Π-Extended Systems Based on Tetracyclic Benzo [4,5] Imidazo [2′,1′:6,1] Pyrido[2,3-D] Pyrimidines
Harutyunyan Artur A1,2*, Ghukasyan Gohar T1 and Danagulyan Gevorg G1,2
1 Russian Armenian (Slavonic) University, Yerevan, Armenia, Europa
2Scientific and technological center of organic and pharmaceutical chemistry, Yerevan, Europa
Submission: March 11, 2019; Published: April 08, 2019
*Corresponding author: Harutyunyan Artur A, Russian Armenian (Slavonic) University, Yerevan, Armenia, Europa
How to cite this article: Harutyunyan A, Ghukasyan GT, Danagulyan G. Π-Extended Systems Based on Tetracyclic Benzo [4,5] Imidazo [2′,1′:6,1] Pyrido[2,3-D] Pyrimidines. Organic & Medicinal Chem IJ. 2019; 8(3): 555737. DOI: 10.19080/OMCIJ.2019.08.555737
Introduction
Recently, we have developed a fundamentally new method of constructing the derivatives of the heterocyclic system of benzo[4′,5] imidazo[2′,1′:6,1]pyrido[2,3-d]pyrimidine [1], which allowed the introduction of methyl and methylene into the molecule groups. It should be noted that some derivatives of the indicated heterocyclic system revealed antibacterial and ant monoamine oxidase properties [2,3].
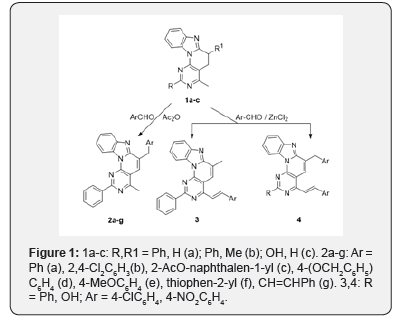
Taking into account, we have developed possibilities for the further functionalization of the class of compounds under discussion, in particular, for obtaining new derivatives on their basis with a lengthy system of conjugated π-bonds that are interesting in terms of biomedical and technical applications. In this context, we developed the reactions of 4-methyl-2-phenyl-5,6-dihydrobenzo[4′,5′] imidazo [2′,1′:6,1] pyrido[2,3-d] pyrimidine 1a, (RS)-4,6-dimethyl-2-phenyl-5,6-di hydrobenzo [4′,5′] imidazo[2′,1′:6,1] pyrido[2,3-d] pyrimidine1b and 4-methyl-5,6-dihydrobenzo [4′,5′] imidazo[2′,1′:6,1]pyrido[2,3-d]pyrimidin-2-ol 1c with aromatic aldehydes in various experimental conditions. This allowed us to obtain benzo [4’,5’]imidazo[2’,1’,6,1]pyrido[2,3-d] pyrimidine derivatives, combining two structural features: an extended π-conjugated chain and a bulky aromatic substituent in the side chain, according to the scheme: (Figure 1).
It was found that under boiling conditions of the starting tetracycle 1a, even with an excess of aromatic aldehyde in acetic anhydride, the reaction proceeds exclusively with the formation of 6-arylmethyl-4-methyl derivatives 2a-g, which is the result of a 1,3-prototropic shift of the 6-benzylidene derivative originally formed and moving double bond from exocyclic position to ring. The signal of the proton H5, which manifests itself in the 7.87 ppm region, characteristic of aromatic structures and not characteristic of protons of the olefinic double bond, is in favor of the intracyclic position of the double bond. Under more severe conditions, namely, with the simultaneous heating of heterocycles 1b or 1c with 4-chloro- and 4-nitrobenzaldehydes in the presence of ZnCl2, either only 4-methyl group is involved in the condensation reaction to form 4-styryl derivative 3, or both reaction centers to form 4-styryl-6-(4-chloro, nitro)benzyl derivatives 4. The specified course of the reaction was confirmed by IR, NMR 1H and 2D NOESY spectroscopy and X-ray crystallography.
References
- Harutyunyan AA (2017) Studies in the field of the pyrimidines and polycyclic azaheterocycles synthesis. The dissertation abstracts of the Doctor of Chemical sciences. Republic of Armenia, Yerevan.
- Harutyunyan AA, Sukasyan RS, Grigoryan AS (2016) Biol J Armenia 68(1): 60-63.
- Harutyunyan AA, Avakimyan JA, Stepanyan HM (2016) Biol J Armenia 68(2): 88-91.






























