Spectroscopy in Wine Industry OEno-NMR: Recent Advances of Nuclear Magnetic Resonance
Jose Enrique Herbert Pucheta*
Consejo Nacional de Ciencia y Tecnología, Laboratorio Nacional de Investigación y Servicio Agroalimentario y Forestal, México
Submission: June 22, 2019; Published: July 03, 2019
*Corresponding author: Jose Enrique Herbert-Pucheta, Consejo Nacional de Ciencia y Tecnología, Laboratorio Nacional de Investigación y Servicio Agroalimentario y Forestal, Universidad Autónoma Chapingo, Km. 38.5 Carretera México-Texcoco, Chapingo 56230, Estado de México, México
How to cite this article: Jose Enrique Herbert-Pucheta. Spectroscopy in Wine Industry OEno-NMR: Recent Advances of Nuclear Magnetic Resonance. Organic & Medicinal Chem IJ. 2019; 8(4): 555741. DOI: 10.19080/OMCIJ.2019.08.555741
Abstract
Present Mini-Review briefly highlights central tasks from the World Organisation of Vine and Wine (OIV) as intergovernmental scientific organism responsible of guaranteeing bests oenological practices in world-wide wine industry. The OIV’s methods of wine and must analysis commission, in charge of conception-execution and vigilance of novel analytical methods applied to wine industry, has recently proposed the nuclear magnetic resonance technology as part of its multivariate portfolio solutions to characterize key aspects in wine such as variety, aging, origin, etc. Accuracy of experiments strongly depend of how spin coherences are prepared in solutions having intense water-to-ethanol solvent signals. It is stressed as well recent advances in one-and two-dimensional NMR schemes coupled with multi-presaturation modules applied in wines’ foot & fingerprinting and target & profiling.
Keywords: Methods of analysis in oenology Nuclear magnetic resonance Solvent multi-presaturation NMR schemes T1 relaxometry DOSY International Organisation of Vine and Wine Oenology Wine profiling
Introduction
Since 1924, the International Organization of Vine and Wine (OIV) has been the intergovernmental scientific organism that harmonizes and regulates world-wide vitivinicultural industry. OIV’s main roles comprise:
i) Conception-execution and vigilance of international policies that guarantees maximum quality of vitivinicultural products;
ii) Promoting research - development and innovations within the OIV’s main axes: viticulture, oenology, economy & vitivinicole laws and wine’s health and security;
iii) Creating statistics and relevant meta-data bases of world’s vitis-vinis sectors describing their complex dynamics and iv) formation of qualified human resources [1]. Each year, a set of hundreds of novel resolution projects are proposed and peer reviewed by world’s certified experts coming from all OIV Member states (Figure 1) as well as by certified observers such as from the Oenological Products and Practices International Association (Oennopia, [2]) or the International Federation of Wines and Spirits (FIVS [3]).
The OIV-CII oenology commission comprises several sub-disciplines: microbiology, oenological specifications, technology and a special sub-commission for methods of wine and must analysis [5]. From the set of hundreds of internationally accepted and on-peer revision methods, currently there is only one accepted [6] and one under-revision methods [7] comprising the use of nuclear magnetic resonance (NMR) technology in wine industry. However, an increased use of proton (1H)-NMR technology for plant metabolomics has been reported over the last years due to improvements in high-throughput automations, NMR sensitivity and solvent suppression routines [8]. NMR spectroscopy has recently found tremendous advances in the profiling – targeting of primary (common metabolites & organic intermediates present in all plant kingdom and essential to their survival such as lipids, steroids, carbohydrates, amino acids or organic acids) and specialized (for plant reproduction,environmental interactions or protection such as polyphenols, flavonoids, terpenoids, coumarins or tannins) metabolites. Highresolution 1H-NMR spectroscopy has recently been accepted and routinely used in the direct study of liquid foods such as fruit juices, beer and wine, with high-throughput instrumentation and in most of the cases with a magnetic field of 9.4 Teslas (400 MHz proton frequency) [9-11]. For oenology, relevant data to obtain from the 1H-NMR spectra includes signal assignment of both primary and specialized metabolites related to grape varieties, geographical origin of wine and year of vintage [12,13].
Geographical discriminations between wines have been initially carried out by combining isotopic -Site-specific Natural Fractionation by Nuclear Magnetic Resonance (SNIF NMR) - and trace elements by Isotope Ratio Monitoring by Mass Spectrometry or NMR (irm-MS/irm-NMR) analysis [14]. For instance, discrimination between geographical regions of Spanish, Slovenian, French and Chinese wines with SNIFIRMS technology [15-18] are some successful examples. Origin authentication by deuterium irm-2H NMR as an official OIV method to said purpose presents at least three major limitations [6]: a) the intrinsic 2H low sensitivity (0.0155% of natural abundance relative to 1H), b) narrow chemical-shift range of 2H (couple of ppm’s, such as its 1H counterpart), producing in many cases, important signal overlap and c) 1H-2H solute-solvent exchanges. Said limitations lead in turn to have long acquisition times per experiment and detection of isotopic fractionation of only the most abundant metabolites, when non-conventional cryoprobes and high-magnetic fields are used [14].
In agreement with the OIV draft resolution project OENOSCMA 17-618, currently at step 5 “Quantitation of glucose, malic acid, acetic acid, fumaric acid, shikimic acid and sorbic acid in wine using proton nuclear magnetic resonance spectroscopy (1H-NMR)” [7], said technique has been recently accepted within the OIV scientific chair as a promising primary quantitative analytical technique for beverage analysis such as wine. Particularly, 1H-NMR can be seen as a non-targeted metabolomics technique, wherein minimal sample preparation is required for identification and quantification of various compounds in wine, in a non-invasive way by means of isotropic chemical-shift, signal integrations and signal’s fine structure analysis of each metabolite [12,19]. However, poor chemical shift dispersion and weak intensities of several resonances in 1H-NMR spectra, severely penalizes identification within overlapped crowded regions.
In particular, the aromatic regions of wine spectra are difficult to assign due to these inconveniences and overall, the above mentioned OIV resolution project proposes the quantification of no more than 6 metabolites. Assignment within crowded regions can be partially alleviated by the addition of a second dimension, generated by the correlation of a spin system with its covalently-bounded or spatial neighbours, bymeans of respectively Correlation Spectroscopy (COSY), Total Correlation Spectroscopy (TOCSY) and Nuclear Over Hauser Effect Spectroscopy (NOESY) NMR schemes [20], as some of the most common techniques to increase the chemical shift dispersion within a spectra. However, said techniques need an evolution t1 period related to spectral resolution. Longer t1 increments will produce better resolved spectra at longer experimental times. For that, a compromise has to be met between experimental time consuming and spectral resolution. Routine users must take into account that wine metabolomic profiles obtained with 2D-shift correlation schemes will have a lower signal to noise ratio or longer experimental times with respect a standard 2D-scheme, as a multi presaturation module to suppress water and ethanol signals has to be done in order to increase signal to noise ratio of weak metabolites. Despite said performances, weak signal intensity, severe signal overlap or the lack of coupling information between different spin segments of molecules within a metabolome, often leads to ambiguous or incomplete assignments.
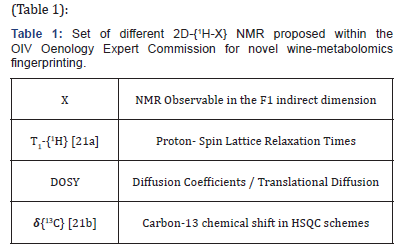
Since 2018, the Mexican delegation at OIV has proposed a set of high-resolution/ bi-dimensional 2D-{1H-X} NMR techniques, which correlates 1H-NMR chemical shifts of metabolites with (Table 1):
1H-NMR chemical shifts of full set of wines’ metabolites are in turn enhanced by the addition of a water-to-ethanol multipresaturation module (Figure 2). Some experimental details, advantages and limitations per 2D-{1H-X} NMR are briefly exposed.
Discussion
Undoubtedly, a successful metabolomics (foot)/ fingerprinting and/or targeted profiling study relies on how observables are observed. In NMR the last means on how chemical shift resonances appear within the NMR spectra, in terms of experimental signal-to-noise ratio. In alcoholic beverages metabolomics studies, signal-to-noise ratio of profiled resonances are strongly dependent on water (4.7 ppm) and ethanol (3.51 and 1.04 ppm) signals’ intensities at specific experimental conditions (mainly temperature and pH) (Figures 2 & 3).
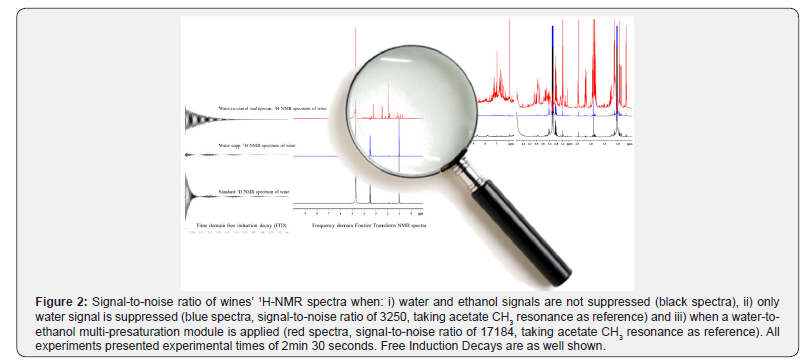
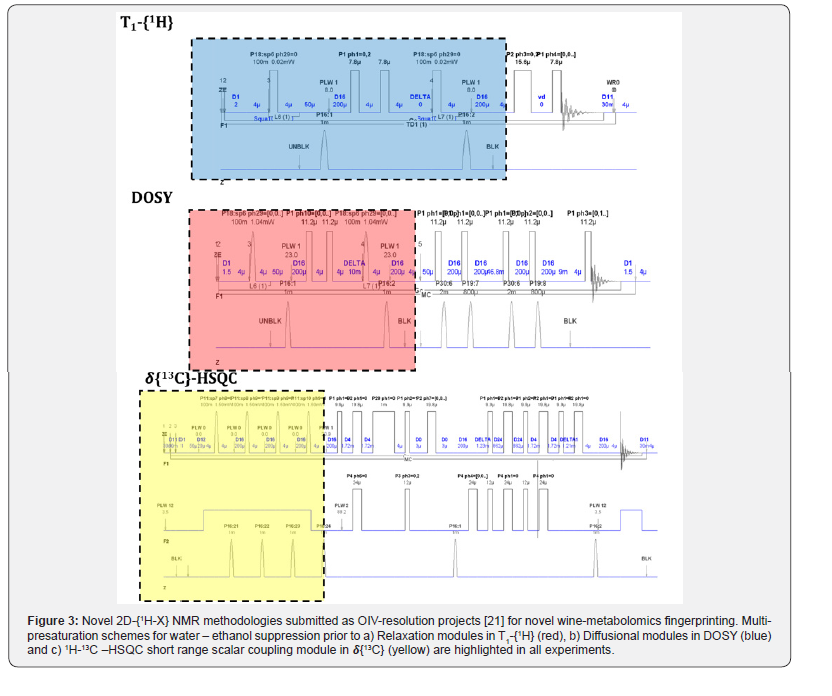
Readers are invited to intuitively deduce the difficulties around developing an accurate multi-presaturation scheme in a one-dimensional experiment and the bigger challenges to circumvent when a multi-presaturation module is added prior to any multi-dimensional scheme (Figure 3) and verify said spectroscopic challenges in recent literature [22,23]. Once the multi-presaturation scheme is optimized to be used in one- or two- dimensional NMR methodologies, novel observables arise from the F1 indirect dimension (relaxometry. Diffusiometry or heteronuclear scalar couplings) and thus can be potentially used in multivariate statistical metabolomics analysis, as complement to single chemical-shift observables (Figure 4). In wine metabolomics, said novel observables are claimed to be related to aging [21a] or grape variety [23] and currently said observation are under OIV peer-review
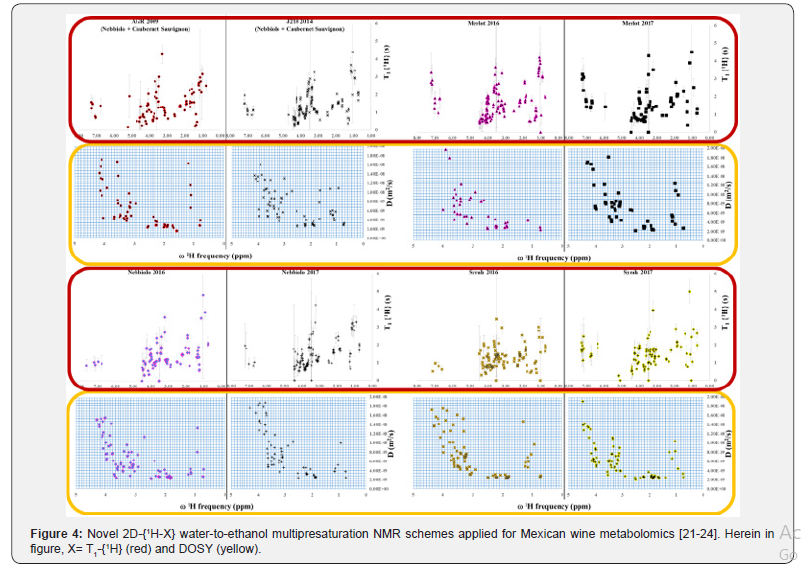
Conclusion
Present Mini-Review highlights key methodological aspects of NMR spectroscopy applied to wine metabolomics, towards to be part of OIV portfolio solutions to quantify oenological practices, authenticity, geographic origin, amongst other important parameters. If water-to-ethanol multi-presaturation scheme is carried out properly for its use in mono- and multidimensional experiments, enormous applications can be proposed. Recenlty our research OIV delegation has presented optimized T1-{1H}, DOSY and {13C} 2D-{1H-X} water-to-ethanol multipresaturation NMR schemes and its applications. Currently our methodologies are under OIV revision in order to validate their use in characterizing wine aging, grape variety, as multitask strategy to quantify Alcohol by Volume strength and oenological practice in non-conventional fermentation processes as well as for cross-checking novel orthogonal technologies prone to detect specialized metabolites such as a polyphenolics fingerprint.
References
- http://www.oiv.int/public/medias/6347/fr-tout-savoir-sur-l-oiv-web.pdf.
- http://www.oenoppia.com/
- Regulating Winemaking Practice Additions in a Rapidly Evolving Global Market - FIVS – 2015.
- http://www.oiv.int/en/the-international-organisation-of-vine-and-wine/member-states-and-observers.
- http://www.oiv.int/public/medias/6619/compendium-2019-en-vol1.pdf.
- OIV-MA-AS311-05
- OENO-SCMA-17-618
- C Deborde, A Moing, L Roch, D Jacob, D Rolin, et al. (2017) Plant metabolism as studied by NMR Spectroscopy. Prog Nucl Magn Reson Spectrosc 102-103: 61-97.
- AM Gil, IF Duarte, M Godejohann, U Braumann, M Maraschin, et al. (2003) Anal Chim Acta 488: 35-51
- Duarte IF, Godejohann M, Braumann U, Spraul M, Gil AM (2003) Application of NMR spectroscopy and LC-NMR/MS to the identification of carbohydrates in beer. J Agric Food Chem 51(17): 4847-4852.
- Ramos, H. Santos (1999) Nmr Studies Of Wine Chemistry and Wine Bacteria. NMR Spectrosc 37: 179-202.
- R Godelman, F Fang, E Hupmfer, B Shütz, M Bansbach, et al. (2013) Targeted and nontargeted wine analysis by 1H NMR spectroscopy combined with multivariate statistical analysis, Differentiation of important parameters: grape variety, geographical origin, year of vintage. J Agric Food Chem 61(23): 5610-5619.
- C Fauhl-Hassek (2019) Quo vadis non-targeted wine analysis? Bio web of conferences 12: 02030.
- T Jézéquel, V Joubert, P Giraudeau, GS Remaud, S Akoka (2017) The new face of isotopic NMR at natural abundance. Magn Reson Chem 55(2): 77-90.
- GJ Martin, M Mazure, C Jouitteau, YL Martin, L Aguile, et al. (1999) Am J Enol Vitic 50: 409-417.
- N Ogrinc, IJ Kosir, M Kocjancic, J Kidric, Determination of Authenticy, Regional Origin, and Vintage of Slovenian Wines Using a Combination of IRMS and SNIF-NMR Analyses. J Agric Food Chem 49(3): 1432-1440.
- JE Giménez-Miralles, DM Salazar, I Solana (1999) Regional origin assignment of red wines from Valencia (Spain) by (2)H NMR and (13)C IRMS stable isotope analysis of fermentative ethanol. J Agric Food Chem 47: 2645-2652.
- J Wei, X Jie, L Xiang, W De-liang, G Yang, et al. (2015) Int J Food Sci Technol 50: 774-781.
- L Mannina, A Sobolev, S Viel (2012) PNAS 66: 1-39.
- J Keeler (2002) Understanding NMR Spectroscopy 2nd ed Wiley, pp. 96-125.
- a) JE Herbert-Pucheta, I Mejía-Fonseca, LG Zepeda-Vallejo, D Milmo-Brittinham, G Padilla-Maya (2019) The “Wine-T1” NMR experiment for novel wine-metabolome fingerprinting with nuclear-spin relaxation. Bio web of conferences 12, 02029; b) a) JE Herbert-Pucheta, C Pino-Villar, F Rodríguez-González, G Padilla-Maya, D Milmo-Brittinham and LG Zepeda-Vallejo (2019) “One-shot” analysis of wine parameters in non-Saccharomyces large-scale alcohol reduction processes with one- and two-dimensional nuclear magnetic resonance.
- W Kew, N GA Bell, I Goodall, D Uhrín (2017) Advanced solvent signal suppression for the acquisition of 1D and 2D NMR spectra of Scotch Whisky. Magn Reson Chem 55(9): 785-796.
- YB Monakhova, SP Mushtakova, T Kuballa, DW Lachenmeier (2014) Investigation into the structural composition of hydroalcoholic solutions as basis for the development of multiple supression pulse sequences for NMR measurement of alcoholic beverages. Magn Reson Chem 52: 755-759.
- M Nilsson, IF Duarte, C Almeida, I Delgadillo, BJ Goodfellow, et al. (2004) High-resolution NMR and Diffusion-Ordered Spectroscopy of Port Wine. J Agric Food Chem 52(12): 3736-3743.






























