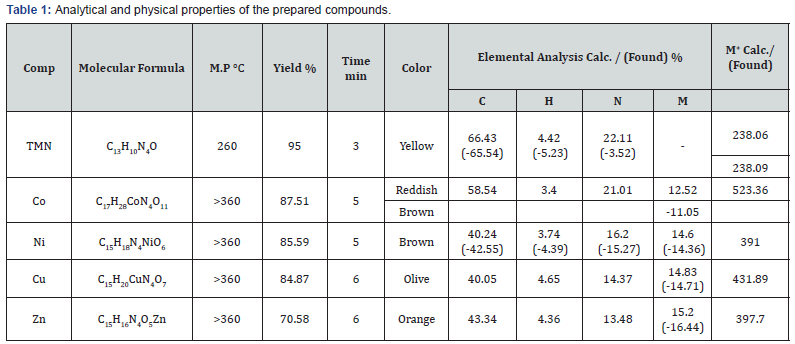
Green Synthesis, Spectral, Thermal Characterization and Biological Activity of Schiff base Ligand Derived from 3-amino-1,2,4-triazol and its Metal Complexes
Ali M Hassan1, Bassem H Heakal2, MA Bedair1, Gamal Abd El-Naeem3, ZI El-Bialy4 and Moataz M Abdelsalam4*
1Chemistry Dept, Faculty of Science, Al-Azhar University, Egypt
2Research Laboratory, Cairo Oil Refining Company, Egypt
3The city of Scientific Research and Technology Application, New Borg El-Arab Alexandria, Egypt
4Egyptian Organization for Standardization and Quality
Submission: February 18, 2019; Published: March 14, 2019
*Corresponding author: MM Abdelsalam, Egyptian Organization for Standardization and Quality, Egypt
How to cite this article: Moataz M A, Ali M H, Bassem H H, M B, Gamal A E-N. Green Synthesis, Spectral, Thermal Characterization and Biological Activity of Schiff base Ligand Derived from 3-amino-1,2,4-triazol and its Metal Complexes. Organic & Medicinal Chem IJ. 2019; 8(3): 555736. DOI: 10.19080/OMCIJ.2019.08.555736
Abstract
Green, efficient and clean synthesis of a Schiff base ligand, 1-(((1H-1,2,4-triazol-3-yl) imino) methyl) naphthalen-2-ol (TMN) has been synthesized in equimolar reaction (1:1) of 3-amino-1,2,4-triazole as a primary amine and 2-hydroxy-1-naphthaldehyde as an aldehyde using microwave irradiation technique. The synthesized Schiff base ligand was then reacted with four transition metals ions Co(II), Ni(II), Cu(II) and Zn(II) in 1:1 molar ratio of ligand and metal acetate using microwave technique. The stereochemistry and the bonding characteristics of the ligand and its metal complexes were achieved based on elemental analysis, FT-IR, UV-Vis., NMR and ESR as well as Thermo-Gravimetric Analysis (TGA). The thermal dehydration and decomposition of Co (II), Ni(II) and Cu(II) complexes were studied kinetically using the integral method applying the Coats-Redfern and Horowitz Metzger equations. The antimicrobial activities of ligand and its Zn (II) complex were studied against the bacterial (positive and negative) grams and fungal strains.
Keywords: Green chemistry Microwave irradiation Schiff base Triazole Spectral Thermal analysis Biological activity
Introduction
Nowadays, the protection of our environment and our health is becoming increasingly important due to the worldwide spread of green chemistry. According to the 12 principles of green chemistry [1], preparation and development of environmentally-friendly and harmless products and technologies are the main tasks. In this context, the application of the microwave (MW) technique in organic, inorganic, medicinal, analytical and polymer chemistry has spread fast [2-7]. Microwave-assisted synthesis is acknowledged as a breakthrough in synthetic chemistry in recent years. This technique has overcome the certain back draws associated with conventional routes i.e. larger reaction time, reduced yields and purity and slow rate of reaction. Microwave synthesis provides more opportunities for organic chemists to expand their synthetic avenues by applying microwave irradiation to a variety of organic reactions with improved results [8].
In the nineteenth century, Hugo Schiff, who was born in Germany, prepared a new family of organic compounds, which now have very wide applications plus its massive importance in thedevelopment of coordination chemistry. These compounds were known after his name as Schiff bases [9]. These compounds were firstly prepared by the condensation of primary amine (R3-NH2) with carbonyl compounds aldehydes or ketones having the carbonyl group (R1R2-C=O) to produce new compounds having the azomethine group (-C=N-). These compounds are also known as anils, imines or azomethines [10]. The produced compound, R1R2C=NR3 is called a Schiff base, where R1 is an aryl group, R2 is a hydrogen atom and R3 is either an alkyl or aryl group [11-14]. It’s also well known that many life activities depend on the heterocyclic compounds, such as purine and pyrimidine bases unit of (DNA and RNA). Five-member N-heterocycle compounds, mainly tetrazole (CH2N4), Triazoles (C2H3N3), and their substituted derivatives [15] are a group of heterocyclic compounds that attracted researchers concern. The presence of three nitrogen hetero-atoms in five-membered ring systems defines an interesting class of compounds, the triazole. This may be of two types, the 1,2,3-triazoles, and the 1,2,4-triazoles.
1,2,4-Triazole and its derivatives are an imperative type of compounds which possess environmental [16], agricultural [17-19] , industrial [20-22], and biological activities such as antimicrobial [17,23-25], antibacterial [18,26,27], anticancer [28-30], antioxidant [18,19,31], anti-inflammatory [32,33] , antiviral [34,35] and many other important applications. In the present study, A Schiff base ligand derived from the reaction between 3-amino-1,2,4-triazole and 2-hydroxy-1-naphthaldehyde and its metal complexes (CoII, NiII, CoII, and ZnII) were synthesized by microwave irradiation as a green approach. All resultant compounds were characterized spectroscopically using different spectroscopic techniques, thermogravimetric analysis and some thermo-kinetic parameter (ΔG, ΔH, and ΔS) were also calculated. The biological activities of the prepared Ligand and its Zn (II) were also evaluated
Experimental
Chemicals used for Ligand Synthesis
a) Primary Amine: 3-amino-1, 2, 4-triazole, White powder, Formula: C2H4N4, Mol. Wt: 84.04 g/mol, from Sigma-Aldrich.
b) Aldehydes: 2-hydroxy-1-naphthaldehyde; Linear formula: C11H8O2, Mol. Wt: 172.18g/mol, color: White, shape: Powder, Furan-2-carboxaldehyde; Linear formula: C5H4O2, Mol. Wt: 96.09 g/mol, color: yellow, shape: liquid, density: 1.16g/ ml, Thiophene-2-carboxaldehyde; Linear formula: C5H4OS, Mol. Wt: 112.15 g/mol, color: Brown, Shape: liquid, Density: 1.22 g/ml, all the above aldehydes were supplied from Sigma- Aldrich.
Metal Salts used for Complexes Synthesis
The selected metal salts for this purpose; Cobalt (II) acetate tetrahydrate; Linear formula: Co(CH3COO)2.4H2O, Mol. Wt: 249.08g/mol, color: Violet, shape: Powder, Nickel (II) acetate tetrahydrate Linear formula: Ni(CH3COO)2.4H2O, Mol. Wt: 248.84g/ mol, color: Blue-Green, shape: Powder, Copper (II) acetate monohydrate Linear formula: Cu(CH3COO)2.H2O, Mol. Wt: 199.65g/mol, color: Greenish-Blue, shape: Powder and Zinc (II) Acetate dihydrate; Linear formula: Zn(CH3COO)2.2H2O, Mol. Wt: 219.51 g/mol, color: White, shape: Powder, all were supplied from Sigma-Aldrich.
Solvents and other Chemicals
Distilled water, Methanol, diethyl ether, dimethylformamide (DMF) and dimethyl sulfoxide (DMSO), were of highest purity and used as received without further purification. Ethylenediaminetetraacetic acid (EDTA), murexide, hexamine, eriochrome black T (EBT) indicator, xylenol orange (XO) all of them were of Aldrich or Analar grade quality.
Physical Measurements
Melting point was measured by electrothermal apparatus and without correction, the electronic absorption spectra (UV-Vis) of ligand and the metal complexes were obtained in DMF solvent from 900-200nm range using Perkin-Elmer Lambda 35 UV/Vis Spectrophotometer fitted with a quartz cell of 1.0cm path, the Fouriertransform infrared spectra of the ligand, as well as the metal complexes dissolved in KBr were recorded on Vertex 70 Analyzer, Bruker, USA from 4000-400cm-1 and metal ions estimation was determined by complexometric titration using general lab glassware [36] at the faculty of science, Al-Azhar University, Cairo, Egypt. The magnetic susceptibility of the solid complexes was carried out at room temperature by the Gouy’s technique for magnetic susceptibility instrument. The thermogravimetric analyses (TGA) of the solid complexes were performed using the Schimadzu TG-50 thermogravimetric analyzer with a heating rate of 10℃/min under nitrogen atmosphere, in the range of 25-800℃ at the faculty of sciences, Cairo University. The ESR spectra of the powdered CuII sample were carried out on Bruker-EMX-(Xbands-9.7GHZ) spectrometer with 100KHZ frequency, microwave power 1.008MW, modulation/amplitude of 4 GAUSSES at National Center for Radiation Research and Technology, Egyptian Atomic Energy Authority.
The elemental analysis for carbon, hydrogen, and nitrogen are carried out using a Flash 2000 organic Elemental Analyzer, Thermo, USA, the mass spectra were performed by an Agilent Technologies- 6890N Network GC System and the biological activity evaluation of the synthesized ligand (TMN) and it’s Zn (II) complex was determined by using 10mg/ml solution in dimethyl sulfoxide (DMSO) at the Regional Center for Mycology and Biotechnology (RCMB) at Al-Azhar University [37] . The 1HNMR and 13C spectra were recorded on an Agilent Technologies model spectrometer NMR400-mercury 400. 1H spectra were run at 300MHz and 13C spectra were run at 75.46 MHz in dimethylsulphoxide (DMSO-d6). Tetramethyl silane (TMS) was used as an internal reference and chemical shifts are quoted in δ(ppm) at the Main Chemical Warfare Laboratories, Chemical Warfare Department, Ministry of Defense, Cairo, Egypt. Microwave-assisted reactions conducted on a commercially household microwave energy output 900 W, frequency 2450 MHz, manufactured by DAEWOO technologies Corporation, model: KOR-9G2B, Korai.
Microwave Irradiation Method
Microwave method for the Synthesis of the Schiff Base Ligand (TMN)
The equimolar ratio (1:1) of 3-amino-1,2,4-triazole (2.44 g, 0.029 mol) and 2-hydroxy-1-naphthaldehyde (5.00g,0.029mol) was ground and mixed thoroughly in a grinder until it reached a satisfactory homogeneity. The reaction mixture was then transferred to a clean crucible and moisturized by adding 3-4ml of methanol as a solvent then transferred to the microwave oven thus irradiated and heated gradually by using on/off cycling to control the temperature. The reaction completed in a short time 1-3 mins at 90-300 watt with higher yields. The products were then recrystallized with hot methanol and finally dried under reduced pressure over anhydrous CaCl2 in a desiccator. The progress of the reaction and purity of the product was monitored by TLC using silica gel G (yield: 85-95 %).
Preparation of the solid Complexes CoII, NiII, CuII and ZnII
Through complexes formation, the same microwave procedures were applied. The metal salts of the prepared Schiff base ligand, Co(CH3COO)2.4H2O, Ni(CH3COO)2.4H2O, Cu(CH3COO)2.2H2O and Zn(CH3COO)2.2H2O were mixed in (1:1) ratio. The reaction mixtures were then irradiated by the microwave oven by adding 1-3ml of methanol as a solvent. The reaction completed in a short time (5-10min) with higher yields. The obtained products were washed by hot methanol and ether then finally dried under reduced pressure over anhydrous CaCl2 in a desiccator. The progress of the reaction and purity of the products were monitored by TLC using silica gel G (yield: 70-90 %), The physical properties of the metal complexes are listed in (Table 1).
Procedure for Antibacterial Activity
Antibacterial and Fungi studies
The Schiff base ligand (TMN) and its respective Zn(II) complex were tested and evaluated for their antimicrobial activity using the agar diffusion technique (Cooper, 1972) [37]. 5mg/ml solution in dimethyl sulfoxide was used. The tested organisms were Gram-negative bacteria (Escherichia Coli (RCMB 010052) ATCC 25955& Salmonella typhimurium RCMB 006 (1) ATCC 14028), Gram-positive bacteria (Bacillus subtilis, RCMB 015 (1) NRRL B-543& Staphylococcus aureus, RCMB010010) and fungi (Aspergillus flavus, RCMB 002008) & Candida albicans RCMB 005003 (1) ATCC 10231). The bacteria and fungi were maintained on nutrient agar medium and Czapeks Dox agar medium, respectively. DMF showed no inhibition zones. The agar media were inoculated with different test microorganism. After 24 hrs. of incubation at 37°C for bacteria and 48 hrs. of incubation at 28°C for fungi, the diameter of inhibition zone (mm) was measured.
Agar Diffusion Method to Determine Antimicrobial activity
The synthesized compounds were screened for their antimicrobial activity against six different test organisms having environmental and clinically importance. The microorganism’s inoculums were uniformly spread using a sterile loop, pure colonies of the bacterial cultural were picked up. The colonies were suspended in 5ml of sterile physiologic saline. Well containing the biomaterial were placed some sterile forceps onto the agar surface and gentlypressed down to ensure contact the plates were pre-incubated for 1hr. at refrigerator followed by incubation at 37°C for 24 hrs. after incubation, the diameter of the inhibition zone was measured (including the diameter of the hole). Antimicrobial disk diffusion was performed as described by the National Committee for Clinical Laboratory Standard [38].
Result and Discussion
Characterization of the Prepared Compounds
All compounds containing the six and four-member rings with (-N- heterocycle), azomethine and hydroxyl groups possess basic characteristics due to the presence of lone pair electron-donating character of the double bond of (-HC=N) and the capability to form metal complexes. The basic strength of the C=N, (-N heteroatom) and -OH groups is insufficient solely to allow the formation of stable complexes by simple coordination of the lone pair to a metal ion. Therefore, a metal ion will attack the -N hetero and -OH groups to form six and four-membered rings for the prepared. The proposed structures of the prepared Schiff base ligand have been confirmed based on the following techniques:
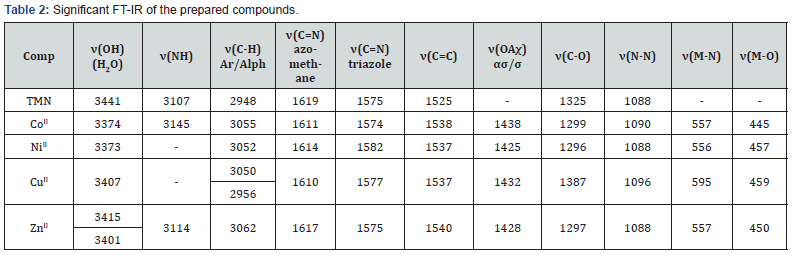
IR Spectra
The important infrared bands exhibited by the Schiff-base ligand and its complexes are given in Table 2. The ligand possessed potential donor sites like azomethine linkage (-C=N), triazole ring nitrogen (-C=N) and hydroxyl (-OH) group. The IR spectra of the ligand showed peaks at 3107, 1575 and 1088 cm-1 respectively due to vibration of (N-H), (C=N) and (N-N) of triazole moiety. Originally, 3-amino-1,2,4-triazole and aldehydes showed peaks at 3325 and 1715 cm-1 respectively, due to amino (NH2) and aldehydic (CHO) group vibrations. In the spectra of the ligand, the peaks at 1715 and 3325 cm-1 due to aldehydic (CHO) and amino (NH2) groups of the original moieties were completely disappeared and in turn, a new sharp band appeared at 1619 cm-1 assigned to the azomethine (-C=N) linkage [39]. The IR spectra of ligand showed bands at 1088 cm-1 for triazole (N-N), 1370 cm-1 for (C-O), 1525 cm-1 for (C=C), 1575 cm-1 for triazole (C=N), 1619 cm-1 for azomethine (C=N), 2885 cm-1 for aliphatic (C-H), 3029 cm-1 for Aromatic (C-H), 3107 cm-1 for triazole (N-H), 3441 cm-1 for phenolic (O-H). The comparison of the IR spectra of Schiff base ligand with corresponding metal complexes gave a different mode of absorption in complexation of the ligand with the metal ions. For ligand (TMN), the disappearance of a broad band of ν(OH) at 3441 cm-1 in spectra of metal complexes and in turn appearance of new band assigned to (C-O) at 1387-1251 cm-1 revealed deprotonation and coordination of hydroxyl-O to the metal atom.
The coordination of metal to oxygen is further justified by the appearance of a new band at 459-445 cm-1 due to M-O. For all the metals (II) complexes, a new band appeared at 595-565 cm-1 due to ν(M-N) vibrations indicating the coordination of nitrogen of (azomethine and triazole) with the metal ions. However, the IR band observed at 1088-1015 cm-1 in triazole ring is assigned to ν(N-N) mode remained unchanged in the spectra of all the metal complexes thus indicating the non-involvement of nitrogen of (N-N) of triazole ring. The C=N stretch shows both a positive and negative shift on complexation. We have observed a positive shift of azomethine to lower frequency at (1610-1617) cm-1 for all complexes the involvement of the azomethine-N in the complex formation. The IR spectra of ligand and its metal (II) complexes conclusively showed that the ligand coordinates to the metal atoms bi and tri-tentative the azomethine-N, triazole-N, and hydroxyl-O groups. Other bands ascribed to negative acetate ion (OAc) were detected in all specimens at 1438-1425cm-1 suggesting carboxylic modes (Table 2).
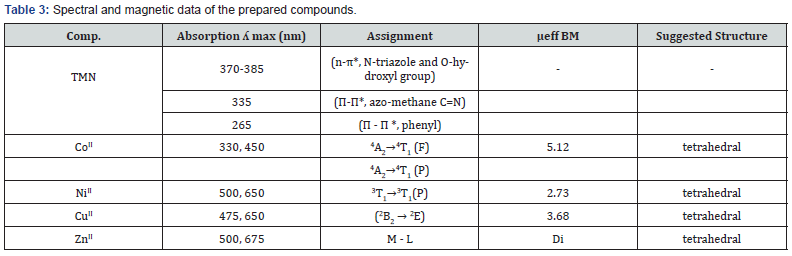
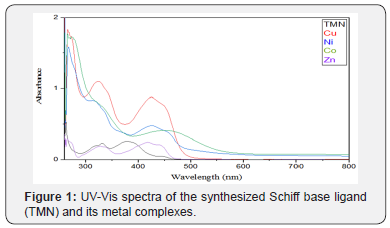
Electronic spectra and Magnetic Properties
The electronic absorption spectra of the Schiff-base ligand and its complexes are shown in Figure 1. and were performed in dimethylformamide (DMF). The position of the band maxima and the magnetic moment values of the prepared complexes are mentioned in Table 3. The electronic spectra of ligand (TMN) showed three absorption bands at 370 -385 cm-1 refers to n π*, of C=N triazole and O-hydroxyl group, respectively. And at 335 cm-1 refers to π π*, of C=N azomethane. The third band was at 265 cm-1 refers to π π*, of C=C phenyl. The electronic spectra of Co(II) complex showed two absorption bands at 330, cm-1 and 460 cm-1 which assigned respectively to the transitions 4A2→4T1 (F) and 4A2→4T1 (P) and the magnetic properties suggested a tetrahedral geometryaround Co(II) ion, the obtained magnetic moment value 5.12 B.M for Co(II) complex. For Ni(II) complex, the absorption band at 425 cm-1 was assigned to the transition 3T1(F) 3T1(P) suggesting a tetrahedral geometry around Ni(II) ion with magnetic moment value was 2.73 B.M for Ni(II). For Cu(II) complex, the spectra showed an absorption band at 425 cm-1 assigned to the transitions (2B2 → 2E) assuming a tetrahedral configuration, the obtained magnetic moment value was 3.68 B.M for Cu(II) complex. For Zn(II) absorption band at 455 cm-1 are assigned to MLCT in a tetrahedral geometry confirmed by the diamagnetic properties.
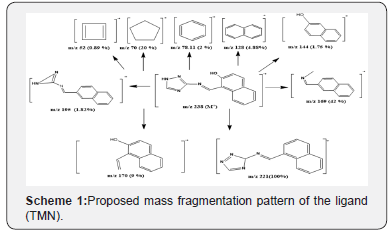
Mass Spectra of the Schiff Base Ligand (TMN)
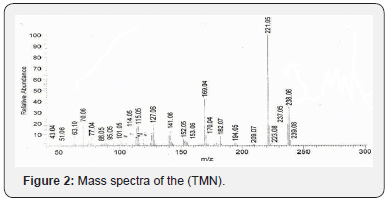
The mass spectrum of ligand (TMN) showed ion peak at m/e = 238 as molecular peak of (C13H10N4O). The ion peak at m/e = m/z 169(42 %) is due to M+(C12H11N), while the ion peak at m/e = 221.0 (100%) corresponds to M+(C13H10N4) which is the base peak, the ion peak at m/e = 170 (9%) corresponds to M+(C12H10O), the ion peak at m/e = 195 (1.82%) is due to M+(C12H9N3), the ion peak at m/e = 144 (1.75 %) points to M+(C10H8O). The ion peak at m/e = 128 (4.88%) refers to M+(C10H8), the ion peak at m/e = 78.11 (2 %) corresponds to M+(C6H6), the ion peak at m/e = 70 (20 %) corresponds to M+(C5H10) and the ion peak at m/e = 52 (0.89%) corresponds to M+(C4H4). The fragmentation patterns of 1-((1H-1,2,4-triazol-3-ylimino) methyl) naphthalen-2-ol (TMN) are shown in Scheme 1 and (Figure 2).
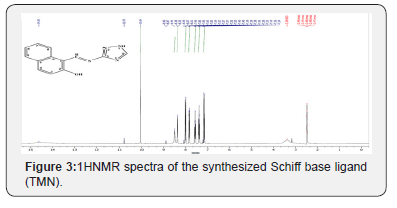
HNMR
The 1H NMR spectral data of the synthesized Schiff base ligand (TMN) are given in (Figure 3). The appeared signals of all the protons of Schiff base Ligand due to heteroaromatic/aromatic groupswere found [40] as to be in their expected region. The spectra of the synthesized ligand (TMN) showed peaks at 7.14-7.14 ppm (d, 1H, naphthalene C3-H), 7.37-7.41 ppm (t, 1H, naphthalene C6-H), 7.56-7.6 ppm (t, 1H, naphthalene C7-H), 7.84-7.86 ppm (d, 1H naphthalene C8-H), 8.29 ppm (s, 1H, triazole C-H), 10.05 ppm (s, 1H, azomethine C-H), 14.65 ppm (s, 1H, OH), 14.19 ppm (s, 1H, triazole NH).
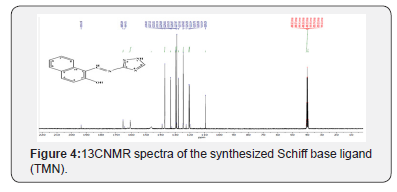
CNMR
The 13CNMR spectra of the Schiff base ligand (TMN) were taken in DMSO-d6. The characteristic absorbtion13CNMR signals are given in Figure 4. The 13C NMR spectral information is reported along with their possible assignments in the experimental section and all the carbons were found in the expected region [40-42]. The 13CNMR (DMSO) spectra of the synthesized TMN showed peaks at 109.291 ppm (C1 naphthalene), 120.386 ppm (C8 naphthalene), 120.699 ppm (C3 naphthalene), 124.39 ppm (C6 naphthalene), 127.829 ppm (C7 naphthalene), 128.973 ppm (C5 naphthalene), 129.248 ppm (C10 naphthalene), 132.191 ppm (C4 naphthalene), 133.015 ppm (C9 naphthalene), 146.284 ppm (C5 triazole), 164.509 ppm (C3 triazole), 165.638 ppm (C azomethine), 193.312 ppm (C2 naphthalene) (Figures 3 & 4).
ESR Spectrum
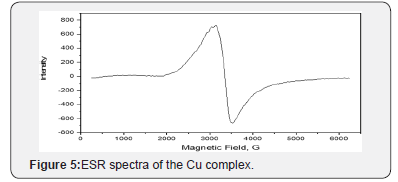
Electron spin resonance (ESR) is a sensitive spectroscopic method for the determination of the geometric and electronic structure, the dynamics and the spatial distribution of paramagnetic species in materials. ESR directly focus on the unpaired electrons and nuclei in their vicinity and is, therefore, the method of choice for studying free radicals, triplet states, compounds with transition metals, rare earth ions, and defect centers. The ESR spectra of the powdered CuII sample was carried out on Bruker- EMX-(Xbands-9.7 GHZ) spectrometer with 100KHZ frequency, microwave power 1.008MW, modulation/amplitude of 4 GAUSSES. The spectra of the complex exhibit a single anisotropic broad signal with hyperstructure indicating the contribution of free acetate ligand with complex formation. The ESR spectra of the Cu complex showed broad signals in (Figure 5). with two “g” values (g\\, g┴). Generally, the g\\ < g┴ < 2.3, characteristic of complex with 2B1(dx2-y2) orbital ground state. The average g value was calculated according to the equation gav = 1/3[g\\ +2g┴] and it was equal to 2.15 for the prepared Cu complex. The g\\ is a moderately function for covalent nature. The prepared cu-complex exhibited g\\ at 2.05 which are less than 2.3, suggesting the covalent character of copper - ligand bonds in the present complexes, where, g\\ < 2.3 concerns ionic metal-ligand bond [43-48]. Fairly high values of g are in conformity with the oxygen, nitrogen and sulfur coordination in these compounds [44]. This coupling, known as the hyperfine interaction. The above complexes have a tetrahedral structure as indicated from the electronic absorption spectra.
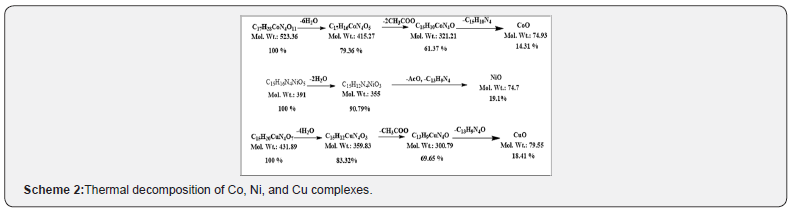
Thermal Analysis (TG)
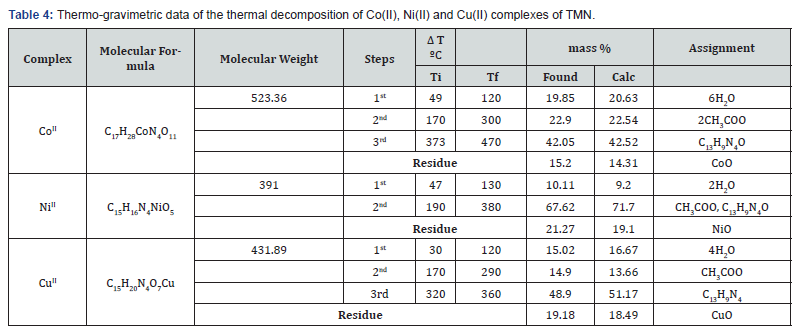
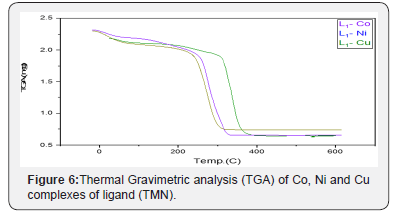
In the present investigation, the heating rates were suitably controlled at 10°C min-1 under nitrogen atmosphere and the weight loss was measured from ambient temperature up to 800°C. The TGA data of the thermal decomposition of the prepared complexes are shown in Table 4, Figure 6. and Scheme 2. The thermogram (TGA) data of CoII complex, Figure 6, exhibited three stages of decomposition. The first stage (30-120°C) corresponds to the loss of six coordinated water molecules (found/calculated=19.85/20.63 %). The second step of decomposition (230-337°C) may be due to the elimination of two coordinated acetate ions (found/calculated =22.9/22.54%), meanwhile the third stage refers to the decomposition of the complex with the formation of CoO as a final product (found/calculated =15.2/14.31%). For NiII complex, the first stage in temperature range (40-130°C) corresponds to the loss of two coordinated water molecules (found/calculated = 10.11/9.20%), while the final stage refers to the decomposition of one acetate group and the organic part leaving the metal oxide NiO as a residue (Found/Calculated = 21.27/19.1). The TGA data of CuII complex, shown in figure 6 and scheme 2, revealedthree stages of decomposition. The first stage (50-120°C) related to the loss of four coordinated water molecules (found/calculated = 18.02/16.67%). The second step of degradation (170-290°C) corresponds to the elimination of one acetate group (found/calculated= 11.9/13.66%). The final stage detected at 290-360°C point to the gradual decomposition of the complex with the formation of CuO at the final stage (found/calculated = 19.18/18.41 %).
Thermo-kinetic Parameters
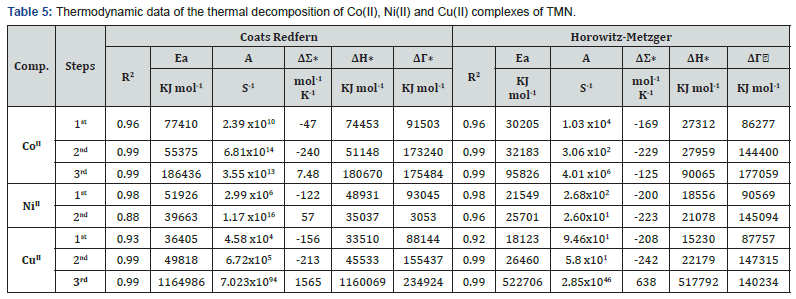
The thermodynamic activation parameters of decomposition processes of dehydrated complexes namely activation energy (E*), enthalpy (ΔH*), entropy (ΔS*) and Gibbs free energy change of the decomposition (ΔG*) are evaluated graphically by employing the Coats-Redfern [49] relation and Horowitz-Metzger [50]. Kinetic parameters of different stages are summarized in Table 5, together with the radii of metal ions. The results showed that the values obtained by various methods are comparable and were in harmony with each other. The kinetic and thermodynamic parameters were determined by non-isothermal methods. The thermodynamic activation parameters for the thermal decomposition steps in complexes were calculated using the relationships:
ΔH*=E*-RT ΔS*=R[ln(Ah/kT)-1] ΔG*=ΔH*-TΔS*

where k is Boltezmann constant and h is Plank’s constant, A is the frequency factor and R is the gas constant. From the results listed in Table 6, we get conclusions as follows:
The negative values of activation entropies ΔS* indicate the reactions are slow and the products more stable than reactants [51], while the positive values of ΔS* indicate that the activated complexes for this decomposition stage have a less ordered structure compared to the reactant. Further, the reaction may be described as faster than normal. The positive values of the ΔH* mean that the decomposition processes are endothermic [52]. The values of ΔG* increases significantly for the subsequent decomposition stages, increasing the values of ΔG* as going from one decomposition step subsequently to another reflects that the rate of removal of the subsequent ligand will be lower than that of the precedent ligand and the reactions are non-spontaneous (Table 5).
Biological Activity
Antimicrobial Activity
To contribute to the field of bioinorganic chemistry, the synthesized ligand and its Zn(II) complex were tested against bacterial and fungal strains by disc diffusion method. The microorganisms used in the present investigations included bacterial and fungal strains. Bacterial strains; Gram-positive bacteria (Staphylococcus aureus (RCMB010010) and Bacillus Subtillus RCMB 015 (1) NRRL B-543 Gram-negative bacteria (Escherichia Coli (RCMB 010052) ATCC 25955and fungal strains; Aspergillus fumigatus (RCMB 002008) and Candida albicans RCMB 005003 (1) ATCC 10231. The results were compared with those of the standard drugs (Gentamicin for Gram-positive bacteria and Gram-negative bacteria and Ketoconazole for fungal strains) and calculated the diameter of inhibition zone for each by mm. The antimicrobial results evidently show that the triazole derivatives and its complexes possess a broad spectrum of activity against the tested organisms.
Antimicrobial activity of Ligand and its Zn(II) complex
The antimicrobial activity of ligand (TMN) and its Zn(II) complex against the bacterial and fungal strains were tested and evaluated. Table 6 shows the antimicrobial activity of ligand against the tested bacterial and fungal strains. Our results showed no inhibition effect on the growth of the bacterial and fungal strains by the ligand excepted the effect on the growth of Bacillus subtillus RCMB 015 (1) NRRL B-543, Aspergillus fumigatus (RCMB 002008) and Candida albicans RCMB 005003 (1) ATCC 10231 with inhibition zones 9 mm, 17 mm and 15 mm, respectively. While the Zn (I) complex exhibited good antimicrobial activity against both bacteria (Gram-negative & Gram-positive bacteria) and fungal strains. It showed higher antibacterial activity againstBacillus subtillus RCMB 015 (1) NRRL B-543, Staphylococcus aureus (RCMB010010) and Escherichia Coli (RCMB 010052) ATCC 25955 with inhibition zone 15mm, 13mm and 14mm respectively, while appearance moderated to weak activity against Aspergillus fumigatus (RCMB 002008) and Candida albicans RCMB 005003 (1) ATCC 10231 with inhibition zone 16 and 14mm, respectively. Hence the antimicrobial activity after complexation with Zn(II) is enhanced as compared to that of the free ligand.
Conclusion
Using the microwave method assures the principals of green chemistry. The structure of the prepared compounds was spectroscopically illustrated by FT-IR, NMR, Mass, and elemental analysis. The newly synthesized Schiff bases ligand obtained from 3-amino- 1,2,4-triazole with 2-hydroxy-1-naphthaldehyde using microwave technique act as bidentate and tridentate ligand, which coordinated through the azomethine-N, methoxy-O and triazole ring to the metal ions; Co(II), Ni(II), Cu(II) and Zn(II). The thermal dehydration and decomposition of Cu(II) complex show elimination of water, acetate then organic content and MO remained as a residue. The antimicrobial activity of the Schiff base ligand (TMN) and its Zn(II) complex against the bacterial and fungal strains showed that the activity of the ligand after complexation with Zn(II) is enhanced as compared to that of the free ligand.
References
- Anastas P, Eghbali N (2010) Green chemistry: principles and practice. Chemical Society Reviews, 39(1): 301-312.
- De la Hoz A, Loupy A (2013) Microwaves in organic synthesis, 2 volume set: John Wiley & Sons United States.
- Kappe CO, Stadler A, Dallinger D (2012) Microwave theory. Microwaves in Organic and Medicinal Chemistry, 2nd edn): 9-39.
- Ameta SC, Punjabi PB, Ameta R, Ameta C (2014) Microwave-assisted organic synthesis: a green chemical approach: CRC Press, USA .
- Horikoshi S, Serpone N (2013) Microwaves in nanoparticle synthesis: fundamentals and applications: John Wiley & Sons, USA.
- De Souza, ROMA (2015) Theoretical Aspects of Microwave Irradiation Practices. In Production of Biofuels and Chemicals with Microwave. Dordrecht: Springer 3-16.
- Chemat F, Cravotto G (2013) Microwave-assisted extraction for bioactive compounds. Theory and practice. Springer.
- Ehsan S, Ishfaq A, Khan B, Saghir T, Ghafoor S (2012) Conventional & Microwave-Assisted Synthesis and Antimicrobial Evaluation of Pyrimidine Azo Compounds. International J. of science and research 3(12): 368-373.
- Abo Dena AS (2014) To the memory of Hugo Schiff: Applications of Schiff bases in potentiometric sensors. Russian Journal of Applied Chemistry 87(3): 383-396.
- Ashraf MA, Mahmood K, Wajid A, Maah MJ, Yusoff I (2011) Synthesis, characterization and biological activity of Schiff bases. IPCBEE 10: 1-7.
- Boceiri N, Benabdallah T, Youcef MH, Reffas H (2016) Synthesis and Characterization of a Novel Series of Amphiphilic Mercapto-1, 2, 4-Triazole Schiff Base Ligands: Investigation of their Behavior in Hydro-Organic Solutions. Journal of Surfactants and Detergents 19(3): 583-597.
- Al-Shemary RK, Al-khazraji AM, Niseaf AN (2016) Preparation, spectroscopic study of Schiff base ligand complexes with some metal ions and Evaluation of antibacterial activity. The Pharma Innovation 5(1, Part B): 81.
- Bader NR (2010) Applications of Schiff’s bases chelates in quantitative analysis: A review. Rasayan Journal of Chemistry 3(4): 660-670.
- Mohamed SA, Ahmed AO, Humada AA (2009) Synthesis and Characterization of Some Schiff Bases (derived from thiazole) and Their Complexes with Co (II), Ni (II) and Cu (II). Journal of Kirkuk University–Scientific Studies 4(2): 37-45.
- Balabin RM (2009) Tautomeric equilibrium and hydrogen shifts in tetrazole and triazoles: Focal-point analysis and ab initio limit. The Journal of chemical physics 131(15): 154307.
- Kavaklı C, Kavaklı PA, Güven O (2014) Preparation and characterization of glycidyl methacrylate grafted 4-amino-1, 2, 4-triazole modified nonwoven fiber adsorbent for environmental application. Radiation Physics and Chemistry 94: 111-114.
- Shuklaa PK, Sonia N, Vermaa A, Jhab AK (2014) Synthesis, characterization and in vitro biological evaluation of a series of 1, 2, 4-triazoles derivatives & triazole based schiff bases. Der Pharma Chemica 6(3): 153-160.
- Hanif M, Saleem M, Hussain MT, Rama NH, Zaib S, Aslam MAM, et al. (2012) Synthesis, urease inhibition, antioxidant and antibacterial studies of some 4-amino-5-aryl-3H-1, 2, 4-triazole-3-thiones and their 3, 6-disubstituted 1, 2, 4-triazolo [3, 4-b] 1, 3, 4-thiadiazole derivatives. Journal of the Brazilian Chemical Society 23(5): 854-860.
- Maste MM, Ainapure R, Patil PB, Bhat AR (2011) Triazolone and their derivatives for anti-tubercular activities. Asian J Research Chem 4(7): 1050-1054.
- Kamal A, Syed MAH, Mohammed SM (2015) Therapeutic potential of benzothiazoles: a patent review (2010–2014). Expert opinion on therapeutic patents 25(3): 335-349.
- Sripriya S, Subha C, Selvaraj A (2013) The inhibition chemistry of 2-amino, 5-phenyl 1, 3, 4-triazole for aluminium in hydrochloric acid solution. IOSR-Journal of Applied Chemistry 6(2): 25-29.
- Murtaza S, Akhtar MS, Kanwal F, Abbas A, Ashiq S, et al. (2017) Synthesis and biological evaluation of schiff bases of 4-aminophenazone as an anti-inflammatory, analgesic and antipyretic agent. Journal of Saudi Chemical Society 21(1): S359-S372.
- Abdulrasool MM, Jawad AH, Shneine JK (2012) Synthesis, characterization and evaluation of biological activity of new heterocyclic compounds containing 1,2,4-triazoleand 1,3, 4-thiadiazole rings. International Journal of Applied Science and Technology 2(10): 155-164.
- Sahoo S, Patwari K, Kumar C, Setty CM (2013) Synthesis and Biological Activity of Certain Mannich Bases Derivatives from 1, 2, 4-Triazoles. Iranian Journal of Pharmaceutical Sciences 9(4): 51-60.
- Al-Khuzaie MG, Al Majidi SM (2014) Synthesis, characterization and evaluation antimicrobial activity of some new substituted 2-mercapto-3-phenyl-4 (3H)-quinazolinone. Iraqi Journal of Science, 55: 582-593.
- Refaee M, Abou-Elmagd W, El-Shahawi M, Hekal M (2014) Novel Fused and Spiro Heterocyclic Compounds Derived from 4-(4-amino-5-mercapto-4H-1, 2, 4-triazol-3-yl) phthalazin-1 (2H)-one. European Chemical Bulletin 3(7): 723-728.
- Jassim WK, Fayad AA, Jassim IK (2011) Synthesis and characterization of some substituted heterocyclic compounds and evaluation of biological activity. karbala journal of pharmaceutical sciences 2: 228-240.
- Hou YP, Sun J, Pang ZH, Lv PC, Li DD, et al. (2011) Synthesis and antitumor activity of 1, 2, 4-triazoles having 1, 4-benzodioxan fragment as a novel class of potent methionine aminopeptidase type II inhibitors. Bioorganic & medicinal chemistry 19(20): 5948-5954.
- Baviskar BA, Khadabadia SS, Deore SL, Shiradkar MR (2012) Synthesis of clubbed triazolyl indeno [1, 2-C] isoquinolines as a novel anticancer agent. Der Pharmacia Sinica 3(1): 24-30.
- Kattimani PP, Kamble RR, Kariduraganavar MY, Dorababu A, Hunnur RK (2013) Synthesis, characterization and in vitro anticancer evaluation of novel 1, 2, 4-triazolin-3-one derivatives. European Eur J Med Chem 62: 232-240.
- Hameed AA, Hassan F (2014) Synthesis, Characterization and Antioxidant Activity of Some 4-Amino-5-Phenyl-4h-1, 2, 4-Triazole-3-Thiol Derivatives. International Journal of Applied 4(2): 202-211.
- Mousa MN, Al-jadaan SA (2012) Evaluation of The Anti-Inflammatory Activity and Ulcerogenic Liability of 5-(3-Chloro-1-benzothien-2-yl)-4-phenyl-4H-1, 2, 4-triazole-3-thiol. Basrah Journal of Veterinary Research 11(1): 122-127.
- Akhter MW, Hassan MZ, Amir M (2014) Synthesis and pharmacological evaluation of 3-diphenylmethyl-6-substituted-1, 2, 4-triazolo [3, 4-b]-1, 3, 4-thiadiazoles: A condensed bridgehead nitrogen heterocyclic system. Arabian Journal of Chemistry 7(6): 955-963.
- Pandey V, Chawla V, Saraf SK (2012) Comparative study of conventional and microwave-assisted synthesis of some Schiff bases and their potential as antimicrobial agents. Medicinal Chemistry Research, 21(6): 844-852.
- Abdullah HM, Jassim IK, Safi MN (2014) Synthesis and characterization of new heterocyclic compounds with studying its biological activity. karbala journal of pharmaceutical sciences 4: 119-135.
- Furniss BS (1989) Vogel's textbook of practical organic chemistry: Pearson Education India Cooper R, Kavanagh F Analytical microbiology 1(2): 1972.
- Kiehlbauch JA, Hannett GE, Salfinger M, Archinal W, Monserrat C, et al. (2000) Use of the National Committee for Clinical Laboratory Standards Guidelines for Disk Diffusion Susceptibility Testing in New York State Laboratories. J Clin Microbiol 38(9): 3341-3348.
- Finney DJ (1971) Probit Analysis: (3rd Edn): Cambridge University Press.
- Cooper R, Kavanagh F (1972) Analytical microbiology 1(2).
- Yoe JH, Jones AL (1944) Colorimetric determination of iron with disodium-1, 2-dihydroxybenzene-3, 5-disulfonate. Industrial & Engineering Chemistry Analytical Edition 16(2): 111-115.
- Chohan ZH, Sumrra SH, Youssoufi MH, Hadda T B (2010) Design and synthesis of triazole Schiff bases and their oxovanadium (IV) complexes as antimicrobial agents. Journal of Coordination Chemistry, 63(22): 3981-3998.
- Prashanthi Y, Raj S (2010) Synthesis and characterization of transition metal complexes with N, O; N, N and S, N-donor Schifff base ligands. Journal of Scientific Research 2(1): 114-126.
- Gup R, Gökçe C, Dilek N (2015) Synthesis, structural characterization and DNA interaction of zinc complex from 2, 6-diacetylpyridine dihydrazone and {4-[(2E)-2-(hydroxyimino) acetyl] phenoxy} acetic acid. Journal of Photochemistry and Photobiology B: Biology 144: 42-50.
- Kivelson D, Neiman R (1961) ESR studies on the bonding in copper complexes. The Journal of Chemical Physics 35(1): 149-155.
- Lever ABP (1968) Inorganic electronic spectroscopy. Journal of chemical education 46(9): A628.
- Calle C, Eichel R A, Finazzo C, Forrer J, Granwehr J, Gromov I, et al. (2001) Electron paramagnetic resonance spectroscopy. CHIMIA International Journal for Chemistry 55(10): 763-766.
- Fouda MFR, Abd-Elzaher MM, Shakdofa MME, El Saied FA, Ayad MI, et al. (2008) Synthesis and characterization of transition metal complexes of N′-[(1, 5-dimethyl-3-oxo-2-phenyl-2, 3-dihydro-1H-pyrazol-4-yl) methylene] thiophene-2-carbohydrazide. Transition Metal Chemistry 33(2): 219-228.
- Carrington A, McLachlan AD (1967) Introduction to magnetic resonance: with applications to chemistry and chemical physics 44(12): 772.
- Coats AW, Redfern JP (1964) Kinetic parameters from thermogravimetric data. Nature 201(4914): 68-69.
- Horowitz HH, Metzger GA (1963) new analysis of thermogravimetric traces. Analytical chemistry, 35(10): 1464-1468.
- Frost AA, Pearson RG (1961) New York. Kinetics and Mechan ism. John Wiley and Sons. Inc New York: 480.
- El-Ayaan U, Kenawy I, El-Reash YA (2007) Synthesis, thermal and spectral studies of first-row transition metal complexes with girard P reagent-based ligand. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 68(2): 211-219.






























