds Thesis, Rowan University: New Jersey, US.
- Silina T A, Gein V L, Gein L F, Voronina E V (2003) Synthesis and antimicrobial activity of 3-hydroxy- and 3-arylamino-5-aryl-4-acyl-1-(pyridyl)-3-pyrrolin-2-ones. Pharm Chem J 37(11): 585–587.
- Gein V L, Bobyleva A A, Levandovskaya E B, Odegova T F, Vakhrin M I, (2012) Synthesis and antimicrobial activity of 5-aryl-4-acyl(heteroyl)-3-hydroxy-1- (3-ethoxypropyl)-3-pyrrolin-2-ones. Pharm Chem J 46(1): 23-25.
- Lovren F, Gaon I D, Bobarevic B, Bobarevic (1990) Synthesis and antimicrobiological activity of N-aryl-substituted 2-methyl-3-carbethoxy-5-pyrrolinone derivatives. Arch Pharm 323(11):901-904.
- Sano T, Horiguchi Y, Toda J, Imafuku K, Tsuda Y (1984) Syntheses of 2-aryl-3-ethoxycarbonyl-2-pyrroline-4,5-diones. Chem Pharm Bull 32(2): 497–503.
- Aasen A J, Culvenor C C (1969) Saturated pyrrolizidinediols II Total synthesis and stereochemistry of macronecine. J Org Chem 34(12): 4143– 4147.
- Goldschmidt B M (1962) Some Substituted pyrrolizidines. J Org Chem 27: 4057–4058.
- Tufariello J J, Tette J P (1975) Synthesis in the pyrrolizidine class of alkaloids DL-Supinidine. J Org Chem 40:3866–3869.
- Chamberlain T World Patent WO 2,001,074,950, 2001
- Morton C J H, Gilmour R, Smith D M, Lightfoot P, Slawin A M Z, et al. (2002) Synthetic studies related to diketopyrrolopyrrole (DPP) pigments Part 1: The search for alkenyl-DPPs Unsaturated nitriles in standard DPP syntheses: a novel cyclopenta[c]pyrrolone chromophore. Tetrahedron 58(27): 5547–5565.
- Ueda A, Sekiya Y, Taniguchi M (2003) Japanese Patent JP 2,003,165,918.
- Pandya K, Patel R (2017) Physical properties, synthetic approach and pharmaceutical use of pyrazole. J Chem & Cheml Sci 7: 1331-1341.
- Adam J M, Dalvi P V, Ekkundi V S, Bacher J P, Tiwari S (2004) World Patent WO 2,004,083,170.
- Adam J M, Dalvi P V, Ekkundi V S , Bacher J P, Sreenivasan R, et al. (2004) World Patent WO 2,004,089,941.
- H Shiraishi, T Nishitani, S Sakaguchi, Y Ishii (1998) Preparation of Substituted Alkylpyrroles via Samarium-Catalyzed Three-Component Coupling Reaction of Aldehydes, Amines, and Nitroalkanes J Org Chem 63(18): 6234.
- X Lin, Z Mao, X Dai, P Lu, Y Wang (2011) Chem Commun 47: 6620.
- O A Attanasi, G Favi, F Mantellini, G Moscatelli, S Santeusanio (2011) Copper (II)/Copper(I)-Catalyzed Aza-Michael Addition/Click Reaction of in Situ Generated α-Azidohydrazones: Synthesis of Novel Pyrazolone−Triazole Framework J Org Chem 76: 2860.
- E Ghabraie, S Balalaie, M Bararjanian, H R Bijanzadeh, F Rominger (2011) Tetrahedron 67: 5415.
- C R Reddy, M D Reddy, B Srikanth, K R Prasad (2011) Org Biomol Chem 9: 6027.
- B M Trost, J -P Lumb, J M Azzarelli (2011) J Am Chem Soc 133: 740.
- A V Gulevich, A S Dudnik, N Chernyak, V Gevorgyan (2013) Transition Metal-Mediated Synthesis of Monocyclic Aromatic Heterocycles. Chem Rev 113: 3084.
- H C Brown, U R Khire, G Narla, U S Racherla (1995) J Org Chem 60: 544.
- Leber J D, Hoover J R E, Holden K G, Johnson R K, Hecht S M (1988) A Revised Structure for Sibiromycin. J Am Chem Soc 110(9): 2992−2993.
- Li W, Khullar A, Chou S, Sacramo A, Gerratana B (2009) Biosynthesis of Sibiromycin, a Potent Antitumor Antibiotic. Appl Environ Microbiol 75(9): 2869−2878.
- Hertel L W, Xu Y C (2000) Pyrrolidine and Pyrroline Derivatives Having Effects on Serotonin Related Systems World Patent WO 2000000196A1, Jan 6. Chem Abstr 132: 78467.
- Kahan J S, Kahan F M, Goegelman R, Currie S A, Jackson M et al. (1979) Thienamycin, a New β-Lactam Antibiotic I Discovery, Taxonomy, Isolation and Physical Properties. J Antibiot 32(1): 1−12.
- Manefield M, Rasmussen TB, Henzter M, et al (2002) Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology 148(4): 1119–1127.
- Albrecht D, Bach T (2007) Synthesis of 4-substituted 1, 5-dihydropyrrol-2-ones and 5, 6- dihydro-1H-pyridin-2-ones by Negishi cross-coupling reactions: Short access to the antidepressant (±)-rolipram. Synlett 10: 1557–1560.
- Li W R, Lin ST, Hsu N M, Chern M S (2002) Efficient total synthesis of pulchellalactam, a CD45 protein tyrosine phosphatase inhibitor. J Org Chem 67(14): 4702–4706.
- Mase N, Nishi T, Takamori Y, Yoda H, Takabe K (1999) First synthesis of (R)-(−)-5-hydroxy- 3-methyl-3-pyrrolin-2-one (jatropham) by lipase-catalyzed kinetic resolution. Tetrahedron Asymmetry 10(23): 4469–4471.
- Shah H P, Shah B R, Bhatt J J, Desai N C, Trivedi P B, et al. (1998) Synthesis of 2,5-disubstituted 1,3,4-oxadiazoles as potential antimicrobial, anticancer and anti-HIV agents. Indian J Chem 37B: 180.
- El Khawas S M, Habib N S (1989) Synthesis of 1,2,4-triazole, 1,2,4-triazolo[3,4-b][1,3,4]thiadiazole and 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine derivatives of benzotriazole. J Hetero Chem 26: 177.
- Somani R R, Shirodkar P Y, Kadam V J (2008) Synthesis and antibacterial activity of some new 2,5-disubstituted-1,3,4-oxadiazole derivatives. Chin J Chem 26: 117-1731.
- Shukla D K, Srivastava S D (2008) Synthesis of some new 5-[2-{(1,2,3-benzotriazole)-1-yl-methyl}-1'-(4'-substituted aryl-3'-chloro-2'-oxo azetidine)]-amino-1,3,4-thiadiazoles: antifungal and antibacterial agents. Indian J Chem 47: 463-469.
- Tabcheh M, Baroudi M, Elomar F, Elzant A, Elkhatib M, et al. (2006) New imidazole compounds derived from pyrrolidonic and piperidonic acids as non-steroidic aromatase inhibitors. Asian J Chem 18: 1771-1782.
- Rose S B, Miller R B (1939) Studies with the Agar Cup-Plate Method: I A Standardized Agar Cup-Plate Technique. J Bacteriol 38: 525-537.
- Jimenez A, Meckes M, Alvarez V, Torres J, Parra R (2005) Secondary metabolites from Chamaedora tepejilote (Palmae) are active against Mycobacterium tuberculosis. Phytother Res 19: 320–322.
- Mendoza Aguilar M, Almaguer Villagran L, Jimenez Arellanes A, Arce Paredes P, Cid Gutierrez J L, et al. (2012) The use of the microplate alamar blue assay (MABA) to assess the susceptibility of Mycobacterium lepraemurium to anti-leprosy and other drugs. J Infect Chemother 18: 652-661.






























